Novel Double-Hit Model of Radiation and Hyperoxia-Induced Oxidative Cell Damage Relevant to Space Travel
Abstract
:1. Introduction
2. Results
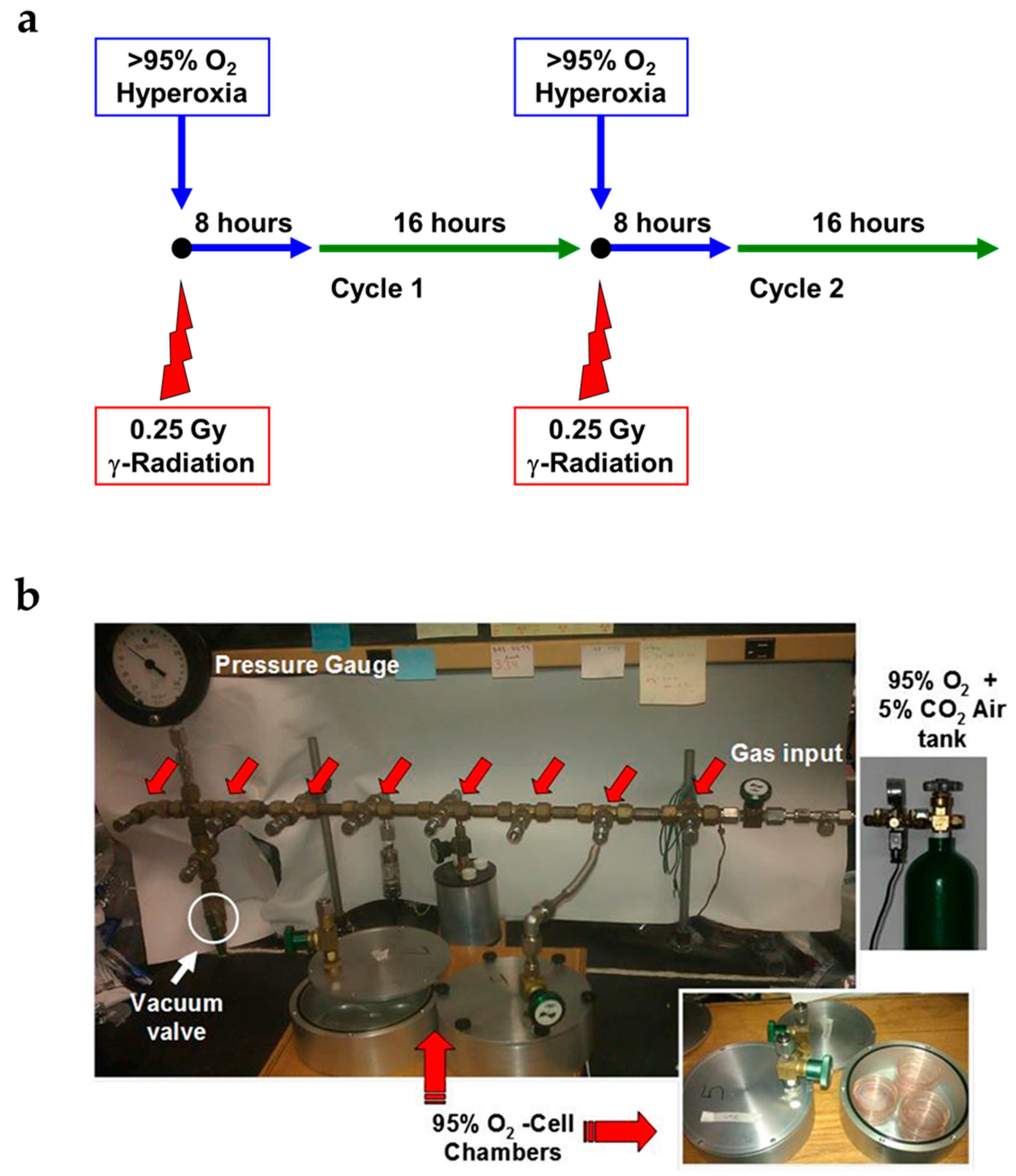
2.1. Novel Design of Airtight Chambers for in Vitro Exposures to Hyperoxia and Radiation
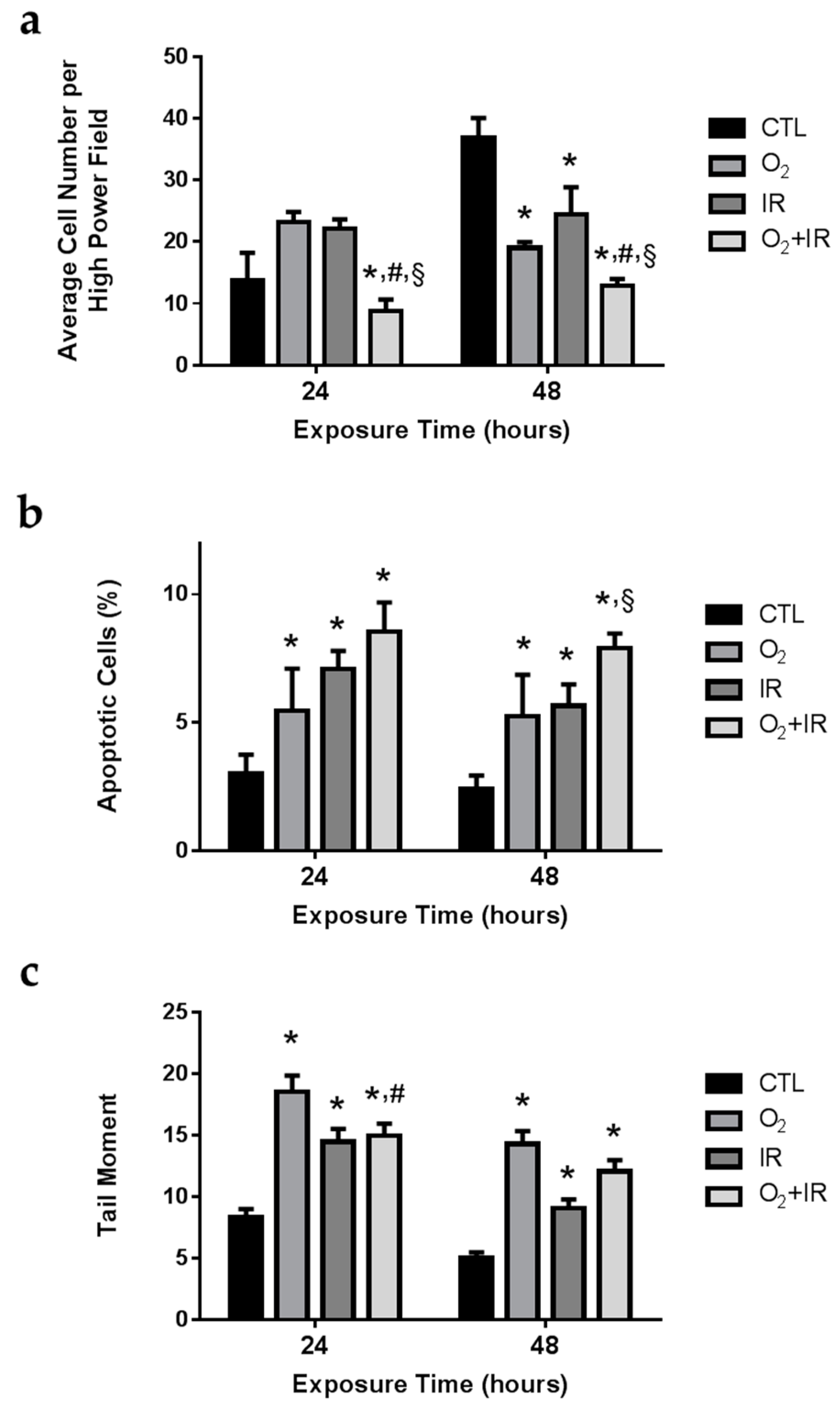
2.2. Cell Survival, Apoptosis, and Oxidative DNA Damage in Lung Epithelial Cells Exposed to Hyperoxia, Low-Level Radiation, and Double-Hit Combination Challenge
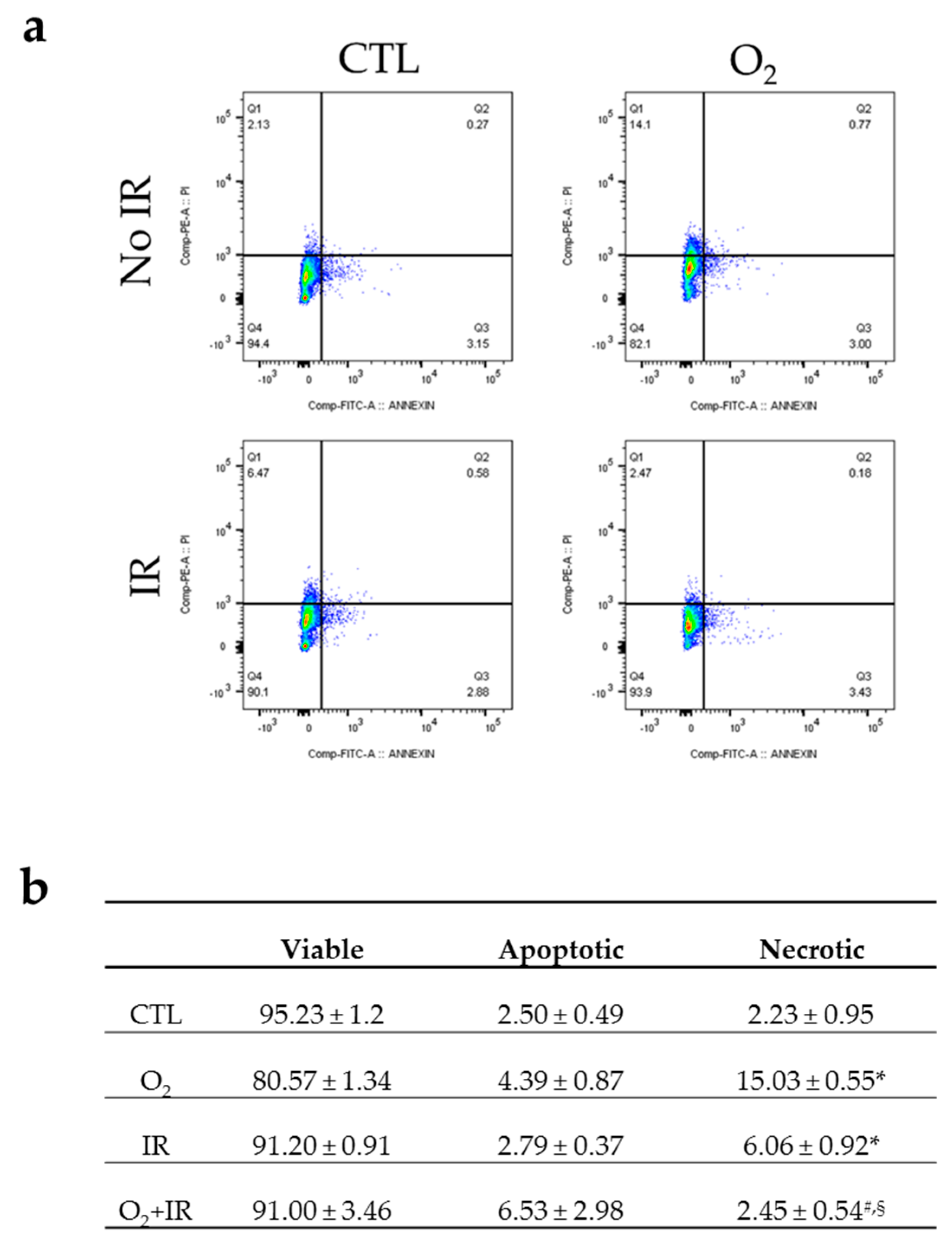
2.3. Double-Hit Combination Challenge Switches Cell Death to Apoptosis in Lung Epithelial Cells
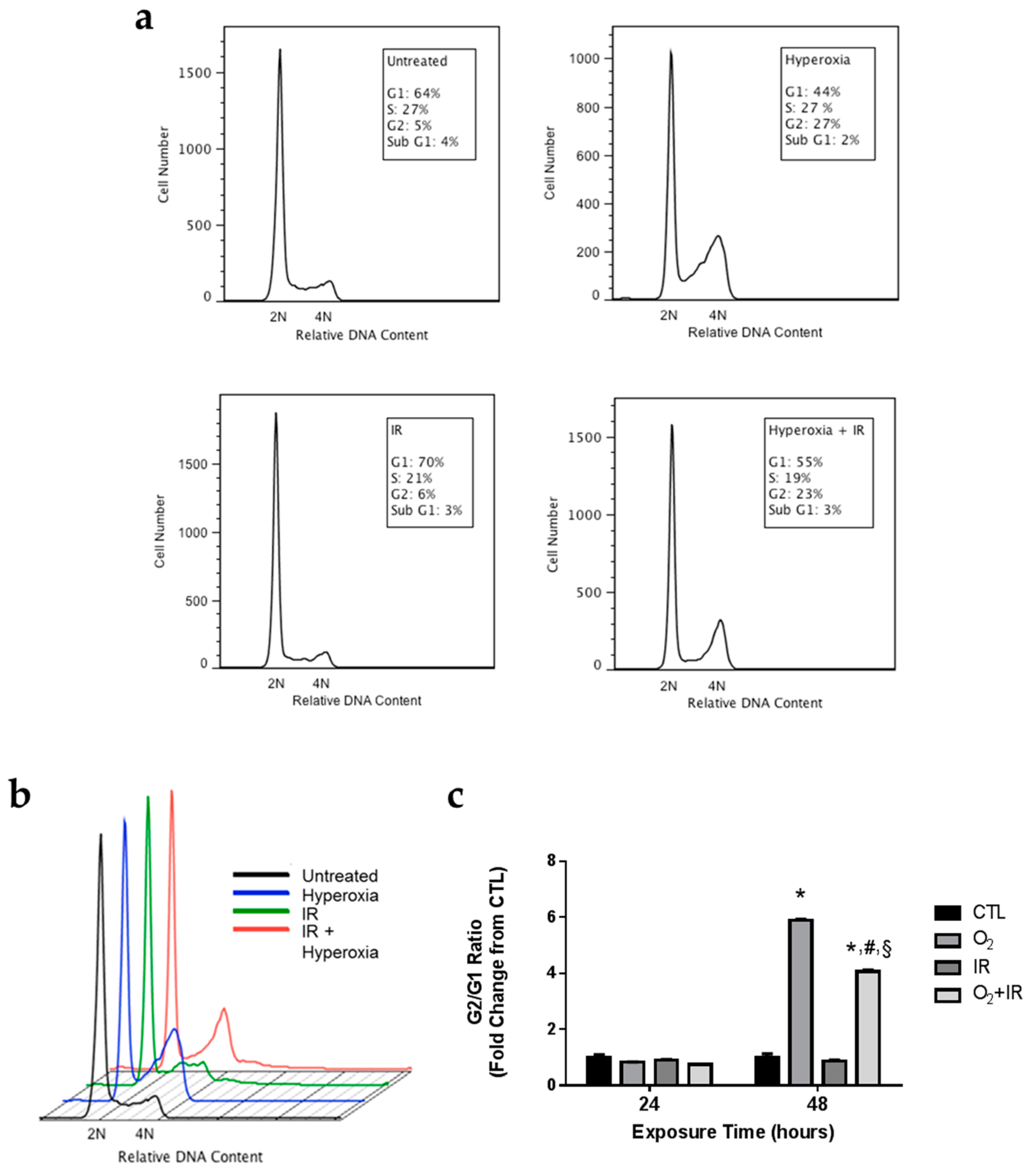
2.4. Alterations in Cell Cycle Progression in Lung Epithelial Cells Exposed to Repeat Cycles of Hyperoxia, Radiation, and Double-Hit Combination Challenge
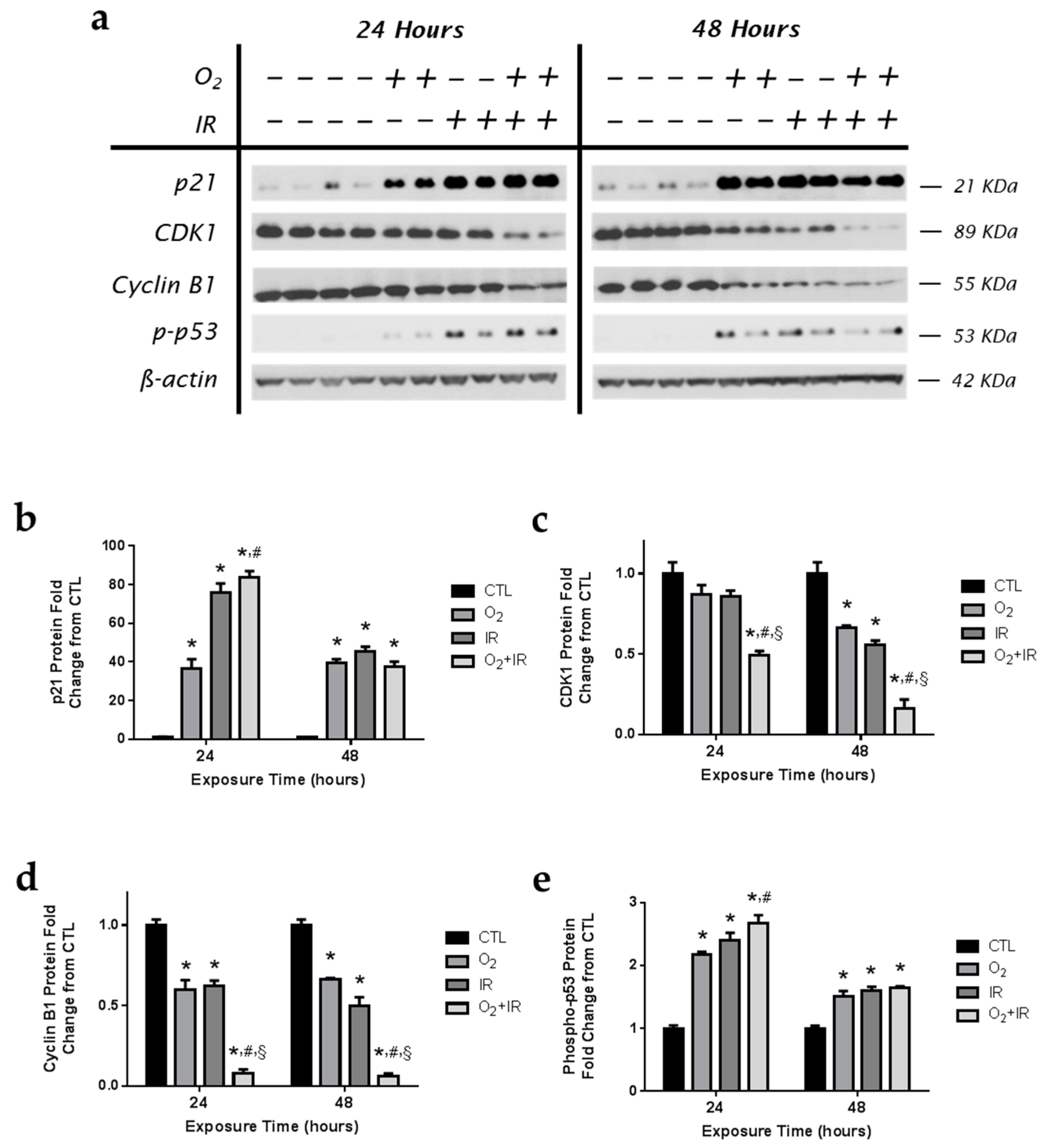
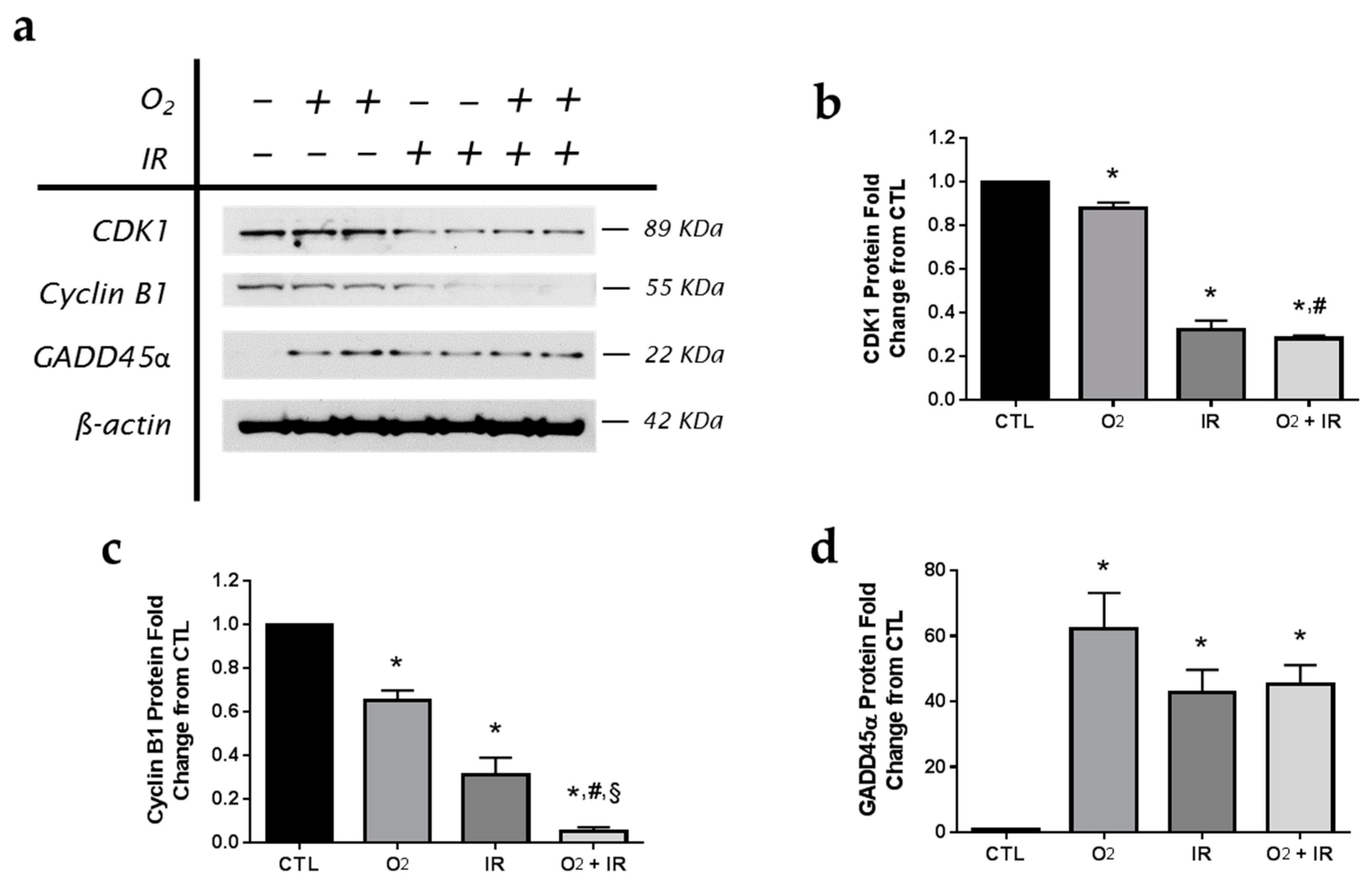
2.5. Modulation of Key Cell Cycle Signaling Protein Levels in Lung Epithelial Cells Exposed to Hyperoxia, Radiation, and Double-Hit Combination Challenge
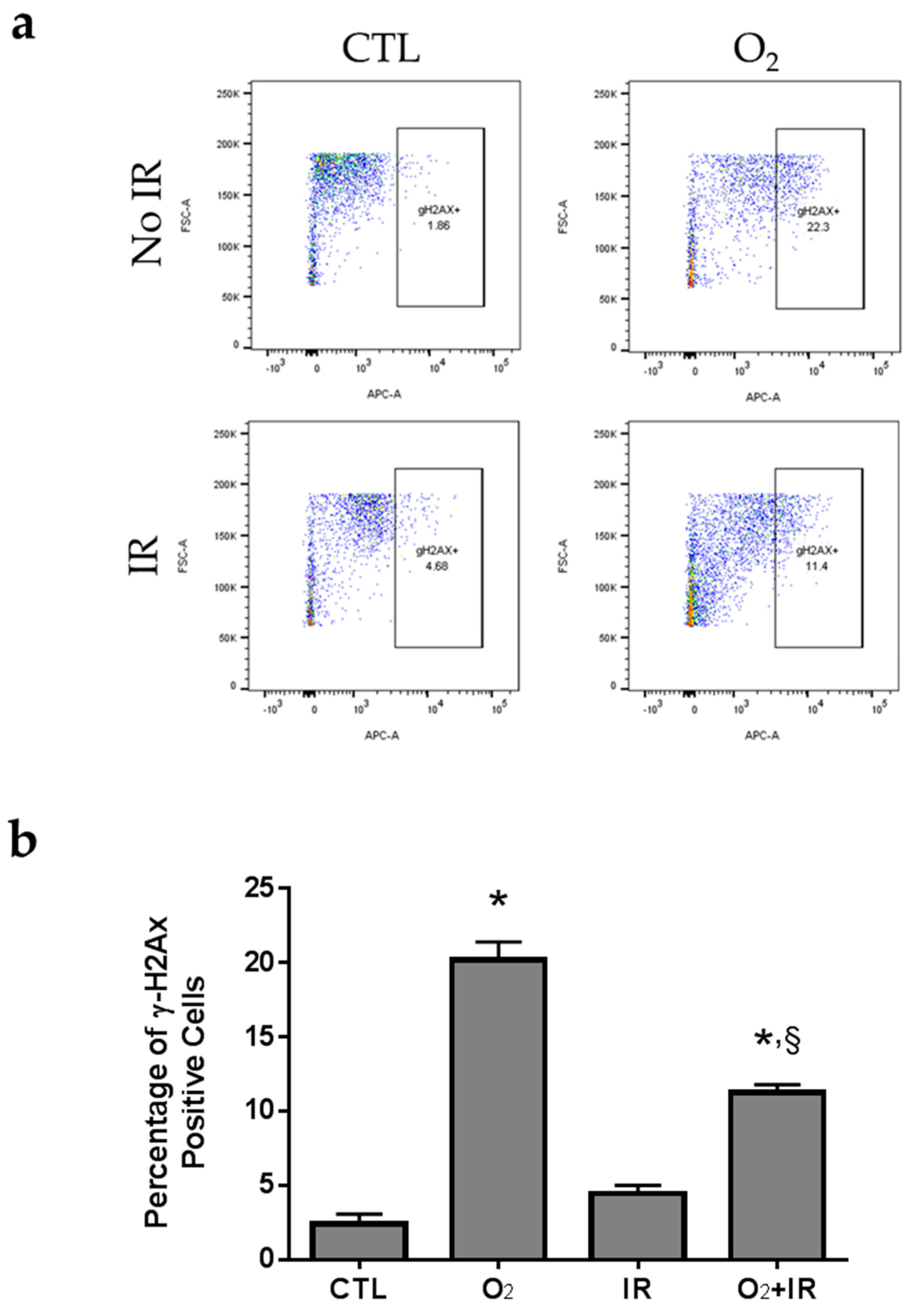
2.6. H2AX Phosphorylation due to DNA Damage in Pulmonary Epithelial Cells Exposed to Repeat Cycles of Radiation, Hyperoxia or Double-Hit Combination Challenge
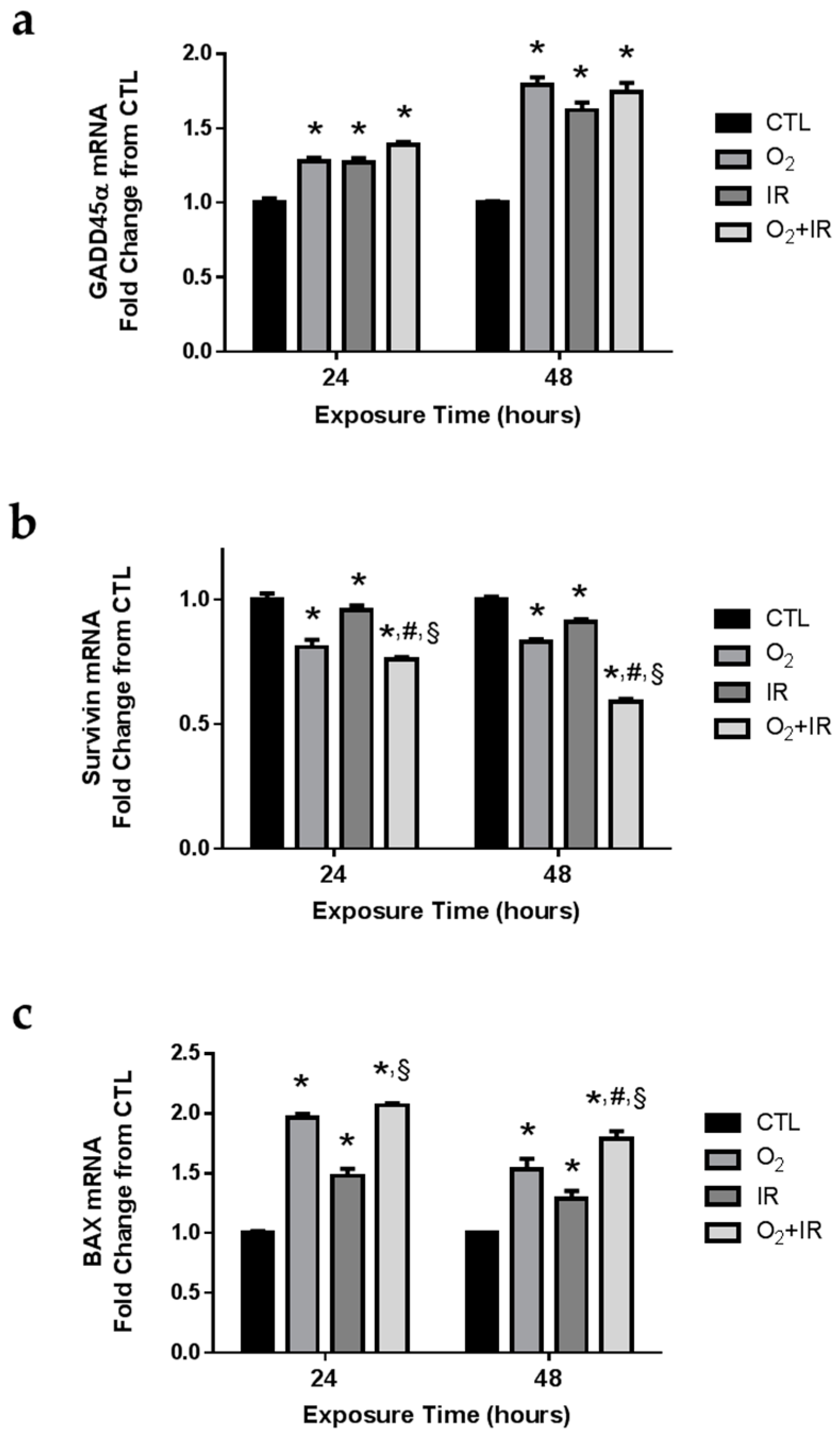
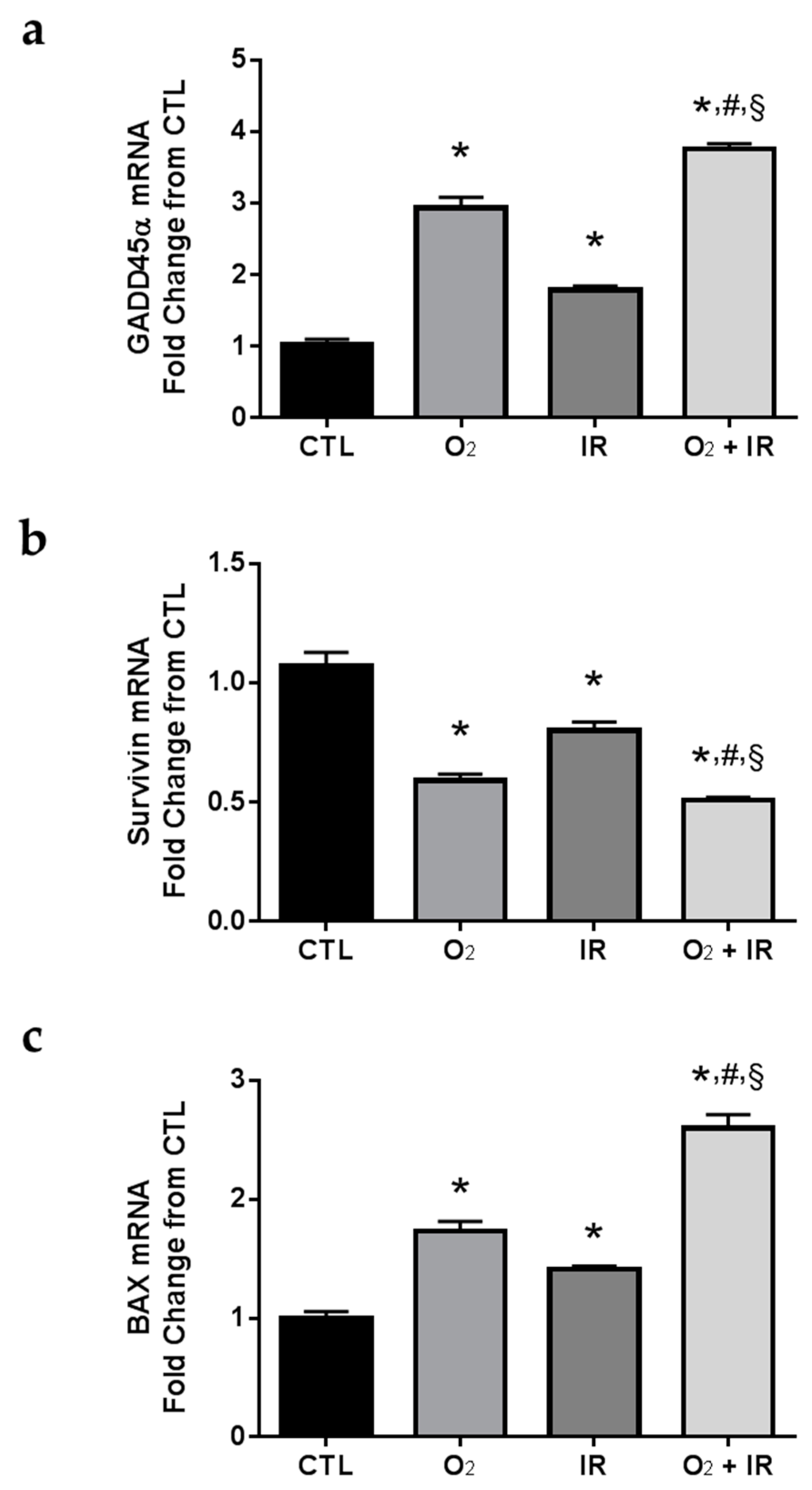
2.7. Gene Expression Changes Related to DNA Damage and Apoptosis in Pulmonary Epithelial Cells Exposed to Repeat Cycles of Radiation, Hyperoxia or Double-Hit Combination Challenge
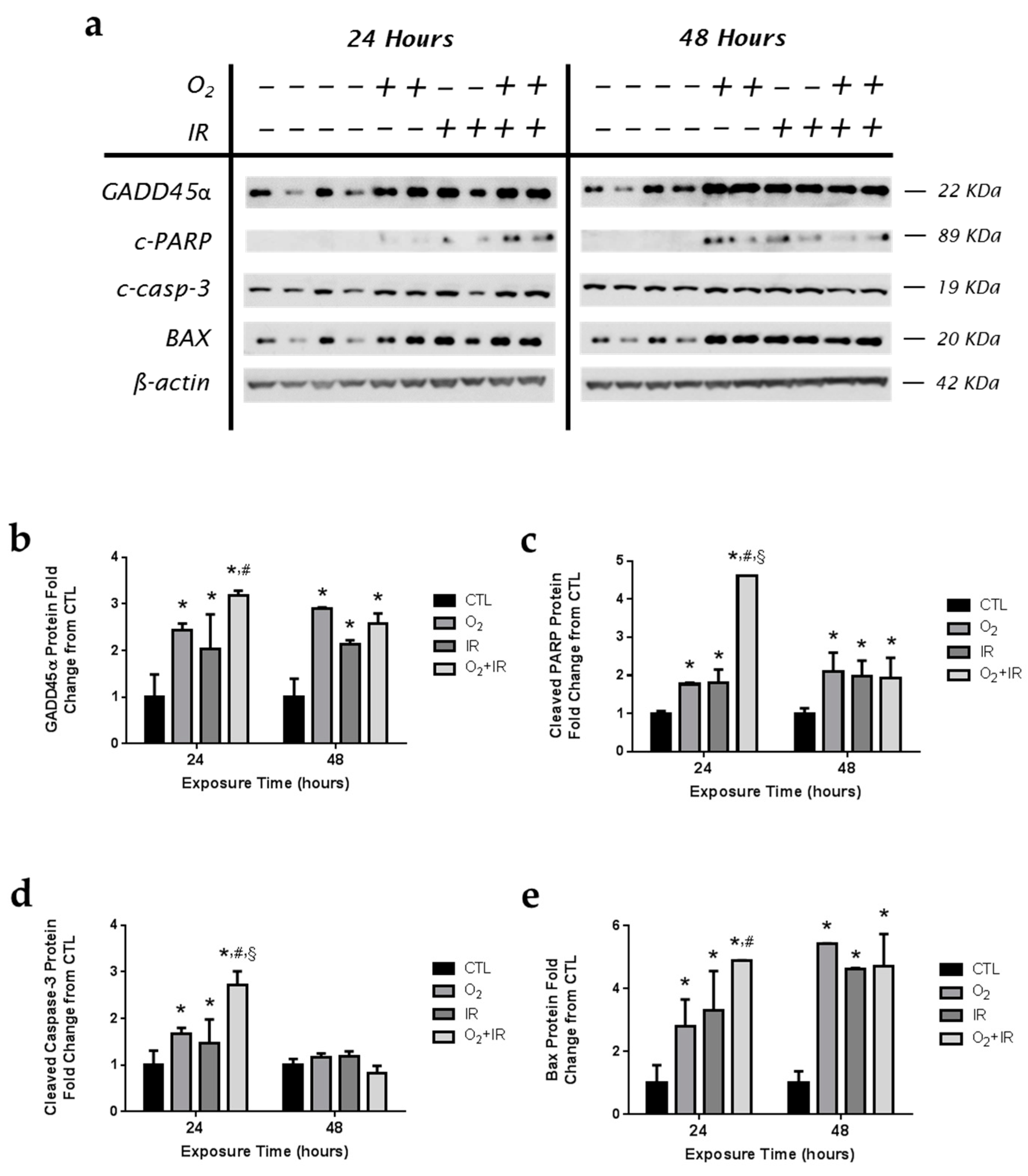
2.8. Expression of Apoptotic and DNA Damage-Induced Proteins in Lung Epithelial Cells Exposed to Hyperoxia, Radiation, and Double-Hit Combination Challenge
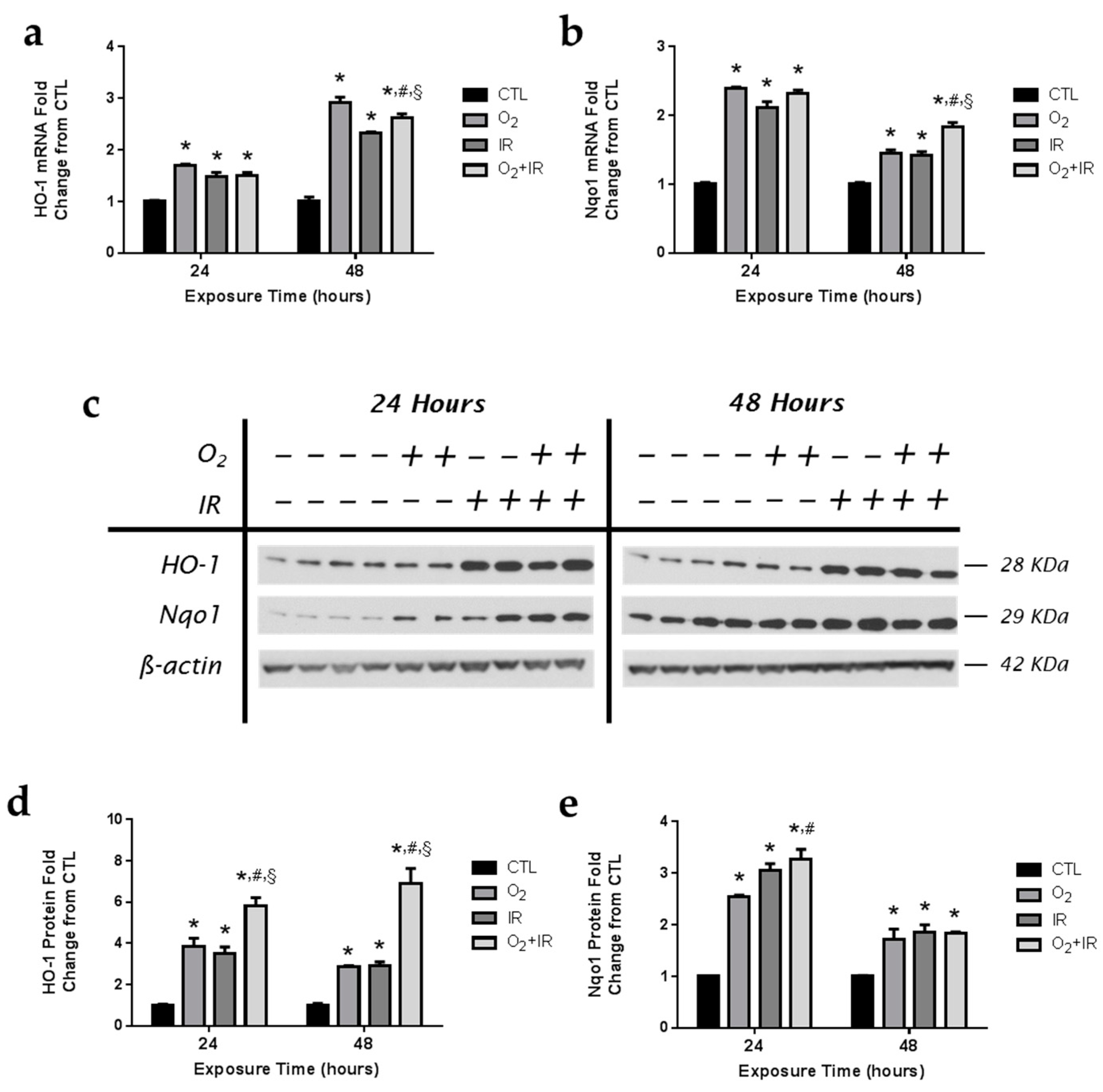
2.9. Exposure to Repeated Cycles of Hyperoxia, Radiation, and Double-Hit Combination Challenge Induces the Expression of Key Antioxidant Enzymes in Pulmonary Epithelial Cells
2.10. Expression of Apoptotic and DNA Damage-Induced Proteins in Lung Epithelial Cells Exposed to Hyperoxia, Radiation, and Double-Hit Combination Challenge
2.11. Expression of Apoptotic and DNA Damage-Induced Proteins in Lung Epithelial Cells Exposed to Hyperoxia, Radiation, and Double-Hit Combination Challenge
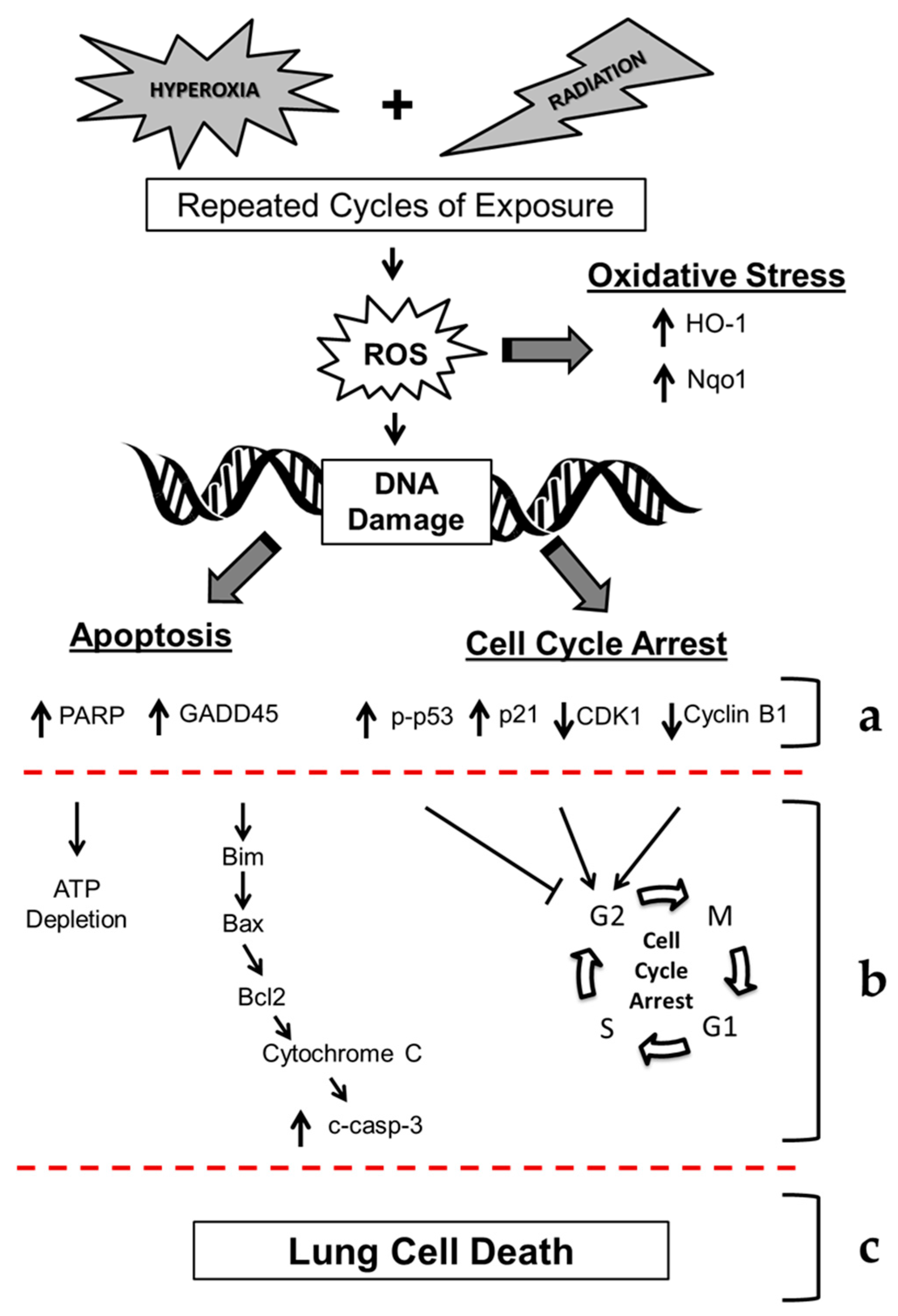
3. Discussion
4. Materials and Methods
4.1. Experimental Plan of in Vitro Cell Exposure
4.2. Exposure of Lung Epithelial Cells to Radiation
4.3. Exposure of Lung Epithelial Cells to Hyperoxia
4.4. Western Blotting
4.5. Lung Epithelial Cell Counts
4.6. Comet Analysis
4.7. Gene Expression Analysis
4.8. Analysis of Cell Cycle by Flow Cytometry
4.9. Analysis of Apoptosis/Necrosis by Flow Cytometry
4.10. Analysis of H2AX Phosphorylation by Flow Cytometry
4.11. In Vivo Animal Exposure Study Design
4.12. Statistical Analysis
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
Abbreviations
| BAX | Bcl-2-associated X protein |
| Casp-3 | caspase-3 |
| Casp-9 | caspase-9 |
| CDK1 | Cyclin-dependent kinase 1 |
| CTL | control |
| DCI | decompression Illness |
| EVA | extravehicular activity |
| GADD45α | growth arrest and DNA damage |
| HO-1 | heme oxygenase-1 |
| IAP | inhibitor of apoptosis |
| Nqo1 | NADPH: quinone oxidoreductase-1 |
| PARP | poly(ADP-ribose) polymerase |
| PBS | phosphate buffered saline |
| qPCR | quantitative polymerase chain reaction |
| RNS | reactive nitrogen species |
| ROS | reactive oxygen species |
| SEM | standard error of mean |
References
- Hu, S.; Kim, M.H.; McClellan, G.E.; Cucinotta, F.A. Modeling the acute health effects of astronauts from exposure to large solar particle events. Health Phys. 2009, 96, 465–476. [Google Scholar] [CrossRef] [PubMed]
- Townsend, L.W. Implications of the space radiation environment for human exploration in deep space. Radiat. Prot. Dosim. 2005, 115, 44–50. [Google Scholar] [CrossRef] [PubMed]
- Holloway, R.J.; Leong, G.F.; Ainsworth, E.J.; Albright, M.L.; Baum, S.J. Recovery from radiation injury in the hamster as evaluated by the split-dose technique. Usnrdl-tr-1111. Res. Dev. Tech. Rep. 1967, 1–26. [Google Scholar]
- Leong, G.F.; Page, N.P.; Ainsworth, E.J.; Hanks, G.E. Injury accumulation in sheep during protracted gamma radiation. Usnrdl-tr-998. Res. Dev. Tech. Rep. 1966, 1–13. [Google Scholar]
- Prisk, G.K. The lung in space. Clin. Chest Med. 2005, 26, 415–438. [Google Scholar] [CrossRef] [PubMed]
- Schmidt, M.A.; Goodwin, T.J. Personalized medicine in human space flight: Using omics based analyses to develop individualized countermeasures that enhance astronaut safety and performance. Metabolomics 2013, 9, 1134–1156. [Google Scholar] [CrossRef] [PubMed]
- Larkin, E.C.; Adams, J.D.; Williams, W.T.; Duncan, D.M. Hematologic responses to hypobaric hyperoxia. Am. J. Physiol. 1972, 223, 431–437. [Google Scholar] [PubMed]
- Madden, L.A.; Chrismas, B.C.; Mellor, D.; Vince, R.V.; Midgley, A.W.; McNaughton, L.R.; Atkin, S.L.; Laden, G. Endothelial function and stress response after simulated dives to 18 msw breathing air or oxygen. Aviat. Space Environ. Med. 2010, 81, 41–45. [Google Scholar] [CrossRef] [PubMed]
- Hellweg, C.E.; Baumstark-Khan, C. Getting ready for the manned mission to mars: The astronauts’ risk from space radiation. Naturwissenschaften 2007, 94, 517–526. [Google Scholar] [CrossRef] [PubMed]
- Cucinotta, F.A.; Kim, M.H.; Willingham, V.; George, K.A. Physical and biological organ dosimetry analysis for international space station astronauts. Radiat. Res. 2008, 170, 127–138. [Google Scholar] [CrossRef] [PubMed]
- Christofidou-Solomidou, M.; Pietrofesa, R.A.; Arguiri, E.; Schweitzer, K.S.; Berdyshev, E.V.; McCarthy, M.; Corbitt, A.; Alwood, J.S.; Yu, Y.; Globus, R.K.; et al. Space radiation-associated lung injury in a murine model. Am. J. Physiol. Lung Cell. Mol. Physiol. 2015, 308, L416–L428. [Google Scholar] [CrossRef] [PubMed]
- Pietrofesa, R.A.; Turowski, J.B.; Arguiri, E.; Milovanova, T.N.; Solomides, C.C.; Thom, S.R.; Christofidou-Solomidou, M. Oxidative lung damage resulting from repeated exposure to radiation and hyperoxia associated with space exploration. J. Pulm. Respir. Med. 2013, 3, 1000158. [Google Scholar] [PubMed]
- Pietrofesa, R.A.; Solomides, C.C.; Christofidou-Solomidou, M. Flaxseed mitigates acute oxidative lung damage in a mouse model of repeated radiation and hyperoxia exposure associated with space exploration. J. Pulm. Respir. Med. 2014, 4, 1000215. [Google Scholar] [CrossRef] [PubMed]
- Movsas, B.; Raffin, T.A.; Epstein, A.H.; Link, C.J., Jr. Pulmonary radiation injury. Chest 1997, 111, 1061–1076. [Google Scholar] [CrossRef] [PubMed]
- Crapo, J.D.; Barry, B.E.; Chang, L.Y.; Mercer, R.R. Alterations in lung structure caused by inhalation of oxidants. J. Toxicol. Environ. Health 1984, 13, 301–321. [Google Scholar] [CrossRef] [PubMed]
- Perkowski, S.; Scherpereel, A.; Murciano, J.C.; Arguiri, E.; Solomides, C.C.; Albelda, S.M.; Muzykantov, V.; Christofidou-Solomidou, M. Dissociation between alveolar transmigration of neutrophils and lung injury in hyperoxia. Am. J. Physiol. Lung Cell. Mol. Physiol. 2006, 291, L1050–L1058. [Google Scholar] [CrossRef] [PubMed]
- Machtay, M.; Scherpereel, A.; Santiago, J.; Lee, J.; McDonough, J.; Kinniry, P.; Arguiri, E.; Shuvaev, V.V.; Sun, J.; Cengel, K.; et al. Systemic polyethylene glycol-modified (pegylated) superoxide dismutase and catalase mixture attenuates radiation pulmonary fibrosis in the c57/bl6 mouse. Radiother. Oncol. 2006, 81, 196–205. [Google Scholar] [CrossRef] [PubMed]
- Taieb, F.; Nougayrede, J.P.; Oswald, E. Cycle inhibiting factors (cifs): Cyclomodulins that usurp the ubiquitin-dependent degradation pathway of host cells. Toxins (Basel) 2011, 3, 356–368. [Google Scholar] [CrossRef] [PubMed]
- Al-Khalaf, H.H.; Mohideen, P.; Nallar, S.C.; Kalvakolanu, D.V.; Aboussekhra, A. The cyclin-dependent kinase inhibitor p16ink4a physically interacts with transcription factor sp1 and cyclin-dependent kinase 4 to transactivate microrna-141 and microrna-146b-5p spontaneously and in response to ultraviolet light-induced DNA damage. J. Biol. Chem. 2013, 288, 35511–35525. [Google Scholar] [CrossRef] [PubMed]
- Pietenpol, J.A.; Stewart, Z.A. Cell cycle checkpoint signaling: Cell cycle arrest versus apoptosis. Toxicology 2002, 181–182, 475–481. [Google Scholar] [CrossRef]
- Alenzi, F.Q. Links between apoptosis, proliferation and the cell cycle. Br. J. Biomed. Sci. 2004, 61, 99–102. [Google Scholar] [CrossRef] [PubMed]
- Zhang, F.; Zhang, T.; Teng, Z.H.; Zhang, R.; Wang, J.B.; Mei, Q.B. Sensitization to γ-irradiation-induced cell cycle arrest and apoptosis by the histone deacetylase inhibitor trichostatin a in non-small cell lung cancer (NSCLC) cells. Cancer Biol. Ther. 2009, 8, 823–831. [Google Scholar] [CrossRef] [PubMed]
- Valerie, K.; Yacoub, A.; Hagan, M.P.; Curiel, D.T.; Fisher, P.B.; Grant, S.; Dent, P. Radiation-induced cell signaling: Inside-out and outside-in. Mol. Cancer Ther. 2007, 6, 789–801. [Google Scholar] [CrossRef] [PubMed]
- Jenkins, R.D.; Sinclair, I.N.; Anand, R.; Kalil, A.G., Jr.; Schoen, F.J.; Spears, J.R. Laser balloon angioplasty: Effect of tissue temperature on weld strength of human postmortem intima-media separations. Lasers Surg. Med. 1988, 8, 30–39. [Google Scholar] [CrossRef] [PubMed]
- Poole, P.S.; Sinclair, D.R.; Armitage, J.P. Real time computer tracking of free-swimming and tethered rotating cells. Anal. Biochem. 1988, 175, 52–58. [Google Scholar] [CrossRef]
- Tong, T.; Ji, J.; Jin, S.; Li, X.; Fan, W.; Song, Y.; Wang, M.; Liu, Z.; Wu, M.; Zhan, Q. GADD45α expression induces bim dissociation from the cytoskeleton and translocation to mitochondria. Mol. Cell. Biol. 2005, 25, 4488–4500. [Google Scholar] [CrossRef] [PubMed]
- Carrier, F.; Smith, M.L.; Bae, I.; Kilpatrick, K.E.; Lansing, T.J.; Chen, C.Y.; Engelstein, M.; Friend, S.H.; Henner, W.D.; Gilmer, T.M.; et al. Characterization of human GADD45, a p53-regulated protein. J. Biol. Chem. 1994, 269, 32672–32677. [Google Scholar] [PubMed]
- Fornace, A.J., Jr.; Alamo, I., Jr.; Hollander, M.C. DNA damage-inducible transcripts in mammalian cells. Proc. Natl. Acad. Sci. USA 1988, 85, 8800–8804. [Google Scholar] [CrossRef] [PubMed]
- Gobeil, S.; Boucher, C.C.; Nadeau, D.; Poirier, G.G. Characterization of the necrotic cleavage of poly(ADP-ribose) polymerase (PARP-1): Implication of lysosomal proteases. Cell Death Differ. 2001, 8, 588–594. [Google Scholar] [CrossRef] [PubMed]
- Zeng, J.; Yang, G.Y.; Ying, W.; Kelly, M.; Hirai, K.; James, T.L.; Swanson, R.A.; Litt, L. Pyruvate improves recovery after PARP-1-associated energy failure induced by oxidative stress in neonatal rat cerebrocortical slices. J. Cereb. Blood Flow Metab. 2007, 27, 304–315. [Google Scholar] [CrossRef] [PubMed]
- Taylor, W.R.; Stark, G.R. Regulation of the G2/M transition by p53. Oncogene 2001, 20, 1803–1815. [Google Scholar] [CrossRef] [PubMed]
- Petrache, I.; Choi, M.E.; Otterbein, L.E.; Chin, B.Y.; Mantell, L.L.; Horowitz, S.; Choi, A.M. Mitogen-activated protein kinase pathway mediates hyperoxia-induced apoptosis in cultured macrophage cells. Am. J. Physiol. 1999, 277, L589–L595. [Google Scholar] [PubMed]
- Lee, H.S.; Kim, C.K. Effect of recombinant IL-10 on cultured fetal rat alveolar type II cells exposed to 65%-hyperoxia. Respir. Res. 2011, 12, 68. [Google Scholar] [CrossRef] [PubMed]
- Barazzone, C.; Horowitz, S.; Donati, Y.R.; Rodriguez, I.; Piguet, P.F. Oxygen toxicity in mouse lung: Pathways to cell death. Am. J. Respir. Cell Mol. Biol. 1998, 19, 573–581. [Google Scholar] [CrossRef] [PubMed]
- Mantell, L.L.; Lee, P.J. Signal transduction pathways in hyperoxia-induced lung cell death. Mol. Genet. Metabol. 2000, 71, 359–370. [Google Scholar] [CrossRef] [PubMed]
- Korpela, E.; Liu, S.K. Endothelial perturbations and therapeutic strategies in normal tissue radiation damage. Radiat. Oncol. 2014, 9, 266. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Saha, J.; Hada, M.; Anderson, J.A.; Pluth, J.M.; O’Neill, P.; Cucinotta, F.A. Novel smad proteins localize to ir-induced double-strand breaks: Interplay between tgfbeta and atm pathways. Nucleic Acids Res. 2013, 41, 933–942. [Google Scholar] [CrossRef] [PubMed]
- Saha, J.; Wang, M.; Cucinotta, F.A. Investigation of switch from atm to atr signaling at the sites of DNA damage induced by low and high let radiation. DNA Repair. (Amst.) 2013, 12, 1143–1151. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Saha, J.; Cucinotta, F.A. Smad7 foci are present in micronuclei induced by heavy particle radiation. Mutat. Res. 2013, 756, 108–114. [Google Scholar] [CrossRef] [PubMed]
- Velalopoulou, A.; Tyagi, S.; Pietrofesa, R.A.; Arguiri, E.; Christofidou-Solomidou, M. The flaxseed-derived lignan phenolic secoisolariciresinol diglucoside (SDG) protects non-malignant lung cells from radiation damage. Int. J. Mol. Sci. 2015, 17. [Google Scholar] [CrossRef] [PubMed]
- Fracica, P.J.; Knapp, M.J.; Crapo, J.D. Patterns of progression and markers of lung injury in rodents and subhuman primates exposed to hyperoxia. Exp. Lung Res. 1988, 14, 869–885. [Google Scholar] [CrossRef] [PubMed]
- Vertrees, R.A.; McCarthy, M.; Solley, T.; Popov, V.L.; Roaten, J.; Pauley, M.; Wen, X.; Goodwin, T.J. Development of a three-dimensional model of lung cancer using cultured transformed lung cells. Cancer Biol. Ther. 2009, 8, 356–365. [Google Scholar] [CrossRef] [PubMed]
- Vertrees, R.A.; Zwischenberger, J.B.; Boor, P.J.; Popov, V.; McCarthy, M.; Solley, T.N.; Goodwin, T.J. Cellular differentiation in three-dimensional lung cell cultures. Cancer Biol. Ther. 2008, 7, 404–412. [Google Scholar] [CrossRef] [PubMed]
- Malkinson, A.M.; Dwyer-Nield, L.D.; Rice, P.L.; Dinsdale, D. Mouse lung epithelial cell lines—Tools for the study of differentiation and the neoplastic phenotype. Toxicology 1997, 123, 53–100. [Google Scholar] [CrossRef]
- McDoniels-Silvers, A.L.; Herzog, C.R.; Tyson, F.L.; Malkinson, A.M.; You, M. Inactivation of both Rb and p53 pathways in mouse lung epithelial cell lines. Exp. Lung Res. 2001, 27, 297–318. [Google Scholar] [CrossRef] [PubMed]
- Koch, C.J. A thin-film culturing technique allowing rapid gas-liquid equilibration (6 s) with no toxicity to mammalian cells. Radiat. Res. 1984, 97, 434–442. [Google Scholar] [CrossRef] [PubMed]
- Allen, C.B.; Schneider, B.K.; White, C.W. Limitations to oxygen diffusion and equilibration in in vitro cell exposure systems in hyperoxia and hypoxia. Am. J. Physiol. Lung Cell. Mol. Physiol. 2001, 281, L1021–L1027. [Google Scholar] [PubMed]
- Lee, J.C.; Bhora, F.; Sun, J.; Cheng, G.; Arguiri, E.; Solomides, C.C.; Chatterjee, S.; Christofidou-Solomidou, M. Dietary flaxseed enhances antioxidant defenses and is protective in a mouse model of lung ischemia-reperfusion injury. Am. J. Physiol. Lung Cell. Mol. Physiol. 2008, 294, L255–L265. [Google Scholar] [CrossRef] [PubMed]
- Christofidou-Solomidou, M.; Tyagi, S.; Pietrofesa, R.; Dukes, F.; Arguiri, E.; Turowski, J.; Grieshaber, P.A.; Solomides, C.C.; Cengel, K.A. Radioprotective role in lung of the flaxseed lignan complex enriched in the phenolic secoisolariciresinol diglucoside (SDG). Radiat. Res. 2012, 178, 568–580. [Google Scholar] [CrossRef] [PubMed]
- Pietrofesa, R.A.; Velalopoulou, A.; Arguiri, E.; Menges, C.W.; Testa, J.R.; Hwang, W.T.; Albelda, S.M.; Christofidou-Solomidou, M. Flaxseed lignans enriched in secoisolariciresinol diglucoside prevent acute asbestos-induced peritoneal inflammation in mice. Carcinogenesis 2016, 37, 177–187. [Google Scholar] [CrossRef] [PubMed]
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pietrofesa, R.A.; Velalopoulou, A.; Lehman, S.L.; Arguiri, E.; Solomides, P.; Koch, C.J.; Mishra, O.P.; Koumenis, C.; Goodwin, T.J.; Christofidou-Solomidou, M. Novel Double-Hit Model of Radiation and Hyperoxia-Induced Oxidative Cell Damage Relevant to Space Travel. Int. J. Mol. Sci. 2016, 17, 953. https://doi.org/10.3390/ijms17060953
Pietrofesa RA, Velalopoulou A, Lehman SL, Arguiri E, Solomides P, Koch CJ, Mishra OP, Koumenis C, Goodwin TJ, Christofidou-Solomidou M. Novel Double-Hit Model of Radiation and Hyperoxia-Induced Oxidative Cell Damage Relevant to Space Travel. International Journal of Molecular Sciences. 2016; 17(6):953. https://doi.org/10.3390/ijms17060953
Chicago/Turabian StylePietrofesa, Ralph A., Anastasia Velalopoulou, Stacey L. Lehman, Evguenia Arguiri, Pantelis Solomides, Cameron J. Koch, Om P. Mishra, Constantinos Koumenis, Thomas J. Goodwin, and Melpo Christofidou-Solomidou. 2016. "Novel Double-Hit Model of Radiation and Hyperoxia-Induced Oxidative Cell Damage Relevant to Space Travel" International Journal of Molecular Sciences 17, no. 6: 953. https://doi.org/10.3390/ijms17060953






