Effect of a Prebiotic Formulation on Frailty Syndrome: A Randomized, Double-Blind Clinical Trial
Abstract
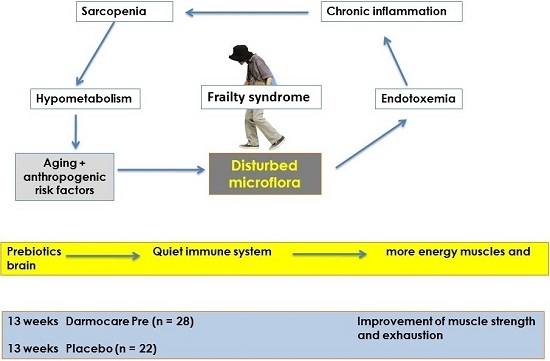
:1. Introduction
2. Results
2.1. Design and Study Population
2.2. Sample and Dropouts
2.3. Effect of Darmocare Pre® Administration on Frailty Criteria
2.4. Effect of Darmocare Pre® on Blood Analytical Parameters
3. Discussion
4. Experimental Section
4.1. Study Population
4.2. Intervention
4.3. Measurement of Frailty Criteria
4.4. Geriatric Assessment
4.5. Blood Analytical Parameters
4.6. Statistical Analysis
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Morley, J.E.; Vellas, B.; van Kan, G.A.; Anker, S.D.; Bauer, J.M.; Bernabei, R.; Cesari, M.; Chumlea, W.C.; Doehner, W.; Evans, J.; et al. Frailty consensus: A call to action. J. Am. Med. Dir. Assoc. 2013, 14, 392–397. [Google Scholar] [CrossRef] [PubMed]
- Fernández-Garrido, J.; Ruiz-Ros, V.; Buigues, C.; Navarro-Martínez, R.; Cauli, O. Clinical features of prefrail older individuals and emerging peripheral biomarkers: A systematic review. Arch. Gerontol. Geriatr. 2014, 59, 7–17. [Google Scholar] [CrossRef] [PubMed]
- Fried, L.P.; Tangen, C.M.; Walston, J.; Newman, A.B.; Hirsch, C.; Gottdiener, J.; Seeman, T.; Tracy, R.; Kop, W.J.; Burke, G.; et al. Frailty in older adults: Evidence for a phenotype. J. Gerontol. Ser. A Biol. Sci. Med. Sci. 2001, 56, M146–M156. [Google Scholar] [CrossRef]
- Cho, I.; Blaser, M.J. The human microbiome: At the interface of health and disease. Nat. Rev. Genet. 2012, 13, 260–270. [Google Scholar] [CrossRef] [PubMed]
- Sekirov, I.; Russell, S.L.; Antunes, L.C.M.; Finlay, B.B. Gut microbiota in health and disease. Physiol. Rev. 2010, 90, 859–904. [Google Scholar] [CrossRef] [PubMed]
- Claesson, M.J.; Cusack, S.; O‘Sullivan, O.; Greene-Diniz, R.; de Weerd, H.; Flannery, E.; Marchesi, J.R.; Falush, D.; Dinan, T.; Fitzgerald, G.; et al. Composition, variability, and temporal stability of the intestinal microbiota of the elderly. Proc. Natl. Acad. Sci. USA 2011, 108 (Suppl. S1), 4586–4591. [Google Scholar] [CrossRef] [PubMed]
- O’Toole, P.W.; Claesson, M.J. Gut microbiota: Changes throughout the lifespan from infancy to elderly. Int. Dairy J. 2010, 20, 281–291. [Google Scholar] [CrossRef]
- Woodmansey, E.J. Intestinal bacteria and aging. J. Appl. Microbiol. 2007, 102, 1178–1186. [Google Scholar] [CrossRef] [PubMed]
- Roberts, C.L.; Keita, A.V.; Duncan, S.H.; O’Kennedy, N.; Söderholm, J.D.; Rhodes, J.M.; Campbell, B.J. Translocation of Crohn’s disease Escherichia coli across M-cells: Contrasting effects of soluble plant fibres and emulsifiers. Gut 2010, 59, 1331–1339. [Google Scholar] [CrossRef] [PubMed]
- Gavini, F.; Cayuela, C.; Antoine, J.-M.; Lecoq, C.; Lefebvre, B.; Membré, J.-M.; Neut, C. Differences in the distribution of bifidobacterial and enterobacterial species in human faecal microflora of three different (children, adults, elderly) age groups. Microb. Ecol. Health Dis. 2001, 13, 40–45. [Google Scholar]
- Hopkins, M.J.; Sharp, R.; Macfarlane, G.T. Variation in human intestinal microbiota with age. Dig. Liver Dis. 2002, 34 (Suppl. S2), S12–S18. [Google Scholar] [CrossRef]
- Franks, A.H.; Harmsen, H.J.; Raangs, G.C.; Jansen, G.J.; Schut, F.; Welling, G.W. Variations of bacterial populations in human feces measured by fluorescent in situ hybridization with group-specific 16S rRNA-targeted oligonucleotide probes. Appl. Environ. Microbiol. 1998, 64, 3336–3345. [Google Scholar] [PubMed]
- Franceschi, C.; Capri, M.; Monti, D.; Giunta, S.; Olivieri, F.; Sevini, F.; Panourgia, M.P.; Invidia, L.; Celani, L.; Scurti, M.; et al. Inflammaging and anti-inflammaging: A systemic perspective on aging and longevity emerged from studies in humans. Mech. Ageing Dev. 2007, 128, 92–105. [Google Scholar] [CrossRef] [PubMed]
- Nagafuchi, S.; Yamaji, T.; Kawashima, A.; Saito, Y.; Takahashi, T.; Yamamoto, T.; Maruyama, M.; Akatsu, H. Effects of a formula containing two types of prebiotics, bifidogenic growth stimulator and galacto-oligosaccharide, and fermented milk products on intestinal microbiota and antibody response to influenza vaccine in elderly patients: A randomized controlled trial. Pharmaceuticals 2015, 8, 351–365. [Google Scholar] [PubMed]
- Vulevic, J.; Drakoularakou, A.; Yaqoob, P.; Tzortzis, G.; Gibson, G.R. Modulation of the fecal microflora profile and immune function by a novel trans-galactooligosaccharide mixture (B-GOS) in healthy elderly volunteers. Am. J. Clin. Nutr. 2008, 88, 1438–1446. [Google Scholar] [PubMed]
- Claesson, M.J.; Jeffery, I.B.; Conde, S.; Power, S.E.; O’Connor, E.M.; Cusack, S.; Harris, H.M.B.; Coakley, M.; Lakshminarayanan, B.; O’Sullivan, O.; et al. Gut microbiota composition correlates with diet and health in the elderly. Nature 2012, 488, 178–184. [Google Scholar] [CrossRef] [PubMed]
- Groeger, D.; O’Mahony, L.; Murphy, E.F.; Bourke, J.F.; Dinan, T.G.; Kiely, B.; Shanahan, F.; Quigley, E.M.M. Bifidobacterium infantis 35624 modulates host inflammatory processes beyond the gut. Gut Microbes 2013, 4, 325–339. [Google Scholar] [CrossRef] [PubMed]
- Cusack, S.; O’Toole, P.W. Diet, the gut microbiota and healthy aging: How dietary modulation of the gut microbiota could transform the health of older populations. Agro FOOD Ind. Hi Tech 2013, 24, 54–57. [Google Scholar]
- Lomax, A.R.; Cheung, L.V.Y.; Tuohy, K.M.; Noakes, P.S.; Miles, E.A.; Calder, P.C. β2-1 Fructans have a bifidogenic effect in healthy middle-aged human subjects but do not alter immune responses examined in the absence of an in vivo immune challenge: Results from a randomized controlled trial. Br. J. Nutr. 2012, 108, 1818–1828. [Google Scholar] [CrossRef] [PubMed]
- Li, F.; Jin, X.; Liu, B.; Zhuang, W.; Scalabrin, D. Follow-up formula consumption in 3- to 4-year-olds and respiratory infections: An RCT. Pediatrics 2014, 133, e1533–e1540. [Google Scholar] [CrossRef] [PubMed]
- Statistical Considerations for Clinical Trials and Scientific Experiments. Massachusetts General Hospital’s Biostatistics Center. Available online: http://hedwig.mgh.harvard.edu/sample_size/size.html (accessed on 8 September 2014).
- Toward, R.; Montandon, S.; Walton, G.; Gibson, G.R. Effect of prebiotics on the human gut microbiota of elderly persons. Gut Microbes 2012, 3, 57–60. [Google Scholar] [CrossRef] [PubMed]
- Bindels, L.B.; Delzenne, N.M. Muscle wasting: The gut microbiota as a new therapeutic target? Int. J. Biochem. Cell Biol. 2013, 45, 2186–2190. [Google Scholar] [CrossRef] [PubMed]
- Woods, N.F.; LaCroix, A.Z.; Gray, S.L.; Aragaki, A.; Cochrane, B.B.; Brunner, R.L.; Masaki, K.; Murray, A.; Newman, A.B. Frailty: Emergence and consequences in women aged 65 and older in the Women’s Health Initiative Observational Study. J. Am. Geriatr. Soc. 2005, 53, 1321–1330. [Google Scholar] [CrossRef] [PubMed]
- Cooper, C.; Dere, W.; Evans, W.; Kanis, J.A.; Rizzoli, R.; Sayer, A.A.; Sieber, C.C.; Kaufman, J.-M.; Abellan van Kan, G.; Boonen, S.; et al. Frailty and sarcopenia: Definitions and outcome parameters. Osteoporos. Int. 2012, 23, 1839–1848. [Google Scholar] [CrossRef] [PubMed]
- Evans, W.J.; Paolisso, G.; Abbatecola, A.M.; Corsonello, A.; Bustacchini, S.; Strollo, F.; Lattanzio, F. Frailty and muscle metabolism dysregulation in the elderly. Biogerontology 2010, 11, 527–536. [Google Scholar] [CrossRef] [PubMed]
- Marzetti, E.E.; Calvani, R.R.; Bernabei, R.R.; Leeuwenburgh, C.C. Apoptosis in skeletal myocytes: A potential target for interventions against sarcopenia and physical frailty—A mini-review. Gerontology 2012, 58, 99–106. [Google Scholar] [CrossRef] [PubMed]
- Buch, A.; Carmeli, E.; Boker, L.K.; Marcus, Y.; Shefer, G.; Kis, O.; Berner, Y.; Stern, N. Muscle function and fat content in relation to sarcopenia, obesity and frailty of old age—An overview. Exp. Gerontol. 2016, 76, 25–32. [Google Scholar] [CrossRef] [PubMed]
- Abizanda, P.; Lopez, M.D.; Garcia, V.P.; de Dios Estrella, J.; da Silva Gonzalez, A.; Vilardell, N.B.; Torres, K.A. Effects of an oral nutritional supplementation plus physical exercise intervention on the physical function, nutritional status, and quality of life in frail institutionalized older adults: The ACTIVNES study. J. Am. Med. Dir. Assoc. 2015, 16, 439.e9–439.e16. [Google Scholar] [CrossRef] [PubMed]
- Dell’Osso, L.; Bazzichi, L.; Baroni, S.; Falaschi, V.; Conversano, C.; Carmassi, C.; Marazziti, D. The inflammatory hypothesis of mood spectrum broadened to fibromyalgia and chronic fatigue syndrome. Clin. Exp. Rheumatol. 2015, 33, S109–S116. [Google Scholar] [PubMed]
- Lasselin, J.; Capuron, L. Chronic low-grade inflammation in metabolic disorders: Relevance for behavioral symptoms. Neuroimmunomodulation 2014, 21, 95–101. [Google Scholar] [PubMed]
- Schiffrin, E.J.; Thomas, D.R.; Kumar, V.B.; Brown, C.; Hager, C.; Van’t Hof, M.A.; Morley, J.E.; Guigoz, Y. Systemic inflammatory markers in older persons: The effect of oral nutritional supplementation with prebiotics. J. Nutr. Health Aging 2007, 11, 475–479. [Google Scholar] [PubMed]
- Jeong, J.-J.; Kim, K.-A.; Jang, S.-E.; Woo, J.-Y.; Han, M.J.; Kim, D.-H. Orally administrated Lactobacillus pentosus var. plantarum C29 ameliorates age-dependent colitis by inhibiting the nuclear factor-kappa B signaling pathway via the regulation of lipopolysaccharide production by gut microbiota. PLoS ONE 2015, 10, e0116533. [Google Scholar]
- Staudacher, H.M.; Whelan, K. Altered gastrointestinal microbiota in irritable bowel syndrome and its modification by diet: Probiotics, prebiotics and the low FODMAP diet. Proc. Nutr. Soc. 2016, 24, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Hubbard, R.E.; O’Mahony, M.S.; Savva, G.M.; Calver, B.L.; Woodhouse, K.W. Inflammation and frailty measures in older people. J. Cell. Mol. Med. 2009, 13, 3103–3109. [Google Scholar] [CrossRef] [PubMed]
- Leng, S.X.; Xue, Q.L.; Tian, J.; Huang, Y.; Yeh, S.-H.; Fried, L.P. Associations of neutrophil and monocyte counts with frailty in community-dwelling disabled older women: Results from the Women’s Health and Aging Studies I. Exp. Gerontol. 2009, 44, 511–516. [Google Scholar] [CrossRef] [PubMed]
- Backhed, F.; Manchester, J.K.; Semenkovich, C.F.; Gordon, J.I. Mechanisms underlying the resistance to diet-induced obesity in germ-free mice. Proc. Natl. Acad. Sci. USA 2007, 104, 979–984. [Google Scholar] [CrossRef] [PubMed]
- Folstein, M.F.; Folstein, S.E.; McHugh, P.R. “Mini-Mental state.” A practical method for grading the cognitive state of patients for the clinician. J. Psychiatr. Res. 1975, 12, 189–198. [Google Scholar] [CrossRef]
- Galvin, J.E.; Sadowsky, C.H. Practical guidelines for the recognition and diagnosis of dementia. J. Am. Board Fam. Med. 2012, 25, 367–382. [Google Scholar] [CrossRef] [PubMed]
- Hendaus, M.A.; Jomha, F.A.; Ehlayel, M. Allergic diseases among children: Nutritional prevention and intervention. Ther. Clin. Risk Manag. 2016, 12, 361–372. [Google Scholar] [CrossRef] [PubMed]
- Pereira, D.I.A.; Gibson, G.R. Effects of consumption of probiotics and prebiotics on serum lipid levels in humans. Crit. Rev. Biochem. Mol. Biol. 2002, 37, 259–281. [Google Scholar] [CrossRef] [PubMed]
- Marteau, P.; Seksik, P. Tolerance of probiotics and prebiotics. J. Clin. Gastroenterol. 2004, 38, S67–S69. [Google Scholar] [CrossRef] [PubMed]
- Radloff, L.S. The CES-D scale: A self-report depression scale for research in the general population. Appl. Psychol. Meas. 1977, 1, 385–401. [Google Scholar] [CrossRef]
- Elosua, R.; Marrugat, J.; Molina, L.; Pons, S.; Pujol, E. Validation of the Minnesota leisure time physical activity questionnaire in Spanish men. Am. J. Epidemiol. 1994, 139, 1197–1209. [Google Scholar] [PubMed]
- Elosua, R.R.; Garcia, M.M.; Aguilar, A.A.; Molina, L.L.; Covas, M.I.M.; Marrugat, J.J. Validation of the Minnesota leisure time physical activity questionnaire in Spanish women. Investigators of the MARATDON group. Med. Sci. Sports Exerc. 2000, 32, 1431–1437. [Google Scholar] [CrossRef] [PubMed]
- Ruiz Comellas, A.; Pera, G.; Baena-Díez, J.M.; Mundet Tudurí, X.; Alzamora Sas, T.; Elosua, R.; Torán Monserrat, P.; Heras, A.; Forés Raurell, R.; Fusté Gamisans, M. Validación de una versión reducida en español del cuestionario de actividad física en el tiempo libre de Minnesota (VREM). Rev. Esp. Salud Pública 2012, 86, 495–508. (In Spanish) [Google Scholar] [PubMed]
- Guralnik, J.M.J.; Simonsick, E.M.E.; Ferrucci, L.L.; Glynn, R.J.R.; Berkman, L.F.L.; Blazer, D.G.D.; Scherr, P.A.P.; Wallace, R.B.R. A short physical performance battery assessing lower extremity function: Association with self-reported disability and prediction of mortality and nursing home admission. J. Gerontol. 1994, 49, M85–M94. [Google Scholar] [CrossRef] [PubMed]
- Ottenbacher, K.J.; Branch, L.G.; Ray, L.; Gonzales, V.A.; Peek, M.K.; Hinman, M.R. The reliability of upper- and lower-extremity strength testing in a community survey of older adults. Arch. Phys. Med. Rehabil. 2002, 83, 1423–1427. [Google Scholar] [CrossRef] [PubMed]
- Mahoney, F.I.; Barthel, D.W. Functional evaluation: The Barthel index. Md. State Med. J. 1965, 14, 61–65. [Google Scholar] [PubMed]
- Soldatos, C.R.; Dikeos, D.G.; Paparrigopoulos, T.J. Athens Insomnia Scale: Validation of an instrument based on ICD-10 criteria. J. Psychosom. Res. 2000, 48, 555–560. [Google Scholar] [CrossRef]

| Variable | Baseline | Significance | Post-Treatment | Significance | ||
|---|---|---|---|---|---|---|
| Placebo Group (n = 22) | Dermocare Pre® (n = 28) | p Value | Placebo Group (n = 22) | Dermocare Pre® (n = 28) | p Value | |
| Exhaustion (score 0–3: 0 “never”; 1 “A few times” (1–2 days per week); 2 “Often” (3–4 days per week); or 3 “Most of the time” (almost each day)) | 1.1 ± 1.7 | 1.4 ± 1.7 | 0.74 | 1.7 ± 1.2 | 0.8 ± 1.4 ** | 0.002 |
| Slow walk (s) (time needed to walk 4.6 m) | 8.6 ± 9.0 | 8.4 ± 6.0 | 0.91 | 8.7 ± 4.2 | 7.9 ± 4.5 | 0.48 |
| Grip strength (right hand, kg) | 11.5 ± 5.7 | 10.6 ± 8.2 | 0.61 | 10.2 ± 4.1 | 12.4 ± 3.2 * | 0.04 |
| Grip strength (left hand, kg) | 10.2 ± 5.8 | 10.1 ± 7.6 | 0.92 | 9.1 ± 3.7 | 9.8 ± 3.5 | 0.50 |
| Self health-perception (score 0–10, being 0 the worst and 10 the best) | 7.1 ± 2.3 | 7.1 ± 2.1 | 0.96 | 6.8 ± 2.4 | 6.8 ± 2.0 | 0.96 |
| Body mass index | 26.1 ± 4.1 | 25.8 ± 4.2 | 0.97 | 26.0 ± 3.8 | 25.9 ± 4.1 | 0.96 |
| Athens insomnia scale | 3.4 ± 3.0 | 4.1 ± 4.7 | 0.77 | 4.5 ± 5.3 | 4.0 ± 4.3 | 0.68 |
| Barthel index | 76.2 ± 13.0 | 74.6 ± 17.7 | 0.69 | 78.3 ± 13.9 | 77.1 ± 29.9 | 0.87 |
| Mini–Mental state examination | 26.1 ± 2.2 | 26.5 ± 3.1 | 0.89 | 25.9 ± 2.1 | 26.4 ± 2.2 | 0.85 |
| Variable | Placebo Group (n = 22) | Darmocare Pre® (n = 28) | p Value |
|---|---|---|---|
| Leukocytes (×103/µL) | 7.6 ± 0.5 | 7.7 ± 0.8 | 0.92 |
| Neutrophils (×103/µL) | 4.5 ± 0.2 | 4.6 ± 0.3 | 0.79 |
| Lymphocytes (×103/µL) | 2.2 ± 0.2 | 2.3 ± 0.1 | 0.64 |
| Monocytes (×103/µL) | 0.61 ± 0.04 | 0.55 ± 0.03 | 0.23 |
| Eosinophils (×103/µL) | 0.22 ± 0.04 | 0.22 ± 0.05 | 0.88 |
| Basophils (×103/µL) | 0.03 ± 0.01 | 0.03 ± 0.01 | 1.00 |
| Platelets (×103/µL) | 220 ± 28 | 225 ± 36 | 0.92 |
| Erythrocytes (×106/µL) | 5.0 ± 0.6 | 4.8 ± 0.7 | 0.83 |
| Haemoglobin (g/dL) | 12.8 ± 1.3 | 12.9 ± 1.1 | 0.95 |
| Glucose (mg/dL) | 95 ± 11 | 92 ± 10 | 0.84 |
| Urea (mg/dL) | 44 ± 5 | 40 ± 3 | 0.48 |
| GOT (U/L) | 28 ± 3 | 27 ± 4 | 0.85 |
| GPT (U/L) | 25 ± 2 | 24 ± 4 | 0.84 |
| HDL cholesterol (mg/dL) | 46 ± 6 | 44 ± 7 | 0.83 |
| LDL cholesterol (mg/dL) | 120 ± 9 | 126 ± 11 | 0.69 |
| Triglycerides (mg/dL) | 131 ± 21 | 122 ± 16 | 0.73 |
| Total proteins (g/dL) | 7.5 ± 0.4 | 7.4 ± 0.5 | 0.88 |
| Creatinine (mg/dL) | 0.71 ± 0.10 | 0.74 ± 0.15 | 0.88 |
| Calcium (mg/dL) | 8.6 ± 1.0 | 8.9 ± 0.8 | 0.81 |
| Sodium (mEq/L) | 140 ± 3 | 141 ± 3 | 0.82 |
| Potassium (mEq/L) | 4.7 ± 0.8 | 4.8 ± 0.5 | 0.91 |
| C-reactive protein (mg/L) | 4.8 ± 1.5 | 4.9 ± 1.8 | 0.97 |
| TNF-α (pg/mL) | 1.8 ± 0.2 | 2.0 ± 0.3 | 0.60 |
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Buigues, C.; Fernández-Garrido, J.; Pruimboom, L.; Hoogland, A.J.; Navarro-Martínez, R.; Martínez-Martínez, M.; Verdejo, Y.; Mascarós, M.C.; Peris, C.; Cauli, O. Effect of a Prebiotic Formulation on Frailty Syndrome: A Randomized, Double-Blind Clinical Trial. Int. J. Mol. Sci. 2016, 17, 932. https://doi.org/10.3390/ijms17060932
Buigues C, Fernández-Garrido J, Pruimboom L, Hoogland AJ, Navarro-Martínez R, Martínez-Martínez M, Verdejo Y, Mascarós MC, Peris C, Cauli O. Effect of a Prebiotic Formulation on Frailty Syndrome: A Randomized, Double-Blind Clinical Trial. International Journal of Molecular Sciences. 2016; 17(6):932. https://doi.org/10.3390/ijms17060932
Chicago/Turabian StyleBuigues, Cristina, Julio Fernández-Garrido, Leo Pruimboom, Aldert J. Hoogland, Rut Navarro-Martínez, Mary Martínez-Martínez, Yolanda Verdejo, Mari Carmen Mascarós, Carlos Peris, and Omar Cauli. 2016. "Effect of a Prebiotic Formulation on Frailty Syndrome: A Randomized, Double-Blind Clinical Trial" International Journal of Molecular Sciences 17, no. 6: 932. https://doi.org/10.3390/ijms17060932
APA StyleBuigues, C., Fernández-Garrido, J., Pruimboom, L., Hoogland, A. J., Navarro-Martínez, R., Martínez-Martínez, M., Verdejo, Y., Mascarós, M. C., Peris, C., & Cauli, O. (2016). Effect of a Prebiotic Formulation on Frailty Syndrome: A Randomized, Double-Blind Clinical Trial. International Journal of Molecular Sciences, 17(6), 932. https://doi.org/10.3390/ijms17060932