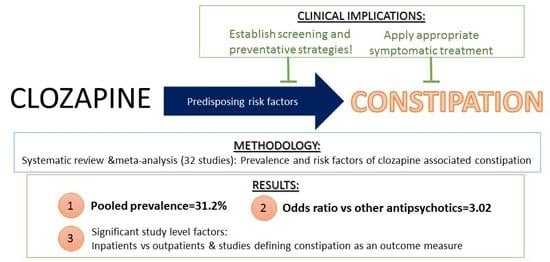
Prevalence and Predictors of Clozapine-Associated Constipation: A Systematic Review and Meta-Analysis
Abstract
:1. Introduction
2. Methods
2.1. Inclusion and Exclusion Criteria
2.2. Information Sources and Searches
2.3. Study Selection
2.4. Data Extraction of Outcomes
2.5. Meta-Analysis
3. Results
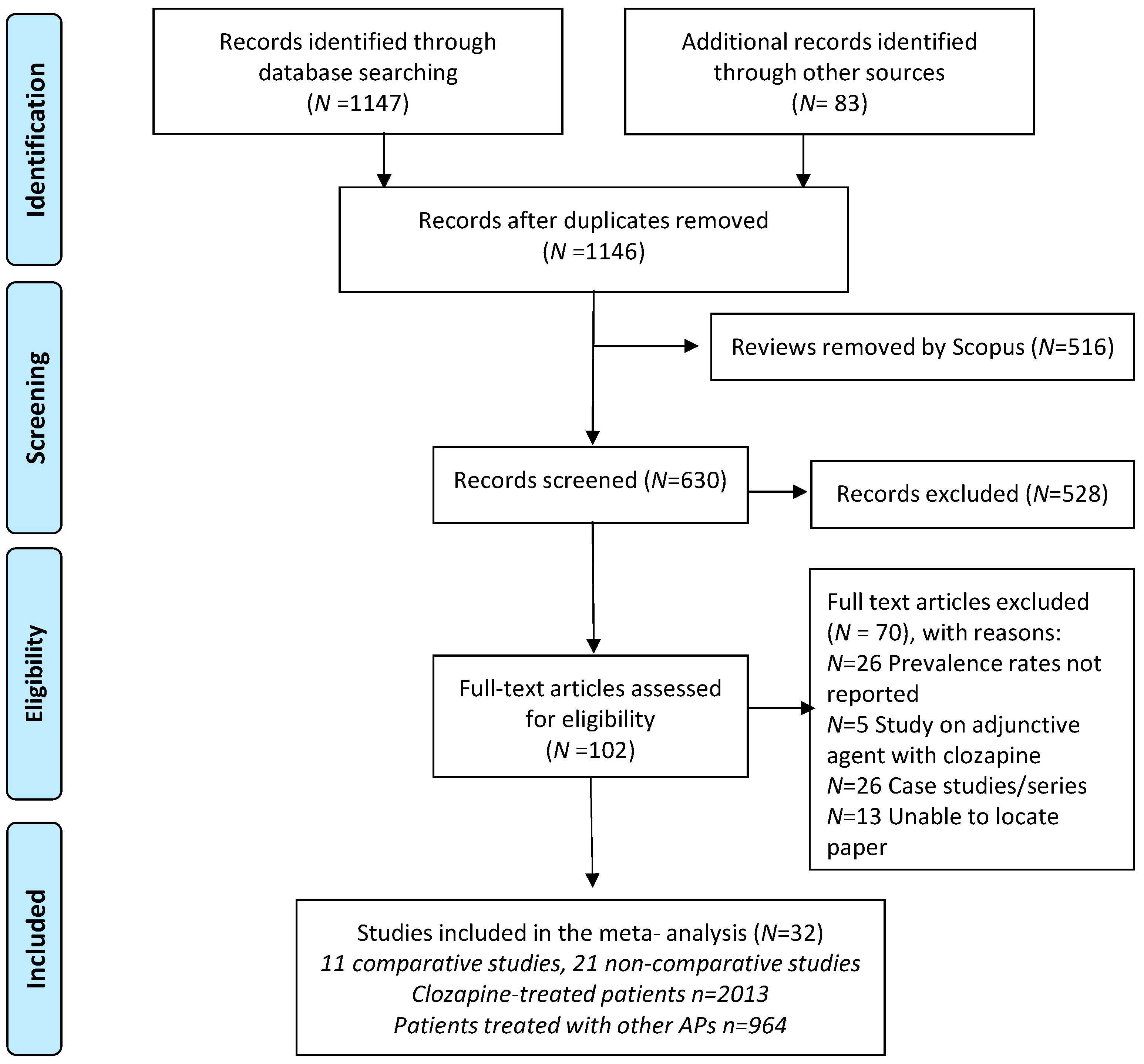
3.1. Search Results and Study Selection
3.2. Study and Participant Characteristics
3.2.1. Included Studies—Non Comparative Studies
3.2.2. Included Studies—Comparative Studies
4. Meta-Analysis
4.1. Prevalence of Constipation on Clozapine
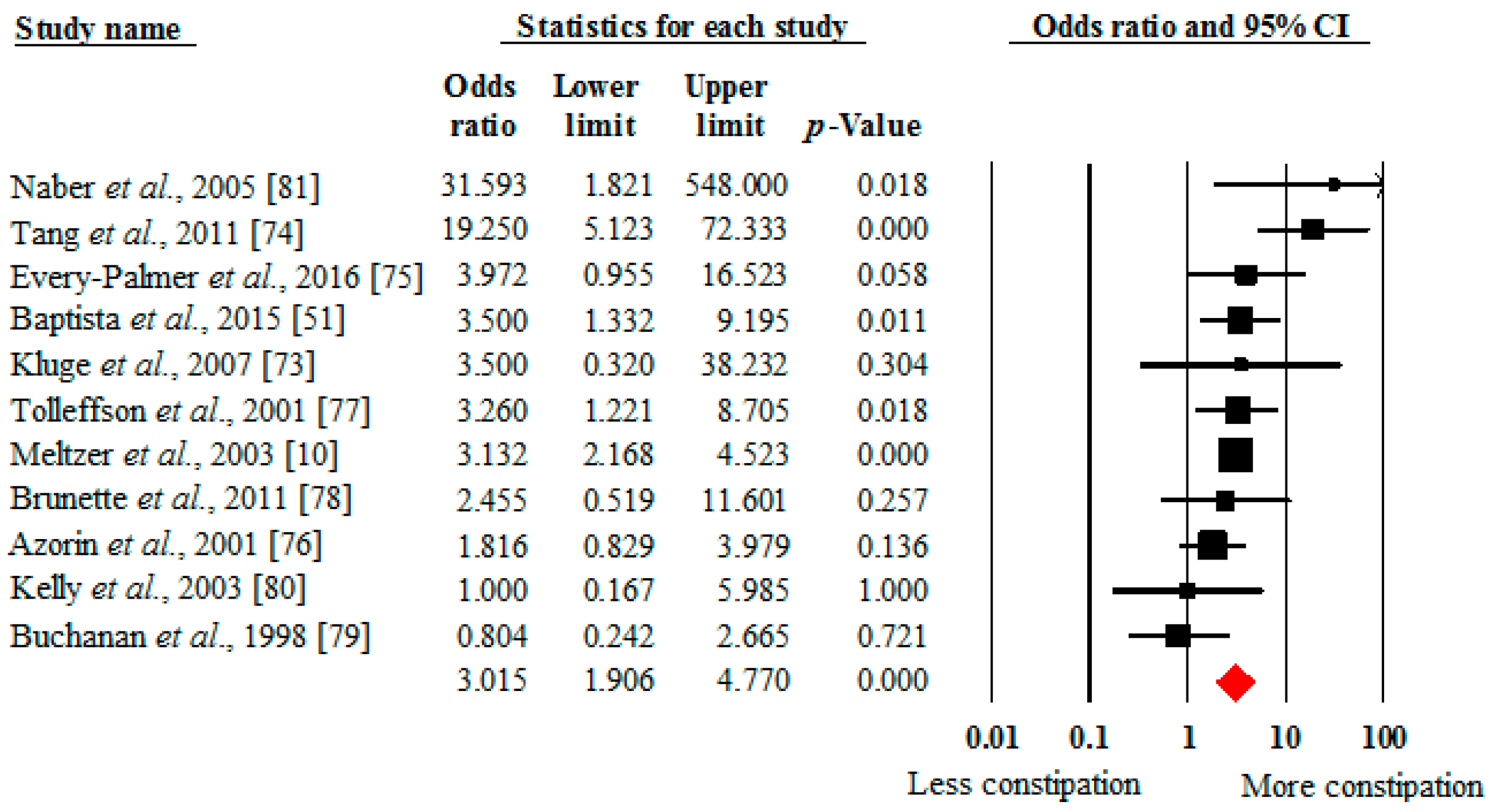
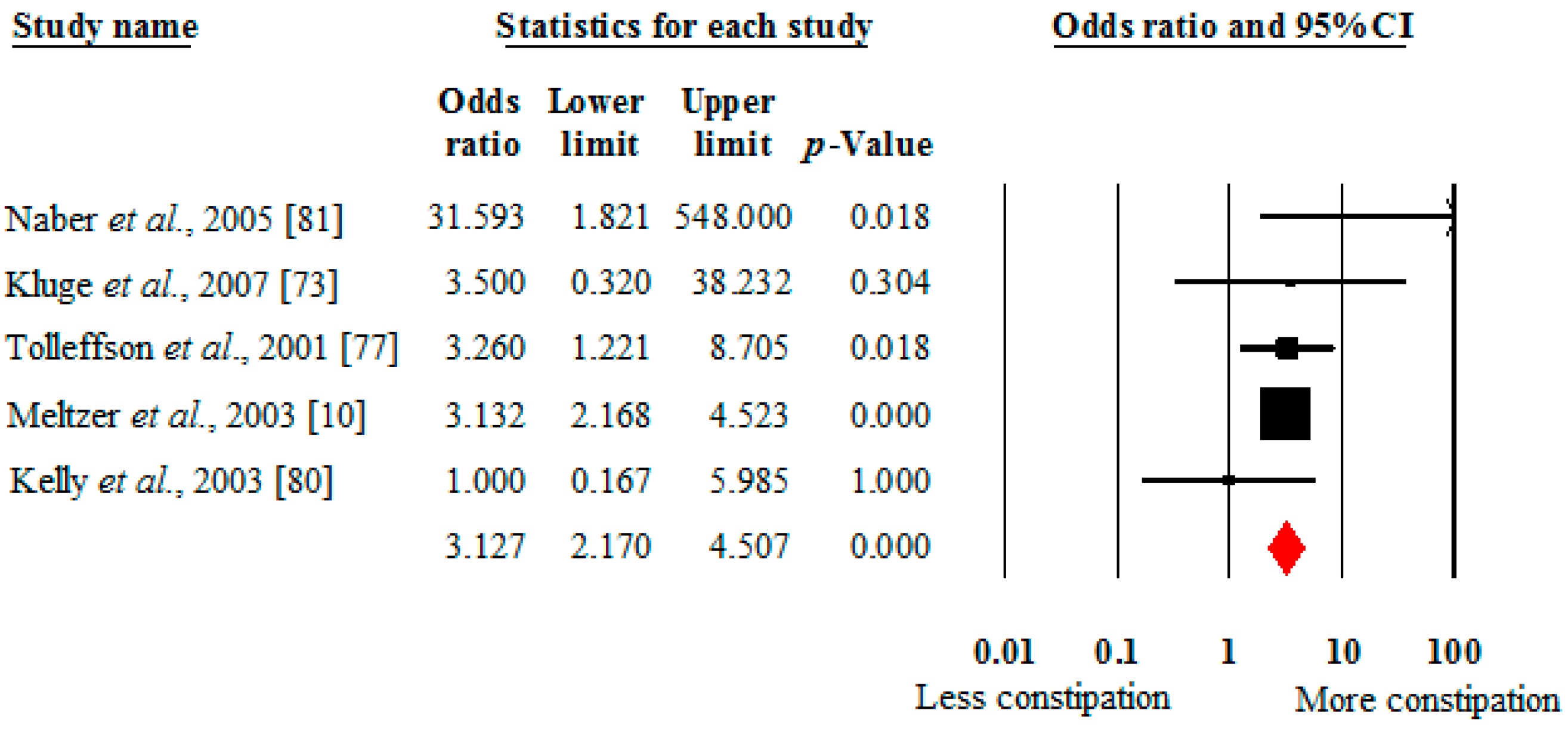
4.2. Prevalence of Constipation in Clozapine versus Other Antipsychotics
4.3. Moderators of Clozapine-Associated Constipation across All Studies
5. Discussion
5.1. General Findings
5.2. What this Study Adds
5.3. Clinical Implications
5.4. Future Directions
5.5. Limitations
6. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
Abbreviations
| TRS | Treatment-resistant schizophrenia |
| MOOSE | Meta-analysis of Observational Studies in Epidemiology |
| PRISMA | Preferred Reporting Items for Systematic Reviews and Meta-Analyses |
| DSM | Diagnostic and Statistical Manual of Mental Disorders |
| ICD | International classification of diseases |
| RCT | Randomized controlled trial |
| OR | odds ratio |
| CYP | Cytochrome P |
References
- Meltzer, H.Y. Treatment-resistant schizophrenia—The role of clozapine. Curr. Med. Res. Opin. 1997, 14, 1–20. [Google Scholar] [CrossRef] [PubMed]
- NICE. Psychosis and Schizophrenia in Adults: Treatment and Management (Clinical Guideline 178); Royal College of Psychiatrists: London, UK, 2014. [Google Scholar]
- Wahlbeck, K.; Cheine, M.; Essali, A.; Adams, C. Evidence of clozapine’s effectiveness in schizophrenia: A systematic review and meta-analysis of randomized trials. Am. J. Psychiatry 1999, 156, 990–999. [Google Scholar] [PubMed]
- Chakos, M.; Lieberman, J.; Hoffman, E.; Bradford, D.; Sheitman, B. Effectiveness of second-generation antipsychotics in patients with treatment-resistant schizophrenia: A review and meta-analysis of randomized trials. Am. J. Psychiatry 2001, 158, 518–526. [Google Scholar] [CrossRef] [PubMed]
- Howes, O.D.; Vergunst, F.; Gee, S.; McGuire, P.; Kapur, S.; Taylor, D. Adherence to treatment guidelines in clinical practice: Study of antipsychotic treatment prior to clozapine initiation. Br. J. Psychiatry 2012, 201, 481–485. [Google Scholar] [PubMed]
- Alvir, J.M.; Lieberman, J.A.; Safferman, A.Z.; Schwimmer, J.L.; Schaaf, J.A. Clozapine-induced agranulocytosis. Incidence and risk factors in the United States. N. Engl. J. Med. 1993, 329, 162–167. [Google Scholar] [CrossRef] [PubMed]
- Honigfeld, G.; Arellano, F.; Sethi, J.; Bianchini, A.; Schein, J. Reducing clozapine-related morbidity and mortality: 5 years of experience with the clozaril national registry. J. Clin. Psychiatry 1998, 59 (Suppl. 3), S3–S7. [Google Scholar]
- Raja, M.; Raja, S. Clozapine safety, 40 years later. Curr. Drug Saf. 2014, 9, 163–195. [Google Scholar] [CrossRef] [PubMed]
- Koch, A.; Voderholzer, W.A.; Klauser, A.G.; Müller-Lissner, S. Symptoms in chronic constipation. Dis. Colon Rectum 1997, 40, 902–906. [Google Scholar] [CrossRef] [PubMed]
- Meltzer, H.Y.; Alphs, L.; Green, A.I.; Altamura, A.C.; Anand, R.; Bertoldi, A.; Bourgeois, M.; Chouinard, G.; Islam, M.Z.; Kane, J. Clozapine treatment for suicidality in schizophrenia: International suicide prevention trial (intersept). Arch. Gen. Psychiatry 2003, 60, 82–91. [Google Scholar] [CrossRef] [PubMed]
- Talley, N.J.; Jones, M.; Nuyts, G.; Dubois, D. Risk factors for chronic constipation based on a general practice sample. Am. J. Gastroenterol. 2003, 98, 1107–1111. [Google Scholar] [CrossRef] [PubMed]
- Palmer, S.E.; McLean, R.M.; Ellis, P.M.; Harrison-Woolrych, M. Life-threatening clozapine-induced gastrointestinal hypomotility: An analysis of 102 cases. J. Clin. Psychiatry 2008, 69, 759–768. [Google Scholar] [CrossRef] [PubMed]
- Hibbard, K.R.; Propst, A.; Frank, D.E.; Wyse, J. Fatalities associated with clozapine-related constipation and bowel obstruction: A literature review and two case reports. Psychosomatics 2009, 50, 416–419. [Google Scholar] [CrossRef] [PubMed]
- Abrams, P.A.K.; Buccafusco, J.J.; Chapple, C.; Groat, W.C.; Fryer, A.D.; Kay, G.; Laties, A.; Nathanson, N.M.; Pasricha, P.J.; Wein, A.J. Muscarinic receptors: Their distribution and function in body systems, and the implications for treating overactive bladder. Br. J. Pharmacol. 2006, 1, 565–578. [Google Scholar] [CrossRef] [PubMed]
- Crowell, M.D. The role of serotonin in the pathophysiology of irritable bowel syndrome. Am. J. Manag. Care 2001, 7, S252–S260. [Google Scholar] [CrossRef] [PubMed]
- Olianas, M.C.; Dedoni, S.; Ambu, R.; Onali, P. Agonist activity of n-desmethylclozapine at δ-opioid receptors of human frontal cortex. Eur. J. Pharmacol. 2009, 607, 96–101. [Google Scholar] [CrossRef] [PubMed]
- De Leon, J.; Odom-White, A.; Josiassen, R.C.; Diaz, F.J.; Cooper, T.B.; Simpson, G.M. Serum antimuscarinic activity during clozapine treatment. J. Clin. Psychopharmacol. 2003, 23, 336–341. [Google Scholar] [CrossRef] [PubMed]
- Weiner, D.; Meltzer, H.; Veinbergs, I.; Donohue, E.; Spalding, T.; Smith, T.; Mohell, N.; Harvey, S.; Lameh, J.; Nash, N. The role of m1 muscarinic receptor agonism of n-desmethylclozapine in the unique clinical effects of clozapine. Psychopharmacology 2004, 177, 207–216. [Google Scholar] [CrossRef] [PubMed]
- De Hert, M.; Correll, C.U.; Bobes, J.; Cetkovich-Bakmas, M.; Cohen, D.; Asai, I.; Detraux, J.; Gautam, S.; Moller, H.J.; Ndetei, D.M.; et al. Physical illness in patients with severe mental disorders. I. Prevalence, impact of medications and disparities in health care. World Psychiatry 2011, 10, 52–77. [Google Scholar]
- Dworkin, R.H. Pain insensitivity in schizophrenia: A neglected phenomenon and some implications. Schizophr. Bull. 1994, 20, 235–248. [Google Scholar] [CrossRef] [PubMed]
- Stubbs, B.; Thompson, T.; Acaster, S.; Vancampfort, D.; Gaughran, F.; Correll, C.U. Decreased pain sensitivity among people with schizophrenia: A meta-analysis of experimental pain induction studies. Pain 2015, 156, 2121–2131. [Google Scholar] [CrossRef] [PubMed]
- Bickerstaff, L.K.; Harris, S.C.; Leggett, R.S.; Cheah, K.-C. Pain insensitivity in schizophrenic patients: A surgical dilemma. Arch. Surg. 1988, 123, 49–51. [Google Scholar] [CrossRef] [PubMed]
- Waddell, L.; Taylor, M. A new self-rating scale for detecting atypical or second-generation antipsychotic side effects. J. Psychopharmacol. 2008, 22, 238–243. [Google Scholar] [CrossRef] [PubMed]
- Hynes, C.; Keating, D.; McWilliams, S.; Madigan, K.; Kinsella, A.; Maidment, I.; Feetam, C.; Drake, R.J.; Haddad, P.M.; Gaughran, F. Glasgow antipsychotic side-effects scale for clozapine—Development and validation of a clozapine-specific side-effects scale. Schizophr. Res. 2015, 168, 505–513. [Google Scholar] [CrossRef] [PubMed]
- Flanagan, R.J.; Ball, R.Y. Gastrointestinal hypomotility: An under-recognised life-threatening adverse effect of clozapine. Forensic Sci. Int. 2011, 206, e31–e36. [Google Scholar] [CrossRef] [PubMed]
- Peyrière, H.; Roux, C.; Ferard, C.; Deleau, N.; Kreft-Jais, C.; Hillaire-Buys, D.; Boulenger, J.P.; Blayac, J.P. Antipsychotics-induced ischaemic colitis and gastrointestinal necrosis: A review of the french pharmacovigilance database. Pharmacoepidemiol. Drug Saf. 2009, 18, 948–955. [Google Scholar] [CrossRef] [PubMed]
- Drew, L.; Herdson, P. Clozapine and constipation: A serious issue. Aust. N. Z. J. Psychiatry 1997, 31, 149–150. [Google Scholar] [PubMed]
- Nielsen, J.; Emborg, C.; Gydesen, S.; Dybbro, J.; Aagaard, J.; Haderup, K.; Glyngdal, P.; Fabricius, S.; Thode, D.; Lublin, H. Augmenting clozapine with sertindole: A double-blind, randomized, placebo-controlled study. J. Clin. Psychopharmacol. 2012, 32, 173–178. [Google Scholar] [CrossRef] [PubMed]
- Leung, J.; Lee, C.C.; Lee, W.K.; Kwong, P. Rapidly fatal clozapine-induced intestinal obstruction without prior warning signs. Aust. N. Z. J. Psychiatry 2008, 42, 1073. [Google Scholar] [PubMed]
- Lavi, E.; Rivkin, L.; Carmon, M.; Reissman, P. Clozapine-induced colonic obstruction requiring surgical treatment. Isr. Med. Assoc. J. 2009, 11, 385–386. [Google Scholar] [PubMed]
- Baptista, T. A fatal case of ischemic colitis during clozapine administration. Rev. Bras. Psiquiatr. 2014, 36, 358–358. [Google Scholar] [CrossRef] [PubMed]
- Nielsen, J.; Meyer, J.M. Risk factors for ileus in patients with schizophrenia. Schizophr. Bull. 2012, 38, 592–598. [Google Scholar] [CrossRef] [PubMed]
- Gliav, A.; Lindberg, G. Quality of life in patients with different types of functional constipation. Scand. J. Gastroenterol. 1997, 32, 1083–1089. [Google Scholar] [CrossRef]
- Dennison, C.; Prasad, M.; Lloyd, A.; Bhattacharyya, S.K.; Dhawan, R.; Coyne, K. The health-related quality of life and economic burden of constipation. Pharmacoeconomics 2005, 23, 461–476. [Google Scholar] [CrossRef] [PubMed]
- De Hert, M.; Hudyana, H.; Dockx, L.; Bernagie, C.; Sweers, K.; Tack, J.; Leucht, S.; Peuskens, J. Second-generation antipsychotics and constipation: A review of the literature. Eur. Psychiatry 2011, 26, 34–44. [Google Scholar] [CrossRef] [PubMed]
- Stroup, D.F.; Berlin, J.A.; Morton, S.C.; Olkin, I.; Williamson, G.D.; Rennie, D.; Moher, D.; Becker, B.J.; Sipe, T.A.; Thacker, S.B. Meta-analysis of observational studies in epidemiology: A proposal for reporting. JAMA 2000, 283, 2008–2012. [Google Scholar] [CrossRef] [PubMed]
- Moher, D.; Liberati, A.; Tetzlaff, J.; Altman, D.G. Preferred reporting items for systematic reviews and meta-analyses: The prisma statement. Ann. Int. Med. 2009, 151, 264–269. [Google Scholar] [CrossRef] [PubMed]
- Essali, M.; Al-Haj Haasan, N.; Li, C.; Rathbone, J. Clozapine versus typical neuroleptic medication for schizophrenia (review). Cochrane Database Syst. Rev. 2010. [Google Scholar] [CrossRef]
- Asenjo Lobos, C.; Komossa, K.; Rummel-Kluge, C.; Hunger, H.; Schmid, F.; Schwarz, S.; Leucht, S. Clozapine versus other atypical antipsychotics for schizophrenia (review). Cochrane Database Syst. Rev. 2010. [Google Scholar] [CrossRef]
- Chengappa, K.N.; Pollock, B.G.; Parepally, H.; Levine, J.; Kirshner, M.A.; Brar, J.S.; Zoretich, R.A. Anticholinergic differences among patients receiving standard clinical doses of olanzapine or clozapine. J. Clin. Psychopharmacol. 2000, 20, 311–316. [Google Scholar] [CrossRef] [PubMed]
- Chowdhury, A.N.; Mukherjee, A.; Ghosh, K.; Chowdhury, S.; Das Sen, K. Horizon of a new hope: Recovery of schizophrenia in Indian. Int. Med. J. 1999, 6, 181–185. [Google Scholar]
- Stroup, T.S.; Lieberman, A.J.; McEvoy, J.P.; Davis, S.M.; Swartz, M.S.; Keefe, R.S.E.; Miller, A.L.; Rosenheck, R.A.; Hsiao, J.K. Results of phase 3 of the catie schizophrenia trial. Schizophr. Res. 2009, 107, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Sengupta, G.; Bhowmick, S.; Hazra, A.; Datta, A.; Rahaman, M. Adverse drug reaction monitoring in psychiatry out-patient department of an Indian teaching hospital. Indian J. Pharmacol. 2011, 43, 36–39. [Google Scholar] [PubMed]
- Kane, J.M.; Marder, S.R.; Schooler, N.R.; Wirshing, W.C.; Umbricht, D.; Baker, R.W.; Wirshing, D.A.; Safferman, A.; Ganguli, R.; McMeniman, M. Clozapine and haloperidol in moderately refractory schizophrenia: A 6-month randomized and double-blind comparison. Arch. Gen. Psychiatry 2001, 58, 965–972. [Google Scholar] [CrossRef] [PubMed]
- Lieberman, J.A.; Phillips, M.; Gu, H.; Stroup, S.; Zhang, P.; Kong, L.; Ji, Z.; Koch, G.; Hamer, R.M. Atypical and conventional antipsychotic drugs in treatment-naive first-episode schizophrenia: A 52-week randomized trial of clozapine vs chlorpromazine. Neuropsychopharmacology 2003, 28, 995–1010. [Google Scholar] [CrossRef] [PubMed]
- Ravanic, D.B.; Dejanovic, S.M.D.; Janjic, V.; Jovic, S.D.; Milovanovic, D.R.; Jakovljevic, V.; Pantovic, V.; Ravanic, B.; Pantovic, M.; Pantovic, M.M. Effectiveness of clozapine, haloperidol and chlorpromazine in schizophrenia during a five-year period. Arq. Neuropsiquiatr. 2009, 67, 195–202. [Google Scholar] [CrossRef] [PubMed]
- Krakowski, M.; Czobor, P. Cholesterol and cognition in schizophrenia: A double-blind study of patients randomized to clozapine, olanzapine and haloperidol. Schizophr. Res. 2011, 130, 27–33. [Google Scholar] [CrossRef] [PubMed]
- Lokshin, P.; Lerner, V.; Miodownik, C.; Dobrusin, M.; Belmaker, R.H. Parenteral clozapine: Five years of experience. J. Clin. Psychopharmacol. 1999, 19, 479–480. [Google Scholar] [CrossRef] [PubMed]
- Dutt, A.; Grover, S.; Chakrabarti, S.; Kulhara, P.; Avasthi, A.; Basu, D.; Das, P.P. Effectiveness of clozapine: A study from north india. Asian J. Psychiatry 2010, 3, 16–19. [Google Scholar] [CrossRef] [PubMed]
- Antonacci, D.J.; de Groot, C.M. Clozapine treatment in a population of adults with mental retardation. J. Clin. Psychiatry 2000, 61, 22–25. [Google Scholar] [CrossRef] [PubMed]
- Baptista, T.; Carrizo, E.; Fernandez, E.; Connell, L.; Servigna, M.; Parra, A.; Quintero, J.; Pabon, A.; Sandia, I.; Uzcateguid, E.; et al. Colonic transit diagnostic test shows significant gastrointestinal hypomotility in clozapine-treated patients in comparison with subjects treated with other antipsychotics. Schizophr. Res. 2015, 166, 207–211. [Google Scholar] [CrossRef] [PubMed]
- DerSimonian, R.; Laird, N. Meta-analysis in clinical trials. Controll. Clin. Trials 1988, 7, 177–188. [Google Scholar] [CrossRef]
- Higgins, J.P.; Thompson, S.G.; Deeks, J.J.; Altman, D.G. Measuring inconsistency in meta-analyses. BMJ 2003, 327, 557–560. [Google Scholar] [CrossRef] [PubMed]
- Egger, M.; Davey Smith, G.; Schneider, M.; Minder, C. Bias in meta-analysis detected by a simple, graphical test. BMJ (Clin. Res. Ed.) 1997, 315, 629–634. [Google Scholar] [CrossRef]
- Fu, R.; Gartlehner, G.; Grant, M.; Shamliyan, T.; Sedrakyan, A.; Wilt, T.J.; Griffith, L.; Oremus, M.; Raina, P.; Ismaila, A.; et al. Conducting quantitative synthesis when comparing medical interventions: Ahrq and the effective health care program. J. Clin. Epidemiol. 2011, 64, 1187–1197. [Google Scholar] [CrossRef] [PubMed]
- Srivastava, S.; Agarwal, A.; Sharma, M. A three-year naturalistic follow-up of patients receiving clozapine: Report from india. Int. J. Psychiatry Clin. Pract. 2002, 6, 167–171. [Google Scholar] [CrossRef] [PubMed]
- Bailey, L.; Varma, S.; Ahmad, N.; Gee, S.; Taylor, D.M. Factors predicting use of laxatives in outpatients stabilized on clozapine. Ther. Adv. Psychopharmacol. 2015, 1, 256–262. [Google Scholar]
- Aamani, M.; Kalyan, K.; Somasundara Babu, R. A study on the efficacy of clozapine in treatment resistant schizphrenia. Int. J. Sci. Res. 2015, 4, 572–574. [Google Scholar]
- Hayes, G.; Gibler, B. Clozapine-induced constipation. Am. J. Psychiatry 1995, 152, 298. [Google Scholar] [PubMed]
- Lieberman, J.A.; Safferman, A.Z.; Pollack, S.; Szymanski, S.; Johns, C.; Howard, A.; Kronig, M.; Bookstein, P.; Kane, J.M. Clinical effects of clozapine in chronic schizophrenia: Response to treatment and predictors of outcome. Am. J. Psychiatry 1994, 151, 1744–1752. [Google Scholar] [PubMed]
- Kishi, T.; Fujita, K.; Furukawa, O.; Suzuki, T.; Moriwaki, M.; Nitta, M.; Hattori, M.; Tsunoka, T.; Chekuri, R.; Kane, J.M.; et al. Efficacy and tolerability of clozapine in japanese patients with treatment-resistant schizophrenia: Results from a 12-week, flexible dose study using raters masked to antipsychotic choice. Asian J. Psychiatry 2013, 6, 200–207. [Google Scholar] [CrossRef] [PubMed]
- Golden, G.; Honigfeld, G. Bioequivalence of clozapine orally disintegrating 100-mg tablets compared with clozapine solid oral 100-mg tablets after multiple doses in patients with schizophrenia. Clin. Drug Investig. 2008, 28, 231–239. [Google Scholar] [CrossRef] [PubMed]
- Honer, W.G.; Thornton, A.E.; Chen, E.Y.; Chan, R.C.; Wong, J.O.; Bergmann, A.; Falkai, P.; Pomarol-Clotet, E.; McKenna, P.J.; Stip, E. Clozapine alone versus clozapine and risperidone with refractory schizophrenia. N. Engl. J. Med. 2006, 354, 472–482. [Google Scholar] [CrossRef] [PubMed]
- Kelly, D.L.; Sullivan, K.M.; McEvoy, J.P.; McMahon, R.P.; Wehring, H.J.; Gold, J.M.; Liu, F.; Warfel, D.; Vyas, G.; Richardson, C.M.; et al. Adjunctive minocycline in clozapine-treated schizophrenia patients with persistent symptoms. J. Clin. Psychopharmacol. 2015, 35, 374–381. [Google Scholar] [CrossRef] [PubMed]
- Ebrahim, G.M.; Gibler, B.; Hayes, G.; Gacono, C.B. Patient response to clozapine in a forensic psychiatric hospital. Psychiatr. Serv. 1994, 45, 271–273. [Google Scholar] [CrossRef]
- Kim, J.H.; Sim, M.; Park, S.-H.; Kim, D. A naturalistic study of high-dose clozapine treatment in refractory schizophrenia using a within-subject design. Clin. Psychopharmacol. Neurosci. 2009, 7, 44–50. [Google Scholar]
- Lu, M.-L.; Lane, H.-Y.; Chen, K.-P.; Jann, M.W.; Chang, W.-H. Fluvoxamine reduces the clozapine dosage needed in refractory schizophrenic patients. J. Clin. Psychiatry 2000, 61, 594–599. [Google Scholar] [CrossRef]
- Spina, E.; Avenoso, A.; Facciolà, G.; Scordo, M.G.; Ancione, M.; Madia, A.G.; Ventimiglia, A.; Perucca, E. Relationship between plasma concentrations of clozapine and norclozapine and therapeutic response in patients with schizophrenia resistant to conventional neuroleptics. Psychopharmacology 2000, 148, 83–89. [Google Scholar] [CrossRef] [PubMed]
- Drew, L.R.; Hodgson, D.M.; Griffiths, K.M. Clozapine in community practice: A 3-year follow-up study in the australian capital territory. Aust. N. Z. J. Psychiatry 1999, 33, 667–675. [Google Scholar] [CrossRef] [PubMed]
- Yusufi, B.; Mukherjee, S.; Flanagan, R.; Paton, C.; Dunn, G.; Page, E.; Barnes, T.R. Prevalence and nature of side effects during clozapine maintenance treatment and the relationship with clozapine dose and plasma concentration. Int. Clin. Psychopharmacol. 2007, 22, 238–243. [Google Scholar] [CrossRef] [PubMed]
- Faasen, N.; Niehaus, D.J.; Koen, L.; Jordaan, E. Undiagnosed metabolic syndrome and other adverse effects among clozapine users of xhosa descent. S. Afr. J. Psychiatry 2014, 20, 54–57. [Google Scholar] [CrossRef]
- Centorrino, F.; Baldessarini, R.J.; Kando, J.C.; Frankenburg, F.R.; Volpicelli, S.A.; Flood, J.G. Clozapine and metabolites: Concentrations in serum and clinical findings during treatment of chronically psychotic patients. J. Clin. Psychopharmacol. 1994, 14, 119–125. [Google Scholar] [CrossRef] [PubMed]
- Kluge, M.; Schuld, A.; Himmerich, H.; Dalal, M.; Schacht, A.; Wehmeier, P.M.; Hinze-Selch, D.; Kraus, T.; Dittmann, R.W.; Pollmächer, T. Clozapine and olanzapine are associated with food craving and binge eating: Results from a randomized double-blind study. J. Clin. Psychopharmacol. 2007, 27, 662–666. [Google Scholar] [CrossRef] [PubMed]
- Tang, X.; Wu, H.; Chen, Q. Randomised controlled trial comparing quetiapine with lithium and clozapine with lithium in the treatment of female patient with mania. Shanghai Arch. Psychiatry 2011, 23, 291–297. [Google Scholar]
- Every-Palmer, S.; Nowitz, M.; Stanley, J.; Grant, E.; Huthwaite, M.; Dunn, H.; Ellis, P.M. Clozapine-treated patients have marked gastrointestinal hypomotility, the probable basis of life-threatening gastrointestinal complications: A cross sectional study. EBioMedicine 2016, 5, 125–134. [Google Scholar] [CrossRef] [PubMed]
- Azorin, J.-M.; Spiegel, R.; Remington, G.; Vanelle, J.-M.; Péré, J.-J.; Giguere, M.; Bourdeix, I. A double-blind comparative study of clozapine and risperidone in the management of severe chronic schizophrenia. Am. J. Psychiatry 2001, 158, 1305–1313. [Google Scholar] [CrossRef] [PubMed]
- Tollefson, G.D.; Birkett, M.A.; Kiesler, G.M.; Wood, A.J. Double-blind comparison of olanzapine versus clozapine in schizophrenic patients clinically eligible for treatment with clozapine. Biol. Psychiatry 2001, 49, 52–63. [Google Scholar] [CrossRef]
- Brunette, M.F.; Dawson, R.; O’Keefe, C.D.; Narasimhan, M.; Noordsy, D.L.; Wojcik, J.; Green, A.I. A randomized trial of clozapine versus other antipsychotics for cannabis use disorder in patients with schizophrenia. J. Dual Diagn. 2011, 7, 50–63. [Google Scholar] [CrossRef] [PubMed]
- Buchanan, R.W.; Breier, A.; Kirkpatrick, B.; Ball, P.; Carpenter, W.T., Jr. Positive and negative symptom response to clozapine in schizophrenic patients with and without the deficit syndrome. Am. J. Psychiatry 1998, 155, 751–760. [Google Scholar] [PubMed]
- Kelly, D.L.; Conley, R.R.; Richardson, C.M.; Tamminga, C.A.; Carpenter, W.T. Adverse effects and laboratory parameters of high-dose olanzapine vs. Clozapine in treatment-resistant schizophrenia. Ann. Clin. Psychiatry 2003, 15, 181–186. [Google Scholar] [CrossRef] [PubMed]
- Naber, D.; Riedel, M.; Klimke, A.; Vorbach, E.U.; Lambert, M.; Kühn, K.U.; Bender, S.; Bandelow, B.; Lemmer, W.; Moritz, S. Randomized double blind comparison of olanzapine vs. Clozapine on subjective well-being and clinical outcome in patients with schizophrenia. Acta Psychiatr. Scand. 2005, 111, 106–115. [Google Scholar] [CrossRef] [PubMed]
- Ozbilen, M.; Adams, C.E. Systematic overview of cochrane reviews for anticholinergic effects of antipsychotic drugs. J. Clin. Psychopharmacol. 2009, 29, 141–146. [Google Scholar] [CrossRef] [PubMed]
- Stewart, W.F.; Liberman, J.N.; Sandler, R.S.; Woods, M.S.; Stemhagen, A.; Chee, E.; Lipton, R.B.; Farup, C.E. Epidemiology of constipation (EPOC) study in the united states: Relation of clinical subtypes to sociodemographic features. Am. J. Gastroenterol. 1999, 94, 3530–3540. [Google Scholar] [CrossRef] [PubMed]
- Peppas, G.; Alexiou, V.G.; Mourtzoukou, E.; Falagas, M.E. Epidemiology of constipation in europe and oceania: A systematic review. BMC Gastroenterol. 2008, 8. [Google Scholar] [CrossRef] [PubMed]
- Meyer, J.M.; Cummings, M.A. Lubiprostone for treatment-resistant constipation associated with clozapine use. Acta Psychiatr. Scand. 2014, 130, 71–72. [Google Scholar] [CrossRef] [PubMed]
- Cahn, W.; Ramlal, D.; Bruggeman, R.; de Haan, L.; Scheepers, F.; Van Soest, M.; Assies, J.; Slooff, C. Prevention and treatment of somatic complications arising from the use of antipsychotics. Tijdschr. Psychiatr. 2007, 50, 579–591. [Google Scholar]
- Taylor, D.; Paton, C.; Kapur, S. The Maudsley Prescribing Guidelines in Psychiatry; Wiley: London, UK, 2015. [Google Scholar]
- Pai, N.B.; Vella, S.C. Reason for clozapine cessation. Acta Psychiatr. Scand. 2012, 125, 39–44. [Google Scholar] [CrossRef] [PubMed]
- Longstreth, G.F.; Thompson, W.G.; Chey, W.D.; Houghton, L.A.; Mearin, F.; Spiller, R.C. Functional bowel disorders. Gastroenterology 2006, 130, 1480–1491. [Google Scholar] [CrossRef] [PubMed]
- Rostami-Hodjegan, A.; Amin, A.M.; Spencer, E.P.; Lennard, M.S.; Tucker, G.T.; Flanagan, R.J. Influence of dose, cigarette smoking, age, sex, and metabolic activity on plasma clozapine concentrations: A predictive model and nomograms to aid clozapine dose adjustment and to assess compliance in individual patients. J. Clin. Psychopharmacol. 2004, 24, 70–78. [Google Scholar] [CrossRef] [PubMed]
- Dobrinas, M.; Cornuz, J.; Oneda, B.; Kohler Serra, M.; Puhl, M.; Eap, C.B. Impact of smoking, smoking cessation, and genetic polymorphisms on CYP1A2 activity and inducibility. Clin. Pharmacol. Ther. 2011, 90, 117–125. [Google Scholar] [CrossRef] [PubMed]
- Hughes, J.R. Effects of abstinence from tobacco: Valid symptoms and time course. Nicot. Tob. Res. 2007, 9, 315–327. [Google Scholar] [CrossRef] [PubMed]
- Lewis, S.; Heaton, K. Stool form scale as a useful guide to intestinal transit time. Scand. J. Gastroenterol. 1997, 32, 920–924. [Google Scholar] [CrossRef] [PubMed]
- Chukhin, E.; Takala, P.; Hakko, H.; Raidma, M.; Putkonen, H.; Rasanen, P.; Terevnikov, V.; Stenberg, J.H.; Eronen, M.; Joffe, G. In a randomized placebo-controlled add-on study orlistat significantly reduced clozapine-induced constipation. Int. Clin. Psychopharmacol. 2013, 28, 67–70. [Google Scholar] [CrossRef] [PubMed]
- Poetter, C.E.; Stewart, J.T. Treatment of clozapine-induced constipation with bethanechol. J. Clin. Psychopharmacol. 2013, 33, 713–714. [Google Scholar] [CrossRef] [PubMed]
- Ramkumar, D.; Rao, S.S. Efficacy and safety of traditional medical therapies for chronic constipation: Systematic review. Am. J. Gastroenterol. 2005, 100, 936–971. [Google Scholar] [CrossRef] [PubMed]
- Every-Palmer, S.; Newton-Howes, G.; Clarke, M.J. Pharmacological treatment for antipsychotic-related constipation. Cochrane Libr. 2014. [Google Scholar] [CrossRef]
- Thompson, S.G.; Higgins, J. How should meta-regression analyses be undertaken and interpreted? Stat. Med. 2002, 21, 1559–1573. [Google Scholar] [CrossRef] [PubMed]
| Analysis | Number of Study Estimates | Meta-Analysis | Heterogeneity | Publication Bias | |||
|---|---|---|---|---|---|---|---|
| Prevalence | 95% CI | Between Group p Value | I2 | Egger Test (p Value) | |||
| Main analysis | 32 | 31.2 | 25.6 | 37.4 | 84 | −0.82 (p = 0.33) | |
| Geographical region | 0.18 | ||||||
| Europe | 6 | 27.7 | 17.4 | 41.0 | 80 | 0.65 (p = 0.46) | |
| North America | 10 | 41.6 | 30.6 | 53.4 | 72 | −0.78 (p = 0.26) | |
| South America | 1 | 33.3 | 10.8 | 67.4 | 0 | N/A | |
| Asia | 6 | 35.0 | 21.8 | 50.9 | 88 | −0.66 (p = 0.37) | |
| Oceania | 2 | 36.9 | 16.0 | 64.2 | 80 | N/A | |
| Africa | 2 | 24.6 | 9.6 | 50.1 | 89 | N/A | |
| Middle East | 1 | 11.9 | 2.9 | 37.7 | 0 | N/A | |
| Various | 4 | 19.5 | 10.8 | 32.7 | 62 | −0.56 (p = 0.44) | |
| Setting | 0.02 | ||||||
| Inpatient | 14 | 40.5 | 31.4 | 50.4 | 87 | −0.66 (p = 0.46) | |
| Outpatient | 14 | 26.2 | 19.2 | 34.3 | 75 | −0.771 (p = 0.29) | |
| Mixed | 4 | 22.2 | 12.2 | 36.8 | 70 | 0.33 (p = 0.40) | |
| Study design | 0.31 | ||||||
| Cross sectional | 6 | 40.7 | 27.4 | 55.3 | 42 | 0.49 (p = 0.75) | |
| Retrospective | 6 | 28.2 | 17.2 | 42.6 | 81 | −0.51 (p = 0.67) | |
| Prospective | 8 | 34.4 | 23.3 | 47.6 | 85 | −0.44 (p = 0.23) | |
| Randomized control trial | 12 | 25.9 | 18.3 | 35.4 | 80 | −0.88 (p = 0.46) | |
| Constipation method | 0.70 | ||||||
| Self-reported | 11 | 26.7 | 18.2 | 37.2 | 59 | 1.13 (p = 0.15) | |
| Checklist | 12 | 34.7 | 24.7 | 46.4 | 90 | −0.59 (p = 0.33) | |
| Clinician diagnosis | 6 | 28.4 | 16.5 | 44.1 | 81 | 0.419 (p = 0.09) | |
| ROME III | 2 | 43.1 | 11.1 | 70.1 | 62 | N/A | |
| Laxative use | 1 | 35.2 | 11.1 | 70.3 | 0 | N/A | |
| Moderator | Number of Studies | β | 95% CI | p Value | R2 | |
|---|---|---|---|---|---|---|
| Mean age | 30 | 0.0090 | −0.0513 | 0.0693 | 0.7704 | 0.08 |
| Percentage of males | 31 | 0.0081 | −0.0071 | 0.0233 | 0.2953 | 0 |
| Percentage of smokers | 8 | 0.0241 | −0.0039 | 0.0522 | 0.0918 | 0 |
| Clozapine mean dose | 30 | 0.0017 | −0.0004 | 0.0039 | 0.1085 | 0 |
| Plasma clozapine | 9 | 3.0455 | −0.6171 | 6.708 | 0.1032 | 0.02 |
| Plasma norclozapine | 7 | 3.3561 | −1.6898 | 8.4021 | 0.1924 | 0.01 |
| Number of weeks clozapine treatment | 28 | 0.0013 | −0.0024 | 0.005 | 0.4888 | 0 |
| % Sample schizophrenia | 27 | −0.0020 | −0.0161 | 0.0121 | 0.7825 | 0 |
| % Sample schizoaffective disorder | 11 | −0.0009 | −0.0223 | 0.0204 | 0.9332 | 0 |
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shirazi, A.; Stubbs, B.; Gomez, L.; Moore, S.; Gaughran, F.; Flanagan, R.J.; MacCabe, J.H.; Lally, J. Prevalence and Predictors of Clozapine-Associated Constipation: A Systematic Review and Meta-Analysis. Int. J. Mol. Sci. 2016, 17, 863. https://doi.org/10.3390/ijms17060863
Shirazi A, Stubbs B, Gomez L, Moore S, Gaughran F, Flanagan RJ, MacCabe JH, Lally J. Prevalence and Predictors of Clozapine-Associated Constipation: A Systematic Review and Meta-Analysis. International Journal of Molecular Sciences. 2016; 17(6):863. https://doi.org/10.3390/ijms17060863
Chicago/Turabian StyleShirazi, Ayala, Brendon Stubbs, Lucia Gomez, Susan Moore, Fiona Gaughran, Robert J. Flanagan, James H. MacCabe, and John Lally. 2016. "Prevalence and Predictors of Clozapine-Associated Constipation: A Systematic Review and Meta-Analysis" International Journal of Molecular Sciences 17, no. 6: 863. https://doi.org/10.3390/ijms17060863
APA StyleShirazi, A., Stubbs, B., Gomez, L., Moore, S., Gaughran, F., Flanagan, R. J., MacCabe, J. H., & Lally, J. (2016). Prevalence and Predictors of Clozapine-Associated Constipation: A Systematic Review and Meta-Analysis. International Journal of Molecular Sciences, 17(6), 863. https://doi.org/10.3390/ijms17060863