Nrf2 Expressions Correlate with WHO Grades in Gliomas and Meningiomas
Abstract
:1. Introduction
2. Results
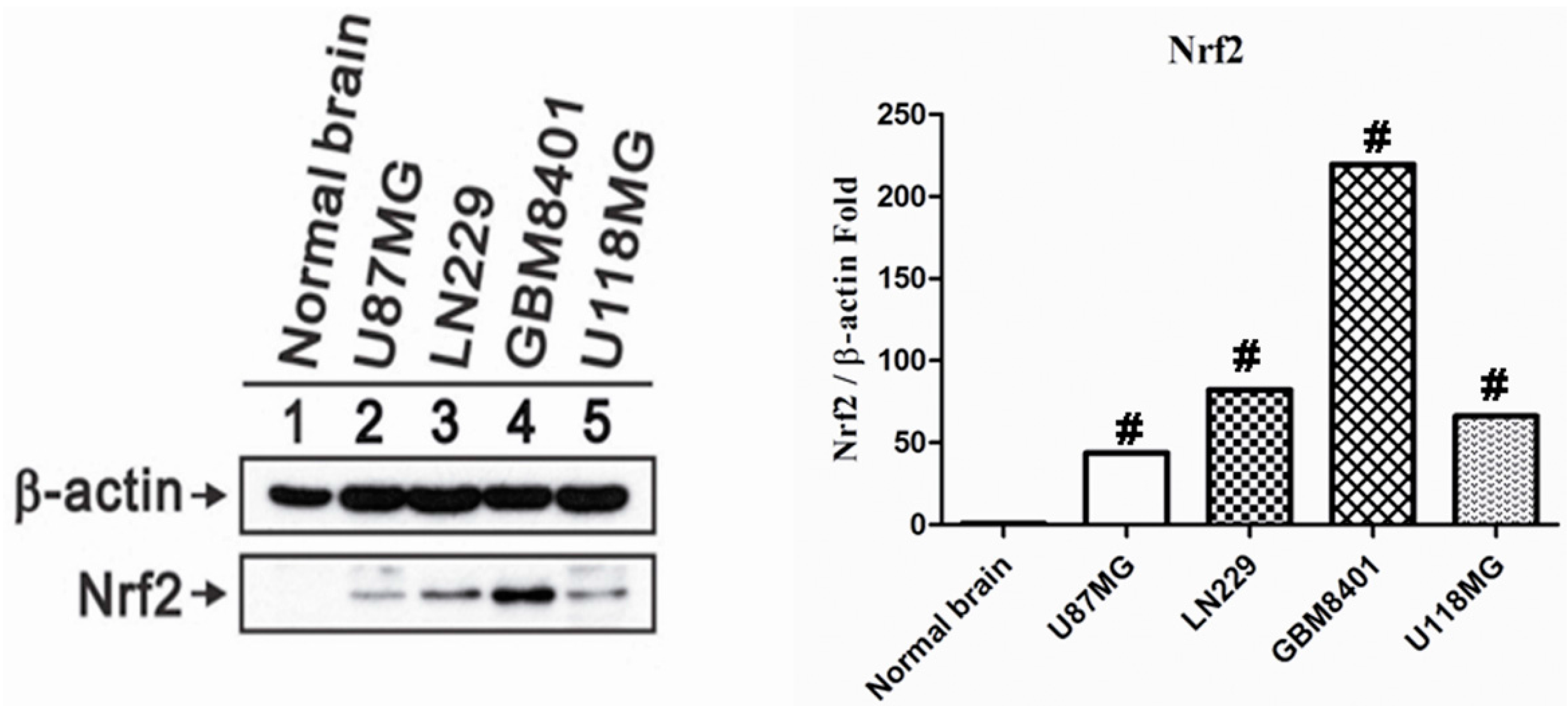
2.1. Nrf2 Protein Overexpression in Human Glioma Cell Lines
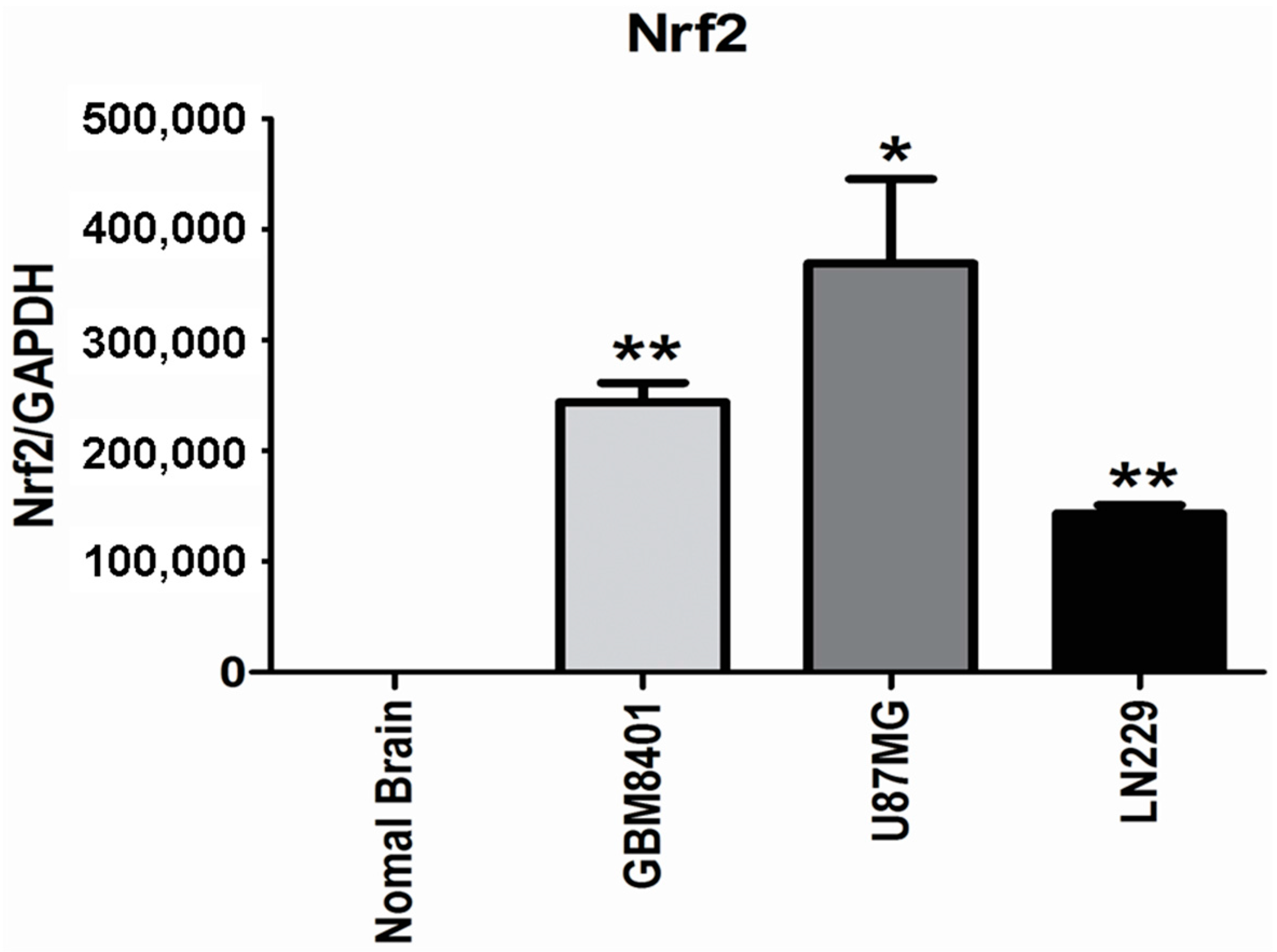
2.2. Higher Nrf2 mRNA Expression in Human Glioma Cell Lines
2.3. Clinicopathological Characteristics in Tissue Microarrays
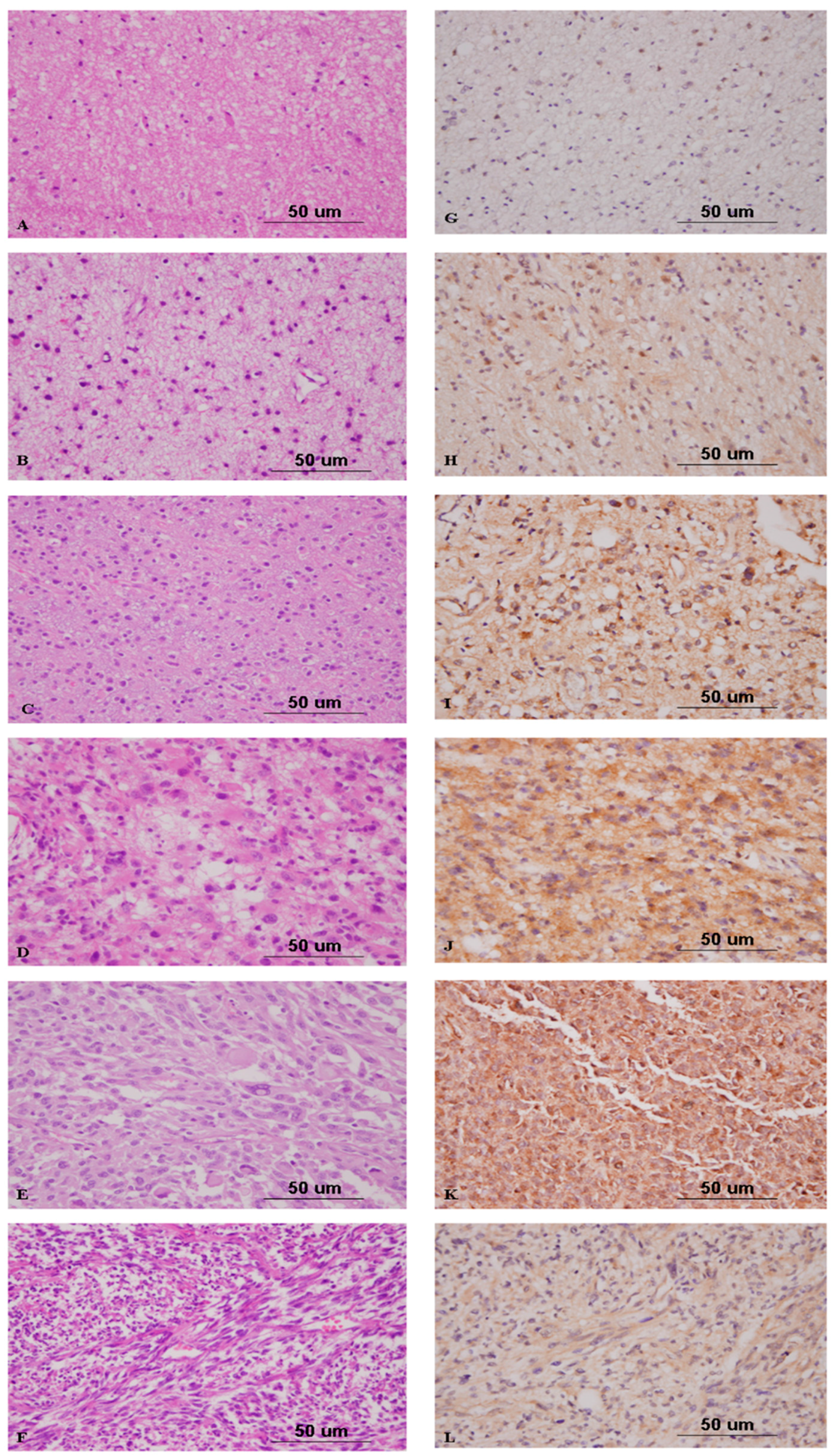
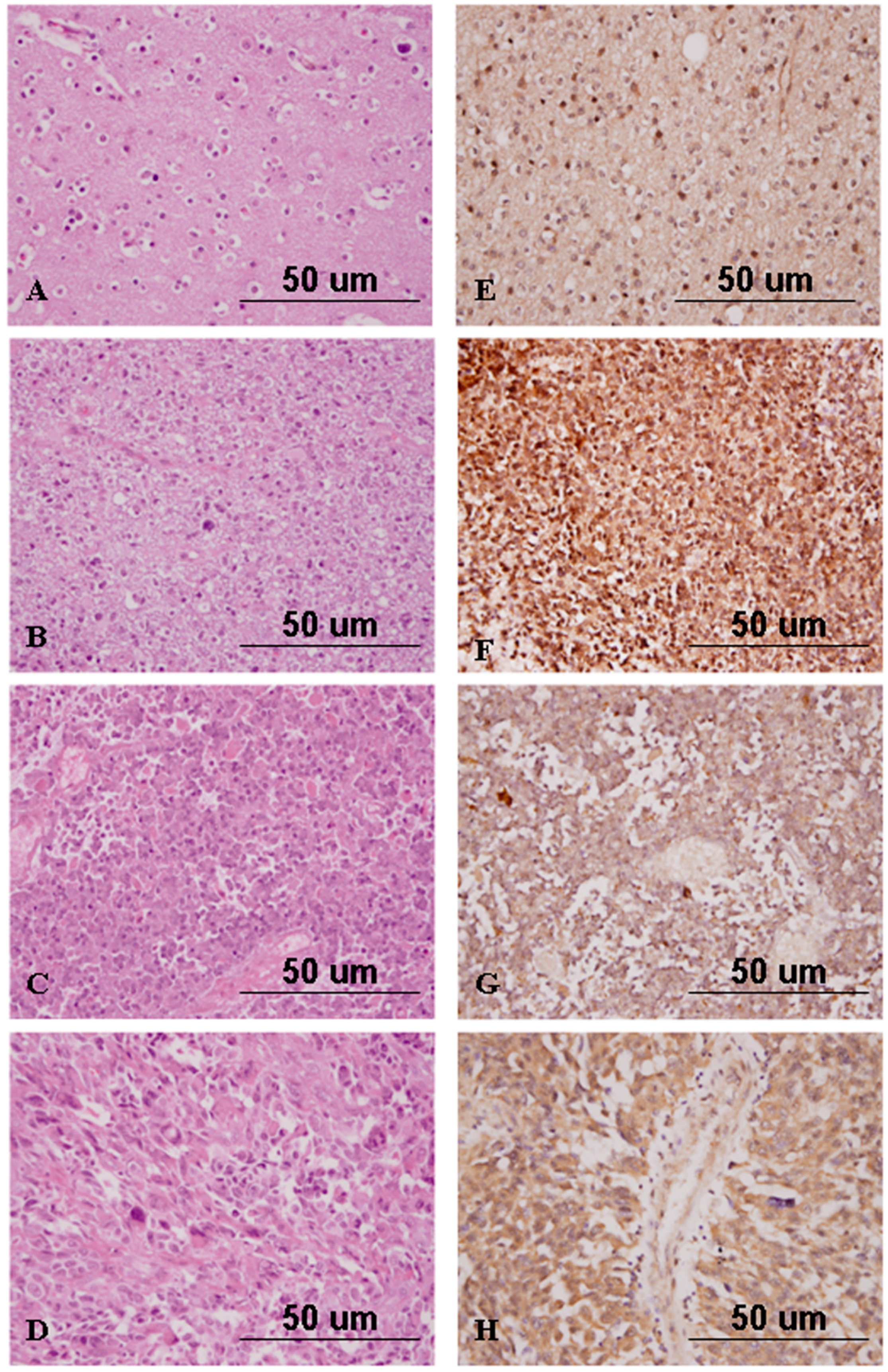
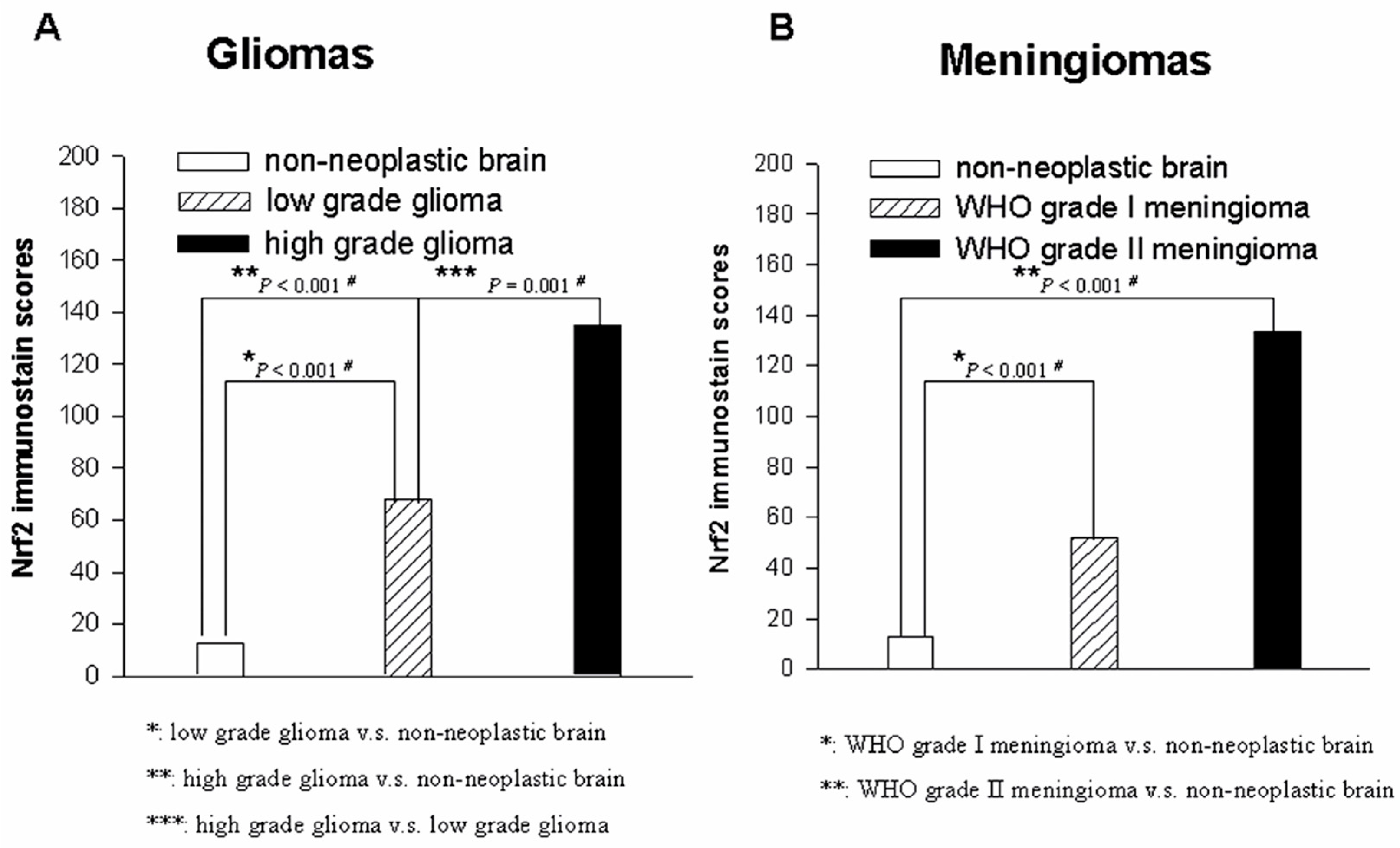
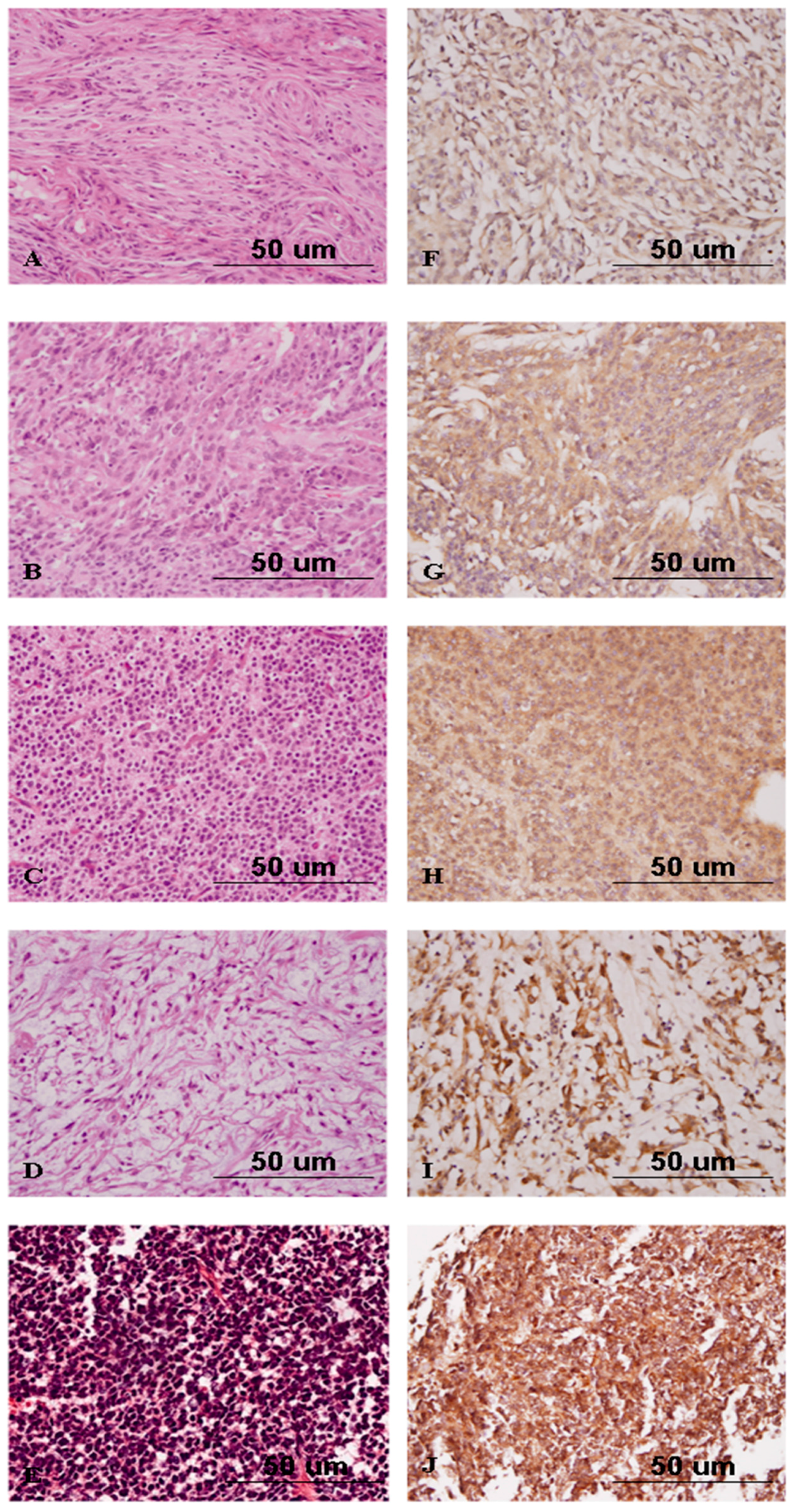
2.4. Nrf2 Expression Correlates with High-Grade Gliomas
2.5. Nrf2 Expression Discriminates Atypical from Benign Meningiomas
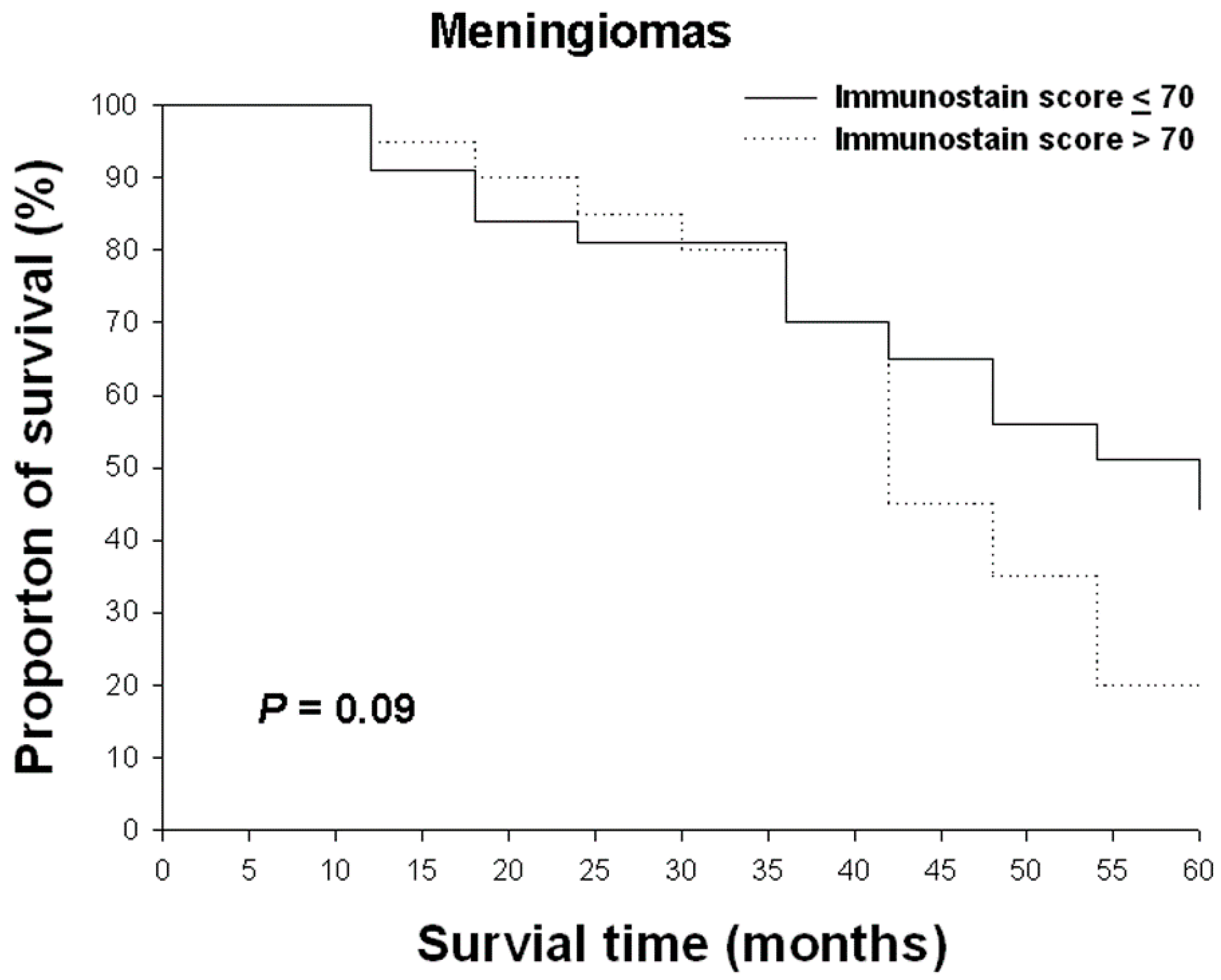
2.6. The Relationship between Nrf2 Imunostain Scores and Overall Survival Rates in Gliomas and Meningiomas
3. Discussion
4. Experimental Section
4.1. Human Glioma Cell Lines and Lysates Preparation
4.2. Western Blots Analysis
4.3. RNA Isolation and Real-Time Reverse Transcription-PCR
4.4. Tissue Microarray Construction
4.5. Immunohistochemistry and Statistical Analysis
4.6. Overall Survival Rate Calculation
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Ostrom, Q.T.; Gittleman, H.; de Blank, P.M.; Finlay, J.L.; Gurney, J.G.; McKean-Cowdin, R.; Stearns, D.S.; Wolff, J.E.; Liu, M.; Wolinsky, Y.; et al. American brain tumor association adolescent and young adult primary brain and central nervous system tumors diagnosed in the United States in 2008–2012. Neuro Oncol. 2016, 18, i1–i50. [Google Scholar] [CrossRef]
- Gu, J.; Liu, Y.; Kyritsis, A.P.; Bondy, M.L. Molecular epidemiology of primary brain tumors. Neurotherapeutics 2009, 6, 427–435. [Google Scholar] [CrossRef]
- Tsai, W.C.; Lee, H.S.; Lin, C.K.; Chen, A.; Nieh, S.; Ma, H.I. The association of osteopontin and LMX1A expression with World Health Organization grade in meningiomas and gliomas. Histopathology 2012, 61, 844–856. [Google Scholar] [CrossRef] [PubMed]
- Louis, D.N.; Ohgaki, H.; Weistler, O.D.; Cavenee, W.K. World Health Organization Classification of Tumours of the Central Nervous System, 4th ed.; IARC Press: Lyon, France, 2007; pp. 8–172. [Google Scholar]
- Bellail, A.C.; Hunter, S.B.; Brat, D.J.; Tan, C.; van Meir, E.G. Microregional extracellular matrix heterogeneity in brain modulates glioma cell invasion. Int. J. Biochem. Cell Biol. 2004, 36, 1046–1069. [Google Scholar] [CrossRef] [PubMed]
- Burger, P.C.; Scheithauer, B.W.; Vogel, F.S. Surgical Pathology of the Central Nervous System and Its Coverings; Churchill Livingstone: London, UK, 1994; pp. 1653–1714. [Google Scholar]
- Kepes, J.J. Meningiomas Biology, Pathology, and Differential Diagnosis, 1st ed.; Masson Publishing: New York, NY, USA, 1982; pp. 3–50. [Google Scholar]
- Lantos, P.L.; VandenBerg, S.R.; Kleihues, P. Tumors of the Nervous System, 3rd ed.; Graham, D.I., Lantos, P.L., Eds.; Greenfield’s neuropathology: London, UK, 1996; pp. 583–879. [Google Scholar]
- Tsai, W.C.; Lee, H.S.; Nieh, S.; Yang, Y.S.; Hueng, D.Y. Cortactin and Fascin-1 Correlate with WHO Grades in Primary Brain Tumors. J. Med. Sci. 2013, 33, 21–27. [Google Scholar]
- Vankalakunti, M.; Vasishta, R.K.; das Radotra, B.; Khosla, V.K. MIB-1 immunolabeling: A valuable marker in prediction of benign recurring meningiomas. Neuropathology 2007, 27, 407–412. [Google Scholar] [CrossRef] [PubMed]
- Nitta, R.T.; Li, G. The invasive nature of glioblastoma. World Neurosurg. 2013, 80, 279–280. [Google Scholar] [CrossRef] [PubMed]
- Niture, S.K.; Jaiswal, A.K. Nrf2-induced antiapoptotic Bcl-xL protein enhances cell survival and drug resistance. Free Radic. Biol. Med. 2013, 57, 119–131. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.J.; Sun, Z.; Villeneuve, N.F.; Zhang, S.; Zhao, F.; Li, Y.; Chen, W.; Yi, X.; Zheng, W.; Wondrak, G.T.; et al. Nrf2 enhances resistance of cancer cells to chemotherapeutic drugs, the dark side of Nrf2. Carcinogenesis 2008, 29, 1235–1243. [Google Scholar] [CrossRef] [PubMed]
- Zhang, D.D. Mechanistic studies of the Nrf2-Keap1 signaling pathway. Drug Metab. Rev. 2006, 38, 769–789. [Google Scholar] [CrossRef] [PubMed]
- Cho, H.Y.; Jedlicka, A.E.; Reddy, S.P.; Kensler, T.W.; Yamamoto, M.; Zhang, L.Y.; Kleeberger, S.R. Role of NRF2 in protection against hyperoxic lung injury in mice. Am. J. Respir. Cell Mol. Biol. 2002, 26, 175–182. [Google Scholar] [CrossRef] [PubMed]
- Shin, S.; Wakabayashi, J.; Yates, M.S.; Wakabayashi, N.; Dolan, P.M.; Aja, S.; Liby, K.T.; Sporn, M.B.; Yamamoto, M.; Kensler, T.W. Role of Nrf2 in prevention of high-fat diet-induced obesity by synthetic triterpenoid CDDO-imidazolide. Eur. J. Pharmacol. 2009, 620, 138–144. [Google Scholar] [CrossRef] [PubMed]
- Burton, N.C.; Kensler, T.W.; Guilarte, T.R. In vivo modulation of the Parkinsonian phenotype by Nrf2. Neurotoxicology 2006, 27, 1094–1100. [Google Scholar] [CrossRef] [PubMed]
- Rangasamy, T.; Guo, J.; Mitzner, W.A.; Roman, J.; Singh, A.; Fryer, A.D.; Yamamoto, M.; Kensler, T.W.; Tuder, R.M.; Georas, S.N.; et al. Disruption of Nrf2 enhances susceptibility to severe airway inflammation and asthma in mice. J. Exp. Med. 2005, 202, 47–59. [Google Scholar] [PubMed]
- Yu, X.; Kensler, T. Nrf2 as a target for cancer chemoprevention. Mutat. Res. 2005, 591, 93–102. [Google Scholar] [CrossRef] [PubMed]
- Rangasamy, T.; Guo, J.; Mitzner, W.A.; Roman, J.; Singh, A.; Fryer, A.D.; Yamamoto, M.; Kensler, T.W.; Tuder, R.M.; Georas, S.N.; et al. Genetic ablation of Nrf2 enhances susceptibility to cigarette smoke-induced emphysema in mice. J. Clin. Investig. 2004, 114, 1248–1259. [Google Scholar] [CrossRef] [PubMed]
- Jiang, T.; Huang, Z.; Lin, Y.; Zhang, Z.; Fang, D.; Zhang, D.D. The protective role of Nrf2 in STZ-induced diabetic nephropathy. Diabetes 2010, 59, 850–860. [Google Scholar] [CrossRef] [PubMed]
- Soini, Y.; Eskelinen, M.; Juvonen, P.; Kärjä, V.; Haapasaari, K.M.; Saarela, A.; Karihtala, P. Nuclear Nrf2 expression is related to a poor survival in pancreatic adenocarcinoma. Pathol. Res. Pract. 2014, 210, 35–39. [Google Scholar] [CrossRef] [PubMed]
- Jiang, T.; Chen, N.; Zhao, F.; Wang, X.J.; Kong, B.; Zheng, W.; Zhang, D.D. High levels of Nrf2 determine chemoresistance in type II endometrial cancer. Cancer Res. 2010, 70, 5486–5496. [Google Scholar] [CrossRef] [PubMed]
- Zhu, J.; Wang, H.; Fan, Y.; Hu, Y.; Ji, X.; Sun, Q.; Liu, H. Differential Nrf2 expression between glioma stem cells and non-stem-like cells in glioblastoma. Oncol. Lett. 2014, 7, 693–698. [Google Scholar] [PubMed]
- Zhu, J.; Wang, H.; Sun, Q.; Ji, X.; Zhu, L.; Cong, Z.; Zhou, Y.; Liu, H.; Zhou, M. Nrf2 is required to maintain the self-renewal of glioma stem cells. BMC Cancer 2013, 13, 380. [Google Scholar] [CrossRef] [PubMed]
- Lee, C.C.; Jan, H.J.; Lai, J.H.; Ma, H.I.; Hueng, D.Y.; Lee, Y.C.; Cheng, Y.Y.; Liu, L.W.; Wei, H.W.; Lee, H.M. Nodal promotes growth and invasion in human gliomas. Oncogene 2010, 29, 3110–3123. [Google Scholar] [CrossRef] [PubMed]
- Ji, X.; Wang, H.; Zhu, J.; Tang, Y.; Zhou, Y.; Zhu, L.; Gao, C.; Li, W.; You, W.; Yu, B.; et al. Correlation of Nrf2 and HIF-1α in glioblastoma and their relationships to clinicopathologic features and survival. Neurol. Res. 2013, 35, 1044–1050. [Google Scholar] [CrossRef] [PubMed]
- Zhao, M.; Xu, H.; Zhang, B.; Hong, B.; Yan, W.; Zhang, J. Impact of nuclear factor erythroid-derived 2-like 2 and p62/sequestosome expression on prognosis of patients with gliomas. Hum. Pathol. 2015, 46, 843–849. [Google Scholar] [CrossRef] [PubMed]
- Kanamori, M.; Higa, T.; Sonoda, Y.; Murakami, S.; Dodo, M.; Kitamura, H.; Taguchi, K.; Shibata, T.; Watanabe, M.; Suzuki, H.; et al. Activation of the NRF2 pathway and its impact on the prognosis of anaplastic glioma patients. Neuro Oncol. 2015, 17, 555–565. [Google Scholar] [CrossRef] [PubMed]
- Boustani, M.R.; Khoshnood, R.J.; Nikpasand, F.; Taleshi, Z.; Ahmadi, K.; Yahaghi, E.; Goudarzi, P.K. Overexpression of ubiquitin-specific protease 2a (USP2a) and nuclear factor erythroid 2-related factor 2 (Nrf2) in human gliomas. J. Neurol. Sci. 2016, 363, 249–252. [Google Scholar] [CrossRef] [PubMed]
- Fletcher, C.D. Diagnostic Histopathology of Tumors, 3rd ed.; Churchill Livingstone: Bosten, MA, USA, 2007; pp. 1653–1721. [Google Scholar]
- Hueng, D.Y.; Tsai, W.C.; Chiou, H.Y.; Feng, S.W.; Lin, C.; Li, Y.F.; Huang, L.C.; Lin, M.H. DDX3X biomarker correlates with poor survival in human gliomas. Int. J. Mol. Sci. 2015, 16, 15578–15591. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Zhu, Q.; He, H.; Jiang, L.; Qiang, Q.; Hu, L.; Hu, G.; Jiang, Y.; Ding, X.; Lu, Y. RIZ1: A potential tumor suppressor in glioma. BMC Cancer 2015, 15, 990. [Google Scholar] [CrossRef] [PubMed]
- Cachia, D.; Kamiya-Matsuoka, C.; Mandel, J.J.; Olar, A.; Cykowski, M.D.; Armstrong, T.S.; Fuller, G.N.; Gilbert, M.R.; de Groot, J.F. Primary and secondary gliosarcomas: Clinical, molecular and survival characteristics. J. Neurooncol. 2015, 125, 401–410. [Google Scholar] [CrossRef] [PubMed]
- Rivera-Zengotita, M.; Yachnis, A.T. Gliosis versus glioma?: Don’t grade until you know. Adv. Anat. Pathol. 2012, 19, 239–249. [Google Scholar] [CrossRef] [PubMed]
- Tsai, W.C.; Chen, Y.; Huang, L.C.; Lee, H.S.; Ma, H.I.; Huang, S.M.; Sytwu, H.K.; Hueng, D.Y. EMMPRIN expression positively correlates with WHO grades of astrocytomas and meningiomas. J. Neurooncol. 2013, 114, 281–290. [Google Scholar] [CrossRef] [PubMed]
- Li, K.; Zhong, C.; Wang, B.; He, J.; Bi, J. Nrf2 expression participates in growth and differentiation of endometrial carcinoma cells in vitro and in vivo. J. Mol. Histol. 2014, 45, 161–167. [Google Scholar] [CrossRef] [PubMed]
| Classification of Tumors | Numbers |
|---|---|
| Meningioma | |
| Meningothelial meningioma (WHO grade I) | 42 |
| Fibrous (fibroblastic) meningioma (WHO grade I) | 7 |
| Psammomatous meningioma (WHO grade I) | 5 |
| Angiomatous meningioma (WHO grade I) | 3 |
| Atypical meningioma(WHO grade II) | 12 |
| Astrocyitc tumors | |
| Pilocytic astrocytoma (WHO grade I) | 7 |
| Diffuse astrocytoma (WHO grade II) | 41 |
| Anaplastic astrocytoma (WHO grade III) | 11 |
| Glioblastoma multiforme (WHO grade IV) | 9 |
| Gliosarcoma (WHO grade IV) | 4 |
| Oligodendroglial tumors | |
| Oligodendroglioma (WHO grade II) | 6 |
| Anaplastic oligodendroglioma (WHO grade III) | 9 |
| Ependymal tumors | |
| Ependymoma (WHO grade II) | 3 |
| Anaplastic ependymoma (WHO grade III) | 2 |
| Other brain tumors | |
| Central neurocytoma (WHO grade II) | 8 |
| Chordoma (WHO grade II) | 4 |
| Medulloblastoma (WHO grade IV) | 5 |
| Classification of Tissue | Average Intensity | Average % Tumor | Average Score | Correlation * |
|---|---|---|---|---|
| Normal brain tissue | 0.67 | 12.78 | 12.78 | - |
| Classification of gliomas | ||||
| Pilocytic astrocytoma | 0.43 | 19.29 | 19.29 | Positive correlation (p = 1.8 × 10−4) |
| Diffuse astrocytoma | 1.29 | 51.95 | 75.98 | |
| Anaplastic astrocytoma | 2.1 | 67.27 | 114 | |
| Glioblastoma multiforme | 1.89 | 83.33 | 160.56 | |
| Gliosarcoma | 1.25 | 32.5 | 42.5 | |
| Oligodendroglioma | 1 | 55 | 71.67 | Positive correlation (p = 0.026) |
| Anaplastic oligodendroglioma | 2.11 | 77.78 | 171.11 | |
| Ependymoma | 1 | 70 | 70 | Positive correlation (p = 0.021) |
| Anaplastic ependymoma | 2.5 | 85 | 212.5 | |
| WHO grades of gliomas | ||||
| WHO grade I | 0.43 | 19.29 | 19.29 | Positive correlation (p = 1.3 × 10−5) |
| WHO grade II | 1.27 | 55.21 | 77.81 | |
| WHO grade III | 2.21 | 75.79 | 173.42 | |
| WHO grade IV | 1.69 | 67.69 | 124.23 | |
| Classification of Tissue | Average Intensity | Average % Tumor | Average Score | Correlation * |
|---|---|---|---|---|
| Classification of tumors | ||||
| Meningothelial meningioma | 1.05 | 41.67 | 55.12 | - |
| Fibrous meningioma | 0.86 | 46.43 | 46.43 | - |
| Psammomatous meningioma | 1 | 53 | 53 | - |
| Angiomatous meningioma | 0.67 | 18.33 | 18.33 | - |
| Atypical meningioma | 1.75 | 70.83 | 133.33 | - |
| WHO grades of meningiomas | ||||
| WHO grade I | 1 | 42.02 | 51.93 | Positive correlation (p = 1.8 × 10−5) |
| WHO grade II | 1.5 | 67.91667 | 105.8333 | |
| Classification of Other Neuroepithelial Tumors | Average Intensity | Average % Tumor | Average Score | WHO Grades |
|---|---|---|---|---|
| Central neurocytoma | 1.57 | 47.86 | 99.29 | II |
| Chordoma | 1.5 | 22.5 | 47.5 | II |
| Medulloblastoma | 2.2 | 78 | 170 | IV |
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tsai, W.-C.; Hueng, D.-Y.; Lin, C.-R.; Yang, T.C.K.; Gao, H.-W. Nrf2 Expressions Correlate with WHO Grades in Gliomas and Meningiomas. Int. J. Mol. Sci. 2016, 17, 722. https://doi.org/10.3390/ijms17050722
Tsai W-C, Hueng D-Y, Lin C-R, Yang TCK, Gao H-W. Nrf2 Expressions Correlate with WHO Grades in Gliomas and Meningiomas. International Journal of Molecular Sciences. 2016; 17(5):722. https://doi.org/10.3390/ijms17050722
Chicago/Turabian StyleTsai, Wen-Chiuan, Dueng-Yuan Hueng, Chii-Ruey Lin, Thomas C. K. Yang, and Hong-Wei Gao. 2016. "Nrf2 Expressions Correlate with WHO Grades in Gliomas and Meningiomas" International Journal of Molecular Sciences 17, no. 5: 722. https://doi.org/10.3390/ijms17050722
APA StyleTsai, W.-C., Hueng, D.-Y., Lin, C.-R., Yang, T. C. K., & Gao, H.-W. (2016). Nrf2 Expressions Correlate with WHO Grades in Gliomas and Meningiomas. International Journal of Molecular Sciences, 17(5), 722. https://doi.org/10.3390/ijms17050722









