Physiological and Molecular Analysis of Aluminium-Induced Organic Acid Anion Secretion from Grain Amaranth (Amaranthus hypochondriacus L.) Roots
Abstract
:1. Introduction
2. Results
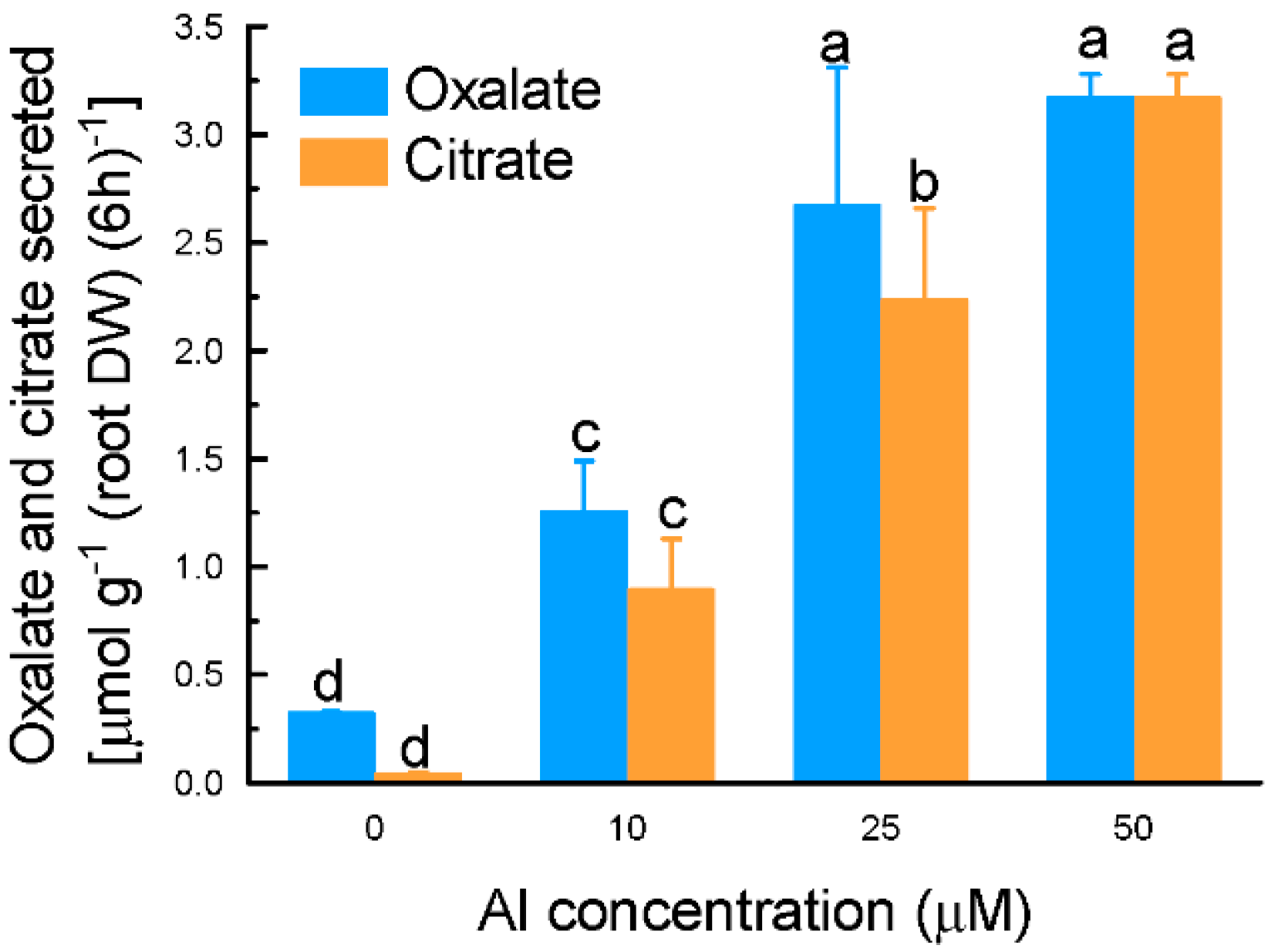
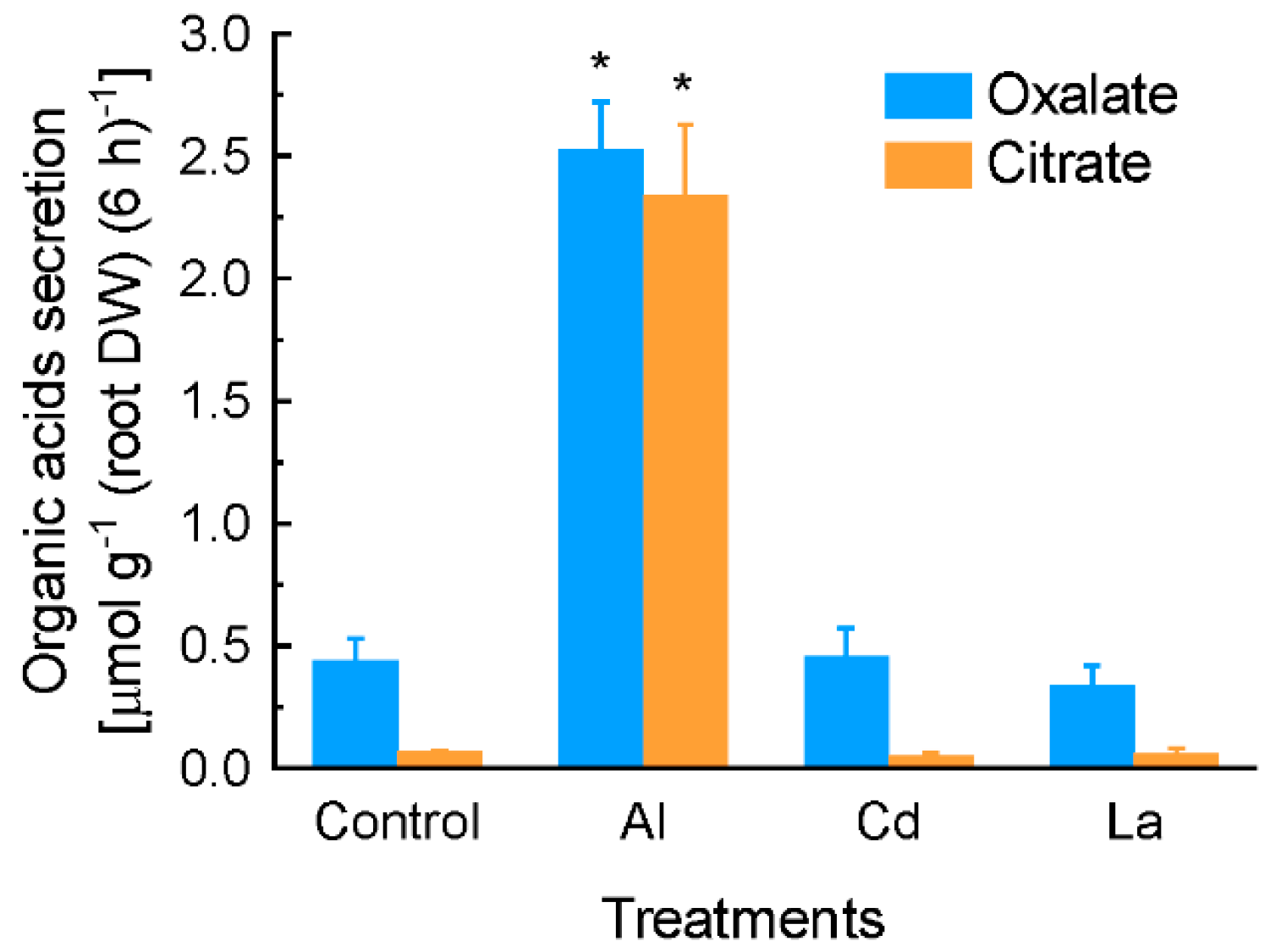
2.1. Characterization of Aluminium (Al)-Induced Organic Acid Anions (OA) Secretion from Grain Amaranth Roots
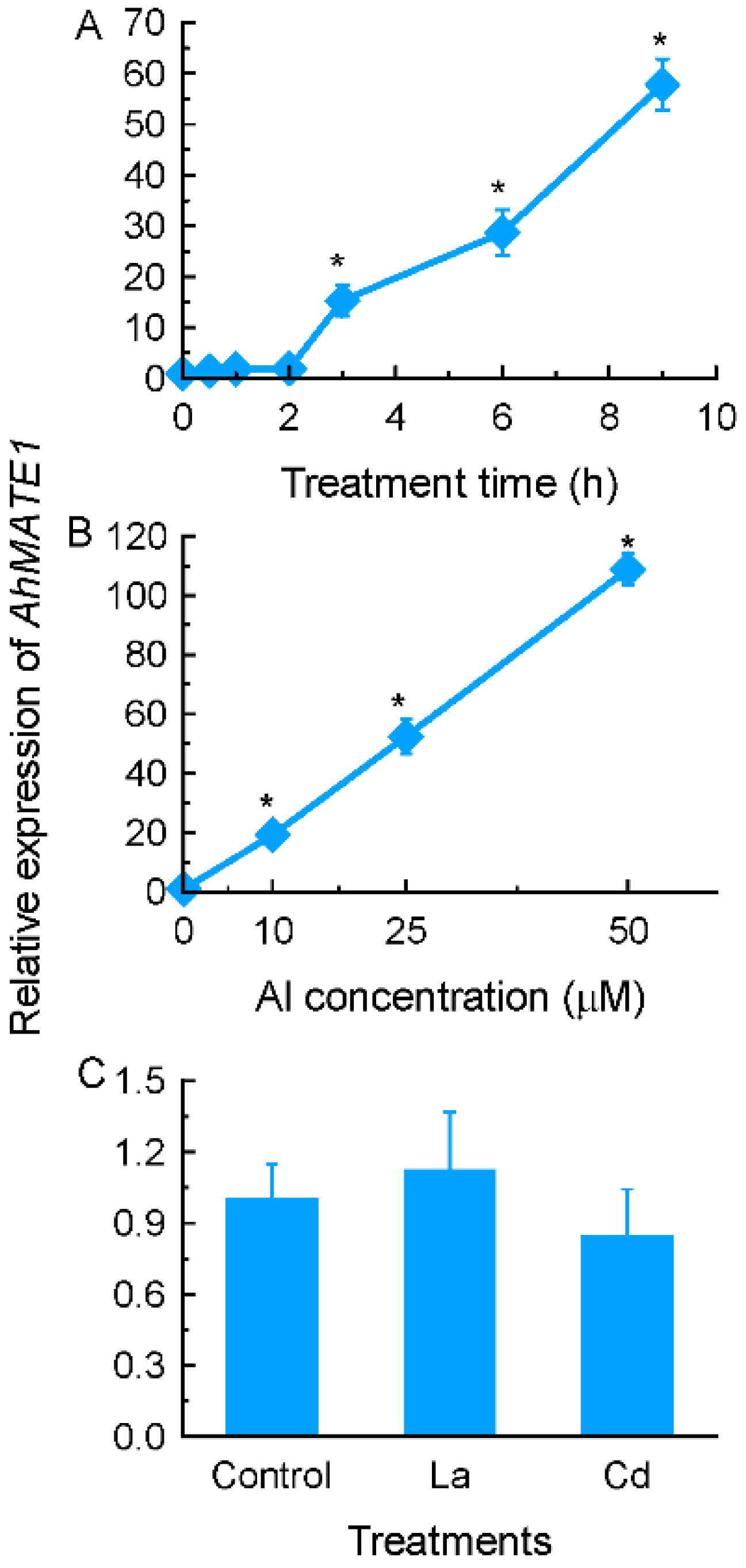
2.2. Identification of up-Regulated Genes by Al Stress in Amaranth Roots
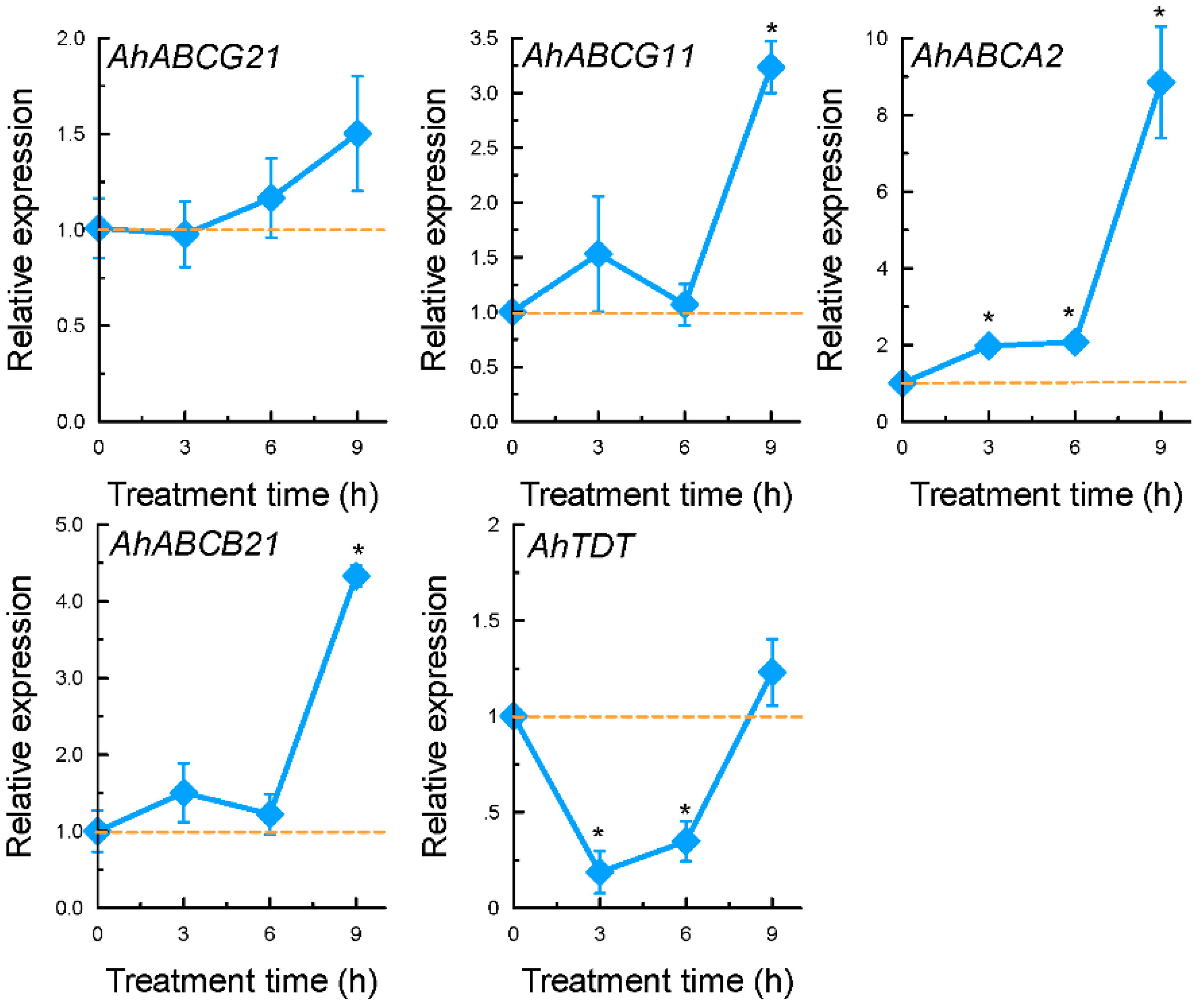
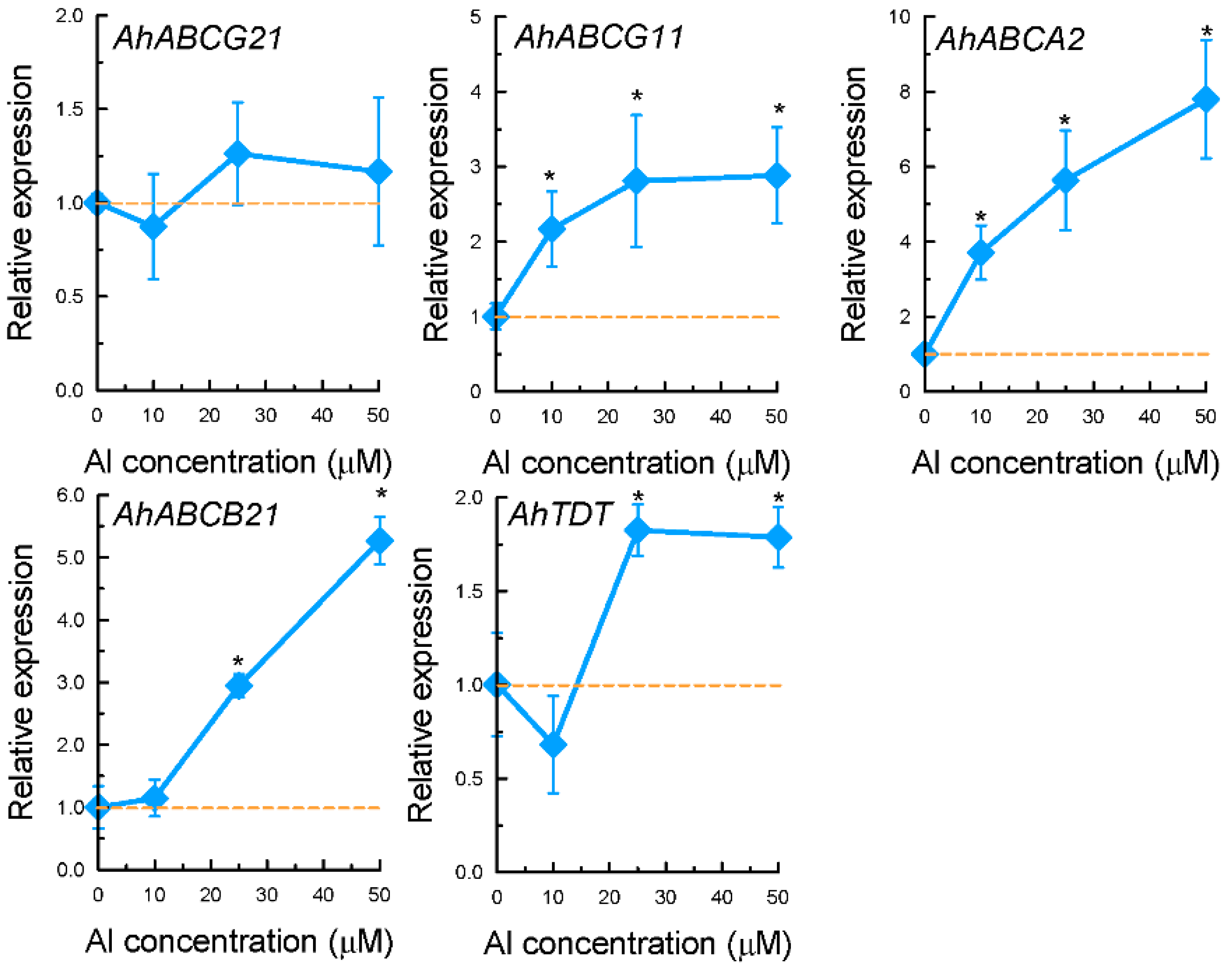
2.3. Expression Patterns of Selected Genes Encoding Transporter Proteins
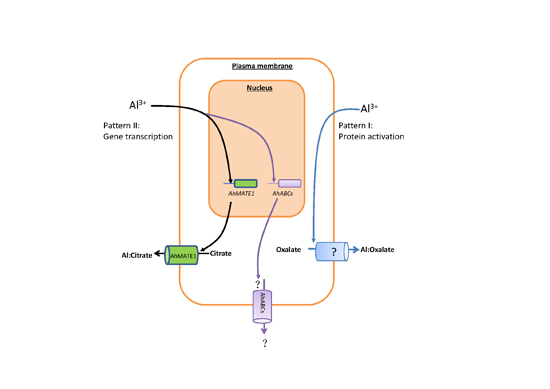
3. Discussion
4. Materials and Methods
4.1. Plant Materials and Growth Conditions
4.2. Treatments
4.3. Collection of Root Exudates
4.4. Measurement of Oxalate and Citrate
4.5. RNA Isolation and Construction of SSH Library
4.6. Sequence Homology and Functional Annotation
4.7. Quantitative Real-Time PCR
4.8. Statistical Analysis
5. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Kochian, L.V. Cellular mechanisms of aluminum toxicity and resistance in plants. Annu. Rev. Plant Physiol. Plant Mol. Biol. 1995, 46, 237–260. [Google Scholar] [CrossRef]
- Barcelo, J.; Poschenrieder, C. Fast root growth responses, root exudates, and internal detoxification as clues to the mechanisms of aluminium toxicity and resistance: A review. Environ. Exp. Bot. 2002, 48, 75–92. [Google Scholar] [CrossRef]
- Matsumoto, H. Cell biology of aluminum toxicity and tolerance in higher plants. Int. Rev. Cytol. 2000, 200, 1–46. [Google Scholar] [PubMed]
- Kochian, L.V.; Hoekenga, O.A.; Piñeros, M.A. How do crop plants tolerate acid soils? Mechanisms of aluminum tolerance and phosphorus efficiency. Annu. Rev. Plant Biol. 2004, 55, 459–493. [Google Scholar] [CrossRef] [PubMed]
- Ma, J.F. Syndrome of aluminum toxicity and diversity of aluminum resistance in higher plants. Int. Rev. Cytol. 2007, 264, 225–252. [Google Scholar] [PubMed]
- Kumari, M.; Taylor, G.J.; Deyholos, M.K. Transcriptomic responses to aluminum stress in roots of Arabidopsis thaliana. Mol. Genet. Genom. 2008, 279, 339–357. [Google Scholar] [CrossRef] [PubMed]
- Goodwin, S.B.; Sutter, T.R. Microarray analysis of Arabidopsis genome response to aluminum stress. Biol. Plant. 2009, 53, 85–99. [Google Scholar] [CrossRef]
- Tsutsui, T.; Yamaji, N.; Huang, C.F.; Motoyama, R.; Nagamura, Y.; Ma, J.F. Comparative genome-wide transcriptional analysis of Al-responsive genes reveals novel Al tolerance mechanisms in rice. PLoS ONE 2012, 7, e48197. [Google Scholar] [CrossRef] [PubMed]
- Yokosho, K.; Yamaji, N.; Ma, J.F. Global transcriptome analysis of Al-induced genes in an Al-accumulating species, common buckwheat (Fagopyrum esculentum Moench). Plant Cell Physiol. 2014, 55, 2077–2091. [Google Scholar] [CrossRef] [PubMed]
- Zhu, H.; Wang, H.; Zhu, Y.; Zou, J.; Zhao, F.J.; Huang, C.F. Genome-wide transcriptomic and phylogenetic analyses reveal distinct aluminum-tolerance mechanisms in the aluminum-accumulating species buckwheat (Fagopyrum tataricum). BMC Plant Biol. 2015, 15, 16. [Google Scholar] [CrossRef] [PubMed]
- Delhaize, E.; Ma, J.F.; Ryan, P.R. Transcriptional regulation of aluminium tolerance genes. Trends Plant Sci. 2012, 17, 341–348. [Google Scholar] [CrossRef] [PubMed]
- Ma, Z.; Miyasaka, S.C. Oxalate exudation by taro in response to Al. Plant Physiol. 1998, 118, 861–865. [Google Scholar] [CrossRef] [PubMed]
- Zheng, S.J.; Ma, J.F.; Matsumoto, H. High aluminum resistance in buckwheat I. Al-induced specific secretion of oxalic acid from root tips. Plant Physiol. 1998, 117, 745–751. [Google Scholar] [CrossRef]
- Yang, J.L.; Zheng, S.J.; He, Y.F.; Matsumoto, H. Aluminium resistance requires resistance to acid stress: A case study with spinach that exudes oxalate rapidly when exposed to Al stress. J. Exp. Bot. 2005, 56, 1197–1203. [Google Scholar] [CrossRef] [PubMed]
- You, J.F.; He, Y.F.; Yang, J.L.; Zheng, S.J. A comparison of aluminum resistance among Polygonum species originating on strongly acidic and neutral soils. Plant Soil. 2005, 276, 143–151. [Google Scholar] [CrossRef]
- Morita, A.; Yanagisawa, O.; Maeda, S.; Takatsu, S.; Ikka, T. Tea plant (Camellia sinensis L.) roots secrete oxalic acid and caffeine into medium containing aluminum. Soil Sci. Plant Nutr. 2011, 57, 796–802. [Google Scholar] [CrossRef]
- Brenner, D.M.; Baltensperger, D.D.; Kulakow, P.A.; Lehmann, J.W.; Myers, R.L.; Slabbert, M.M.; Sleugh, B.B. Genetic resources and breeding of Amaranthus. Plant Breed. Rev. 2000, 19, 227–285. [Google Scholar]
- Miller, T.E.; Wing, J.S.; Huete, A.R. The agricultural potential of selected C4 plants in arid environments. J. Arid Environ. 1984, 7, 275–286. [Google Scholar]
- Johnson, B.L.; Henderson, T.L. Water use patterns of grain amaranth in the northern Great Plains. Agron. J. 2002, 94, 1437–1443. [Google Scholar] [CrossRef]
- Huerta-Ocampo, J.A.; LeÓn-Galván, M.F.; Ortega-Cruz, L.B.; Barrera-Pacheco, A.; de León-Rodríguez, A.; Mendoza-Hernández, G.; de la Rosa, A.P. Water stress induces up-regulation of DOF1 and MIF1 transcription factors and down-regulation of proteins involved in secondary metabolism in amaranth roots (Amaranthus hypochondriacus L.). Plant Biol. 2011, 13, 472–482. [Google Scholar] [CrossRef] [PubMed]
- Délano-Frier, J.P.; Martínez-Gallardo, N.A.; Martínez-de la Vega, O.; Salas-Araiza, M.D.; Barbosa-Jaramillo, E.R.; Torres, A.; Vargas, P.; Borodanenko, A. The effect of exogenous jasmonic acid on induced resistance and productivity in amaranth (Amaranthus hypochondriacus) is influenced by environmental conditions. J. Chem. Ecol. 2004, 30, 1001–1034. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.L.; Zheng, L.; Zheng, S.J. Aluminum-activated oxalate secretion does not associate with internal content among some oxalate accumulators. J. Integr. Plant Biol. 2008, 50, 1103–1107. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; He, Z.; Tian, H.; Zhu, G.; Peng, X. Identification of aluminium-responsive genes in rice cultivars with different aluminium sensitivities. J. Exp. Bot. 2007, 58, 2269–2278. [Google Scholar] [CrossRef] [PubMed]
- Mao, C.; Yi, K.; Yang, L.; Zheng, B.; Wu, Y.; Liu, F.; Wu, P. Identification of aluminium-regulated genes by cDNA-AFLP in rice (Oryza sativa L.): Aluminium-regulated genes for the metabolism of cell wall components. J. Exp. Bot. 2004, 55, 137–143. [Google Scholar] [CrossRef] [PubMed]
- Maron, L.G.; Kirst, M.; Mao, C.; Milner, M.J.; Menossi, M.; Kochian, L.V. Transcriptional profiling of aluminum toxicity and tolerance responses in maize roots. New Phytol. 2008, 179, 116–128. [Google Scholar] [CrossRef] [PubMed]
- Watt, D.A. Aluminium-responsive genes in sugarcane: Identification and analysis of expression under oxidative stress. J. Exp. Bot. 2003, 54, 1163–1174. [Google Scholar] [CrossRef] [PubMed]
- Arenhart, R.A.; Bai, Y.; de Oliveira, L.F.; Neto, L.B.; Schunemann, M.; Maraschin Fdos, S.; Mariath, J.; Silverio, A.; Sachetto-Martins, G.; Margis, R.; et al. New insights into aluminum tolerance in rice: ASR5 protein binds in the STAR1 promoter and other aluminum responsive genes. Mol. Plant 2014, 7, 709–721. [Google Scholar] [CrossRef] [PubMed]
- Sasaki, T.; Yamamoto, Y.; Ezaki, B.; Katsuhara, M.; Ahn, S.J.; Ryan, P.R.; Delhaize, E.; Matsumoto, H. A wheat gene encoding an aluminum-activated malate transporter. Plant J. 2004, 37, 645–653. [Google Scholar] [CrossRef] [PubMed]
- Eticha, D.; Zahn, M.; Bremer, M.; Yang, Z.; Rangel, A.F.; Rao, I.M.; Horst, W.J. Transcriptomic analysis reveals differnetial gene expression in response to aluminium in common bean (Phaseolus vulgaris) genotypes. Ann. Bot. 2010, 105, 1119–1128. [Google Scholar] [CrossRef] [PubMed]
- Chen, Q.; Zhang, X.D.; Wang, S.S.; Wang, Q.F.; Wang, G.Q.; Hian, H.J.; Li, K.Z.; Yu, Y.X.; Chen, L.M. Transcriptional and physiological changes of alfalfa in response to aluminium stress. J. Agric. Sci. 2011, 149, 737–751. [Google Scholar] [CrossRef]
- Fan, W.; Lou, H.Q.; Gong, Y.L.; Liu, M.Y.; Wang, Z.Q.; Yang, J.L.; Zheng, S.J. Identification of early Al-responsive genes in rice bean (Vigna umbellata) roots provides new clues to molecular mechanism of Al toxicity and tolerance. Plant Cell Environ. 2014, 37, 1586–1597. [Google Scholar] [CrossRef] [PubMed]
- Kobayashi, Y.; Kobayashi, Y.; Sugimoto, M.; Lakshmanan, V.; Luchi, S.; Kobayashi, M.; Bais, H.P.; Koyama, H. Characterization of the complex regulation of AtALMT1 expression in response to phytohormones and other inducers. Plant Physiol. 2013, 162, 732–740. [Google Scholar] [CrossRef] [PubMed]
- Tian, Q.; Zhang, X.; Ramesh, S.; Gilliham, M.; Tyerman, S.D.; Zhang, W.H. Ethylene negatively regulates aluminium-induced malate efflux from wheat roots and tobacco cells transformed with TaALMT1. J. Exp. Bot. 2014, 65, 2415–2426. [Google Scholar] [CrossRef] [PubMed]
- Ma, J.F.; Ryan, P.R.; Delhaize, E. Aluminium tolerance in plants and the complexing role of organic acids. Trends Plant Sci. 2001, 6, 273–278. [Google Scholar] [CrossRef]
- Ma, J.F.; Zheng, S.J.; Matsumoto, H. Specific secretion of citric acid induced by Al stress in Cassia tora L. Plant Cell Physiol. 1997, 38, 1019–1025. [Google Scholar] [CrossRef]
- Delhaize, E.; Ryan, P.R.; Randall, P.J. Aluminum tolernace in wheat (Triticum aestivum L.) II. Aluminum-stimulated excretion of malic acid from root apices. Plant Physiol. 1993, 103, 695–702. [Google Scholar] [PubMed]
- Li, X.F.; Ma, J.F.; Matsumoto, H. Pattern of aluminum-induced secretion of organic acids differs between rye and wheat. Plant Physiol. 2000, 123, 1537–1544. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.P.; Magalhaes, J.V.; Shaff, J.E.; Kochian, L.V. Aluminum-activated citrate and malate transporters from the MATE and ALMT families function independently to confer Arabidopsis aluminum tolerance. Plant J. 2009, 57, 389–399. [Google Scholar] [CrossRef] [PubMed]
- Ryan, P.R.; Raman, H.; Gupta, S.; Horst, W.J.; Delhaize, E. A second mechanism for aluminum resistance in wheat relies on the constitutive efflux of citrate from roots. Plant Physiol. 2009, 149, 340–351. [Google Scholar] [CrossRef] [PubMed]
- Ma, J.F. Role of organic acids in detoxification of aluminum in higher plants. Plant Cell Physiol. 2000, 41, 383–390. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.L.; Zhu, X.F.; Peng, Y.X.; Zheng, C.; Ming, F.; Zheng, S.J. Aluminum regulates oxalate secretion and plasma membrane H+-ATPase activity independently in tomato roots. Planta 2011, 234, 281–291. [Google Scholar] [CrossRef] [PubMed]
- Zhu, X.F.; Zheng, C.; Hu, Y.T.; Jiang, T.; Dong, N.Y.; Yang, J.L.; Zheng, S.J. Cadmium-induced oxalate secretion from root apex is associated with cadmium exclusion and resistance in Lycopersicon esculentum. Plant Cell Environ. 2011, 34, 1055–1064. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.L.; Zhang, L.; Li, Y.Y.; You, J.F.; Wu, P.; Zheng, S.J. Citrate transporters play a critical role in aluminium-stimulated citrate efflux in rice bean (Vigna umbellata) roots. Ann. Bot. 2006, 97, 579–584. [Google Scholar] [CrossRef] [PubMed]
- Nunes-Nesi, A.; Brito, D.S.; Inostroza-Blancheteau, C.; Fernie, A.R.; Araújo, W.L. The complex role of mitochondrial metabolism in plant aluminum resistance. Trends Plant Sci. 2014, 19, 399–407. [Google Scholar] [CrossRef] [PubMed]
- Omote, H.; Hiasa, M.; Matsumoto, T.; Otsuka, M.; Moriyama, Y. The MATE proteins as functional transporters of metabolic and xenobiotic organic cations. Trends Pharmacol. Sci. 2007, 11, 587–593. [Google Scholar]
- Magalhaes, J.V.; Liu, J.; Guimarães, C.T.; Lana, U.G.; Alves, V.M.; Wang, Y.H.; Schaffert, R.E.; Hoekenga, O.A.; Piñeros, M.A.; Shaff, J.E.; et al. A member of the multidrug and toxic compound extrusion (MATE) family confers aluminum tolerance in sorghum. Nat. Genet. 2007, 39, 1156–1161. [Google Scholar] [CrossRef] [PubMed]
- Furukawa, J.; Yamaji, N.; Wang, H.; Mitani, N.; Murata, Y.; Sato, K.; Katsuhara, M.; Takeda, K.; Ma, J.F. An aluminum-activated citrate transporter in barley. Plant Cell Physiol. 2007, 48, 1081–1091. [Google Scholar] [CrossRef] [PubMed]
- Yokosho, K.; Yamaji, N.; Ma, J.F. An Al-inducible MATE gene is involved in external detoxification of Al in rice. Plant J. 2011, 68, 1061–1069. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.Y.; Yang, J.L.; Zhou, Y.; Piñeros, M.A.; Kochian, L.V.; Li, G.X.; Zheng, S.J. A de novo synthesis citrate transporter, Vigna umbellate multidrug and toxic compound extrusion, implicates in Al-activated citrate efflux in rice bean (Vigna umbellata) root apex. Plant Cell Environ. 2011, 34, 2138–2148. [Google Scholar] [CrossRef] [PubMed]
- Sawaki, Y.; Kihara-Doi, T.; Kobayashi, Y.; Nishikubo, N.; Kawazu, T.; Kobayashi, Y.; Koyama, H.; Sato, S. Characterization of Al-responsive citrate excretion and citrate-transporting MATEs in Eucalyptus camaldulensis. Planta 2012, 237, 979–989. [Google Scholar] [CrossRef] [PubMed]
- Martinoia, E.; Klein, M.; Geisler, M.; Bovet, L.; Forestier, C.; Kolukisaoglu, U.; Müller-Röber, B.; Schulz, B. Multifunctionality of plant ABC transporters—More than just detoxifiers. Planta 2002, 214, 345–355. [Google Scholar] [CrossRef] [PubMed]
- Lee, M.; Choi, Y.; Burla, B.; Kim, Y.Y.; Jeon, B.; Maeshima, M.; Yoo, J.Y.; Martinoia, E.; Lee, Y. The ABC transporter AtABCB14 is a malate importer and modulates stomatal response to CO2. Nat. Cell Biol. 2008, 10, 1227–1223. [Google Scholar] [CrossRef] [PubMed]
- Badri, D.V.; Loyola-Vargas, V.M.; Broeckling, C.D.; de-la-Peña, C.; Jasinski, M.; Sanelia, D.; Martinoia, E.; Sumner, L.W.; Banta, L.M.; Stermitz, F.; et al. Altered profile of secondary metabolites in the root exudates of Arabidopsis ATP-binding cassette transporter mutants. Plant Physiol. 2008, 146, 762–771. [Google Scholar] [CrossRef] [PubMed]
- Sasaki, T.; Ezaki, B.; Matsumoto, H. A gene encoding multidrug resistance (MDR)-like protein is induced by aluminum and inhibitors of calcium flux in wheat. Plant Cell Physiol. 2002, 43, 177–185. [Google Scholar] [CrossRef] [PubMed]
- Larsen, P.B.; Cancel, J.; Rounds, M.; Ochoa, V. Arabidopsis ALS1 encodes a root tip and stele localized half type ABC transporter required for root growth in an aluminum toxic environment. Planta 2007, 225, 1447–1458. [Google Scholar] [CrossRef] [PubMed]
- Larsen, P.B.; Geisler, M.J.B.; Jones, C.A.; Williams, K.M.; Cancel, J.D. ALS3 encodes a phloem-localized ABC transporter-like protein that is required for aluminum tolerance in Arabidopsis. Plant J. 2005, 41, 353–363. [Google Scholar] [CrossRef] [PubMed]
- Huang, C.F.; Yamaji, N.; Mitani, N.; Yano, M.; Nagamura, Y.; Ma, J.F. A bacterial-type ABC transporter is involved in aluminum tolerance in rice. Plant Cell 2009, 21, 655–667. [Google Scholar] [CrossRef] [PubMed]
- Ryan, P.R.; Delhaize, E. The convergent evolution of aluminium resistance in plants exploits a convenient currency. Funct. Plant Biol. 2010, 37, 275–284. [Google Scholar] [CrossRef]
- Zhao, Z.; Ma, J.F.; Sato, K.; Takeda, K. Differential Al resistance and citrate secretion in barley (Hordeum vulgare L.). Planta 2003, 217, 794–800. [Google Scholar] [CrossRef] [PubMed]
- BLAST®. Available online: http://blast.ncbi.nlm,nig.gov/Blast.cgi (accessed on 23 February 2016).


| Gene Name a | Annotation | Primer Pairs | Amplicon Size (bp) |
|---|---|---|---|
| AhABCG21 | ABC transporter G family member 21 | for: AGGTGACTTGCCTATGGAACT | 107 |
| rev: TCGTAAGGGTAAGGATAAATG | |||
| AhABCG11 | ABC transporter G family member 11 | for: AAACACACTTTCTTCAATCCCAT | 205 |
| rev: ACCCGTTATGATACCCATTAGAA | |||
| AhABCA2 | ABC transporter A family member 2 | for: ACATCGCAAGACAAGCCG | 116 |
| rev: CCCCACATACCTGGCTCC | |||
| AhABCB21 | ABC transporter B family member 21 | for: TGCTATGGGGGAGAAGGT | 122 |
| rev: AAAGGGGTATGGACGAAA | |||
| AhTDT | Tonoplast dicarboxylate transporter | for: TACAGCGACTTCCGACGACTA | 267 |
| rev: ACAAGCAACAAAGAACACCCC | |||
| AhMATE1 | MATE protein | for: GGTCCTTTGGTGCTCCTGC | 163 |
| rev: CCACTGACACCCAAACGACAT |
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fan, W.; Xu, J.-M.; Lou, H.-Q.; Xiao, C.; Chen, W.-W.; Yang, J.-L. Physiological and Molecular Analysis of Aluminium-Induced Organic Acid Anion Secretion from Grain Amaranth (Amaranthus hypochondriacus L.) Roots. Int. J. Mol. Sci. 2016, 17, 608. https://doi.org/10.3390/ijms17050608
Fan W, Xu J-M, Lou H-Q, Xiao C, Chen W-W, Yang J-L. Physiological and Molecular Analysis of Aluminium-Induced Organic Acid Anion Secretion from Grain Amaranth (Amaranthus hypochondriacus L.) Roots. International Journal of Molecular Sciences. 2016; 17(5):608. https://doi.org/10.3390/ijms17050608
Chicago/Turabian StyleFan, Wei, Jia-Meng Xu, He-Qiang Lou, Chuan Xiao, Wei-Wei Chen, and Jian-Li Yang. 2016. "Physiological and Molecular Analysis of Aluminium-Induced Organic Acid Anion Secretion from Grain Amaranth (Amaranthus hypochondriacus L.) Roots" International Journal of Molecular Sciences 17, no. 5: 608. https://doi.org/10.3390/ijms17050608
APA StyleFan, W., Xu, J.-M., Lou, H.-Q., Xiao, C., Chen, W.-W., & Yang, J.-L. (2016). Physiological and Molecular Analysis of Aluminium-Induced Organic Acid Anion Secretion from Grain Amaranth (Amaranthus hypochondriacus L.) Roots. International Journal of Molecular Sciences, 17(5), 608. https://doi.org/10.3390/ijms17050608