Unfractionated Heparin Promotes Osteoclast Formation in Vitro by Inhibiting Osteoprotegerin Activity
Abstract
1. Introduction
2. Results
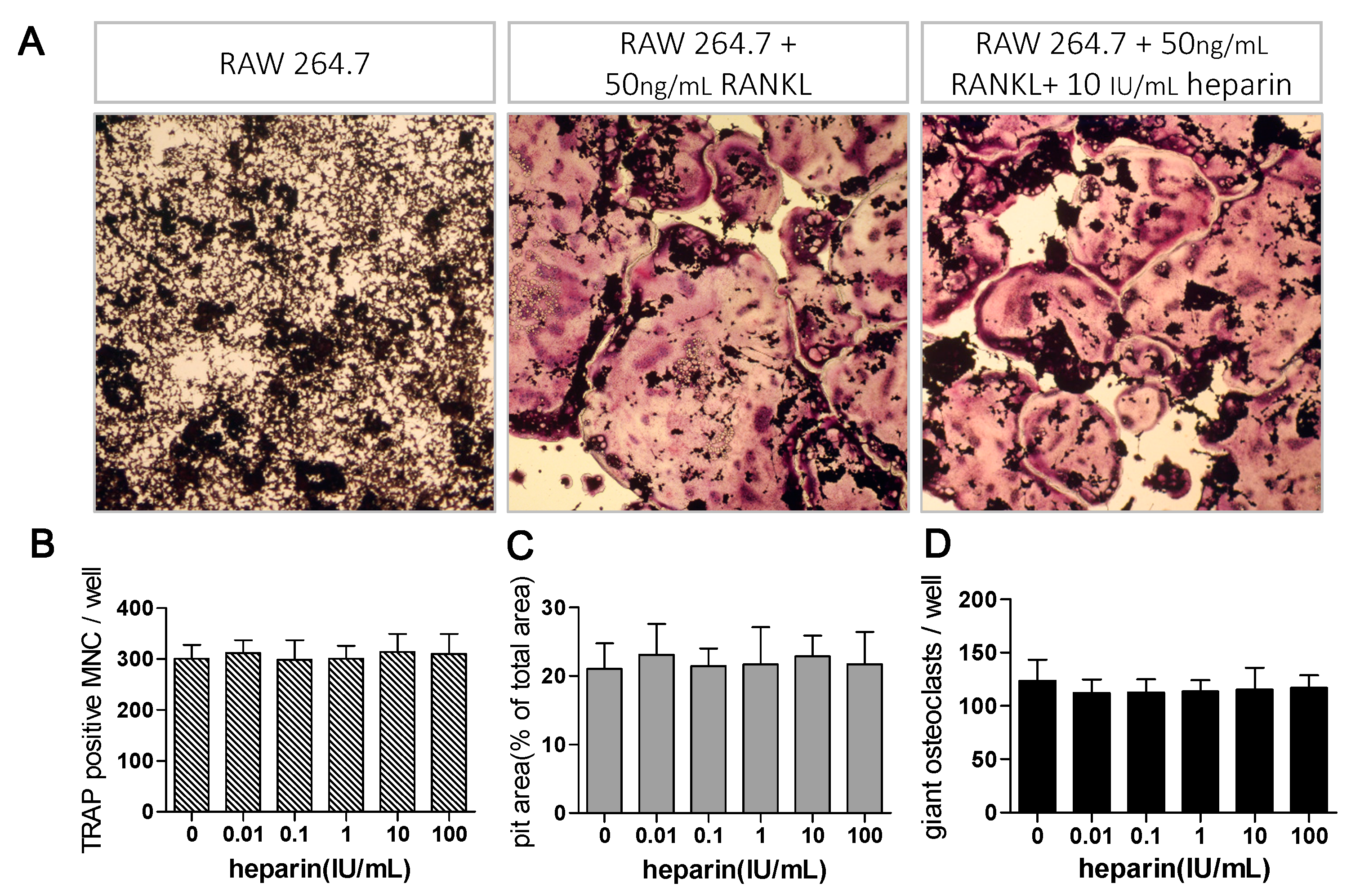
2.1. Heparin Enhances Osteoclastogenesis and Osteoclastic Bone Resorption
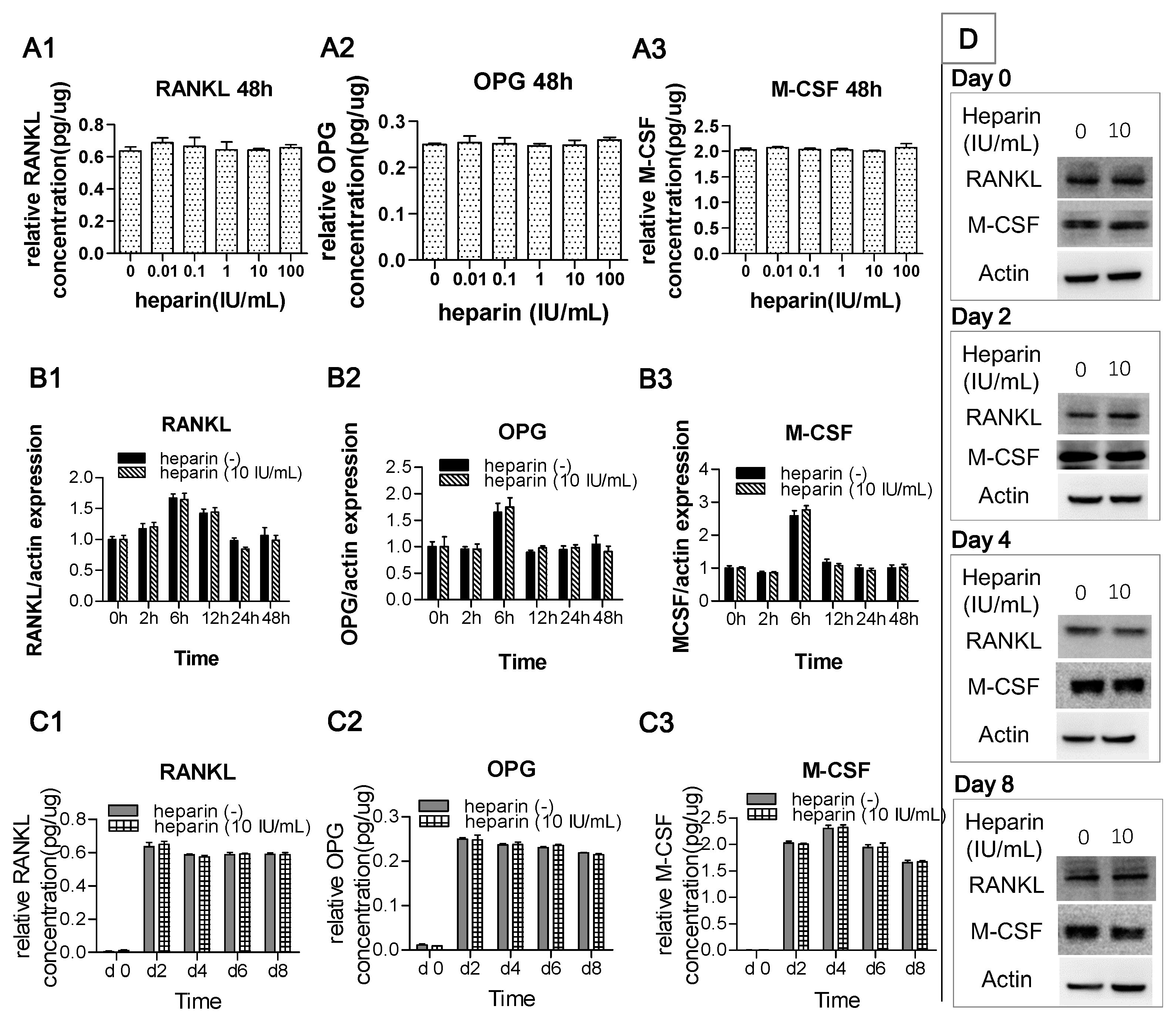
2.2. Heparin Has No Effect on the Production of RANKL, OPG, and M-CSF
2.3. Heparin Has No Effects on RANKL Induced Osteoclast Formation
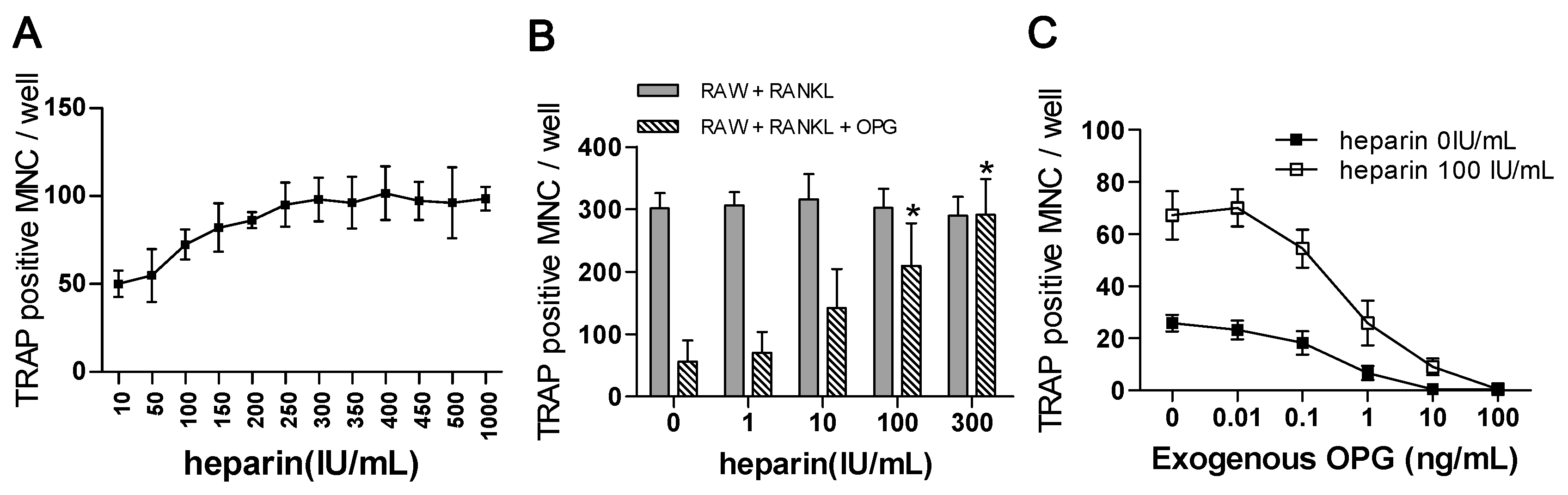
2.4. The Effect of Heparin Was Related to the Inhibition of OPG Activity
2.4.1. The “Saturation Phenomenon”
2.4.2. The Effects of Heparin on RANKL-Induced Osteoclastogenesis with the Addition of OPG
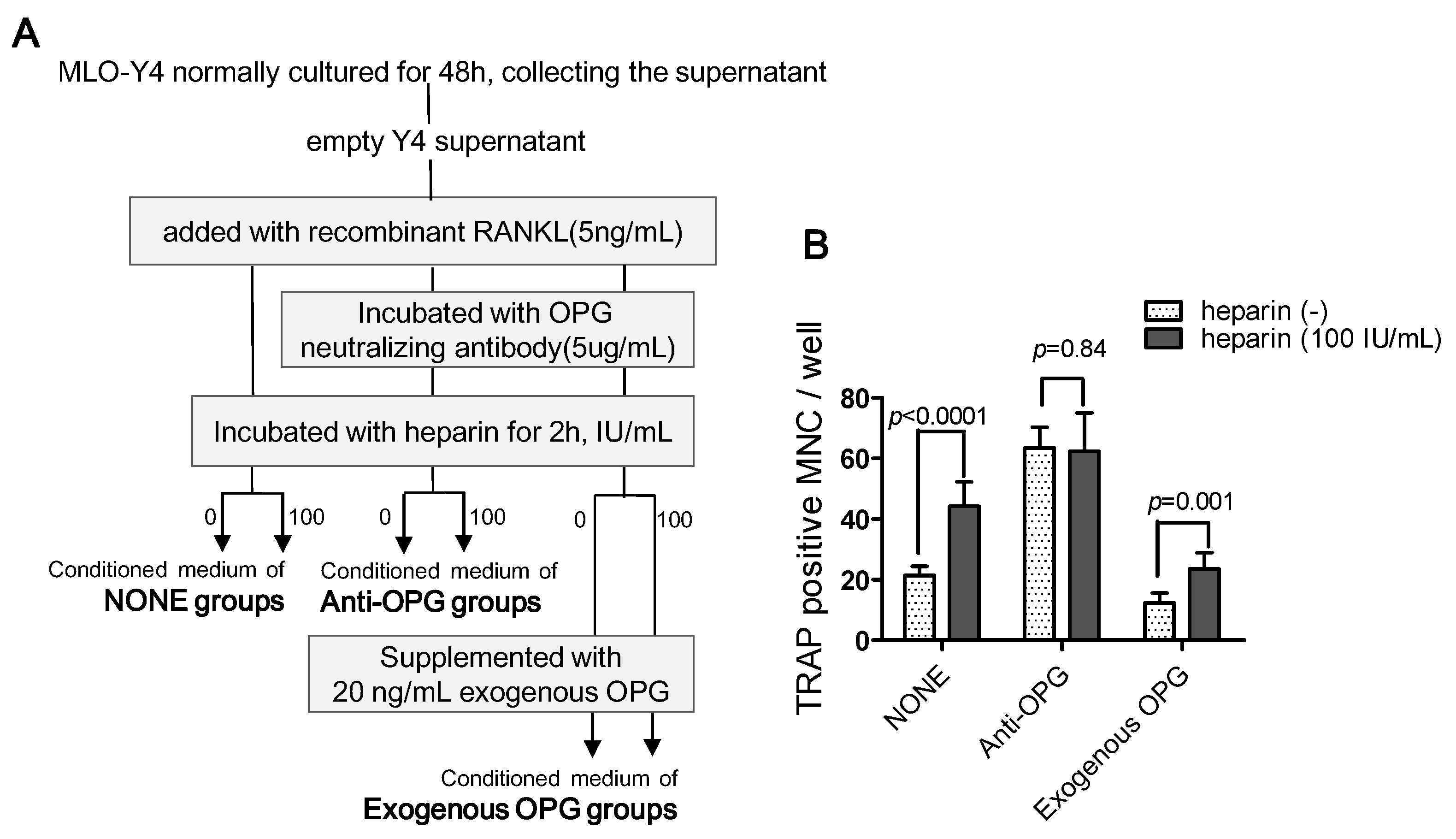
2.4.3. The Use of Exogenous OPG to Attenuate the Effects of Heparin
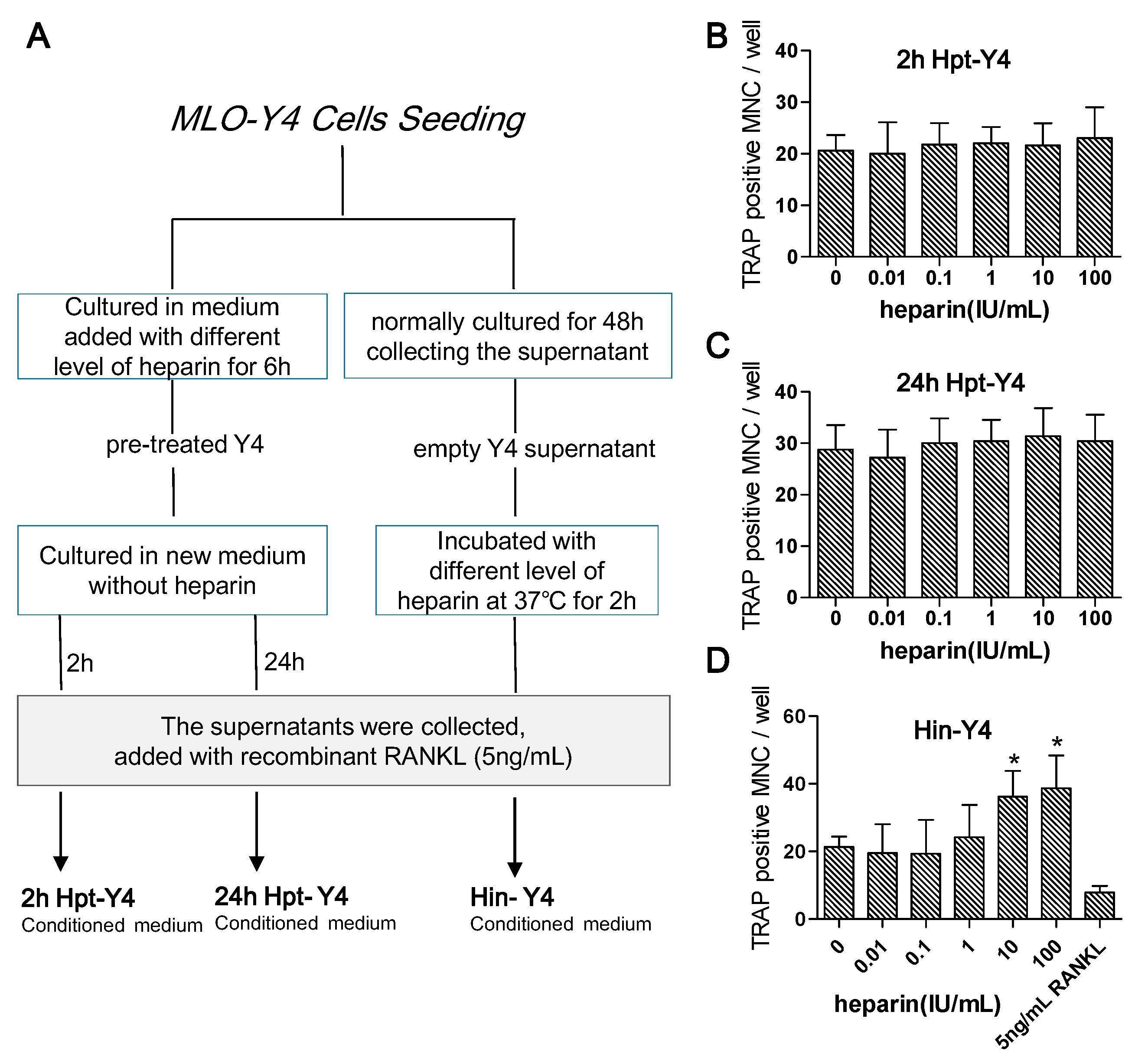
2.4.4. Osteoclast Formation Induced by the Conditioned Media of MLO-Y4 Cells
3. Discussion
4. Materials and Methods
4.1. Materials
4.2. Cell Culture
4.3. Co-Culture of MLO-Y4 Cells with RAW 264.7 Cells
4.4. Tartrate-Resistant Acid Phosphate (TRAP) Stain Assay
4.5. Pit Formation Assay
4.6. Real-Time Quantitative Polymerase Chain Reaction
4.7. ELISA for RANKL and M-CSF
4.8. Western Blot
4.9. Verification Tests
4.9.1. The “Saturation Phenomenon” Test
4.9.2. The Effects of Heparin on the Monoculture
4.9.3. The Effects of Exogenous OPG on Heparin Activity
4.9.4. Osteoclast Formation Supported by Conditioned Media of MLO-Y4 Cells
- (1)
- MLO-Y4 cells were pre-treated with different levels of heparin for six hours and then cultured in fresh medium. After two hours (to test the early response) or 24 h (to test the late response), the medium was collected and supplemented with 5 ng/mL RANKL (the optimal minimum concentration to induce osteoclast formation) and reserved as heparin pre-treated MLO-Y4 conditioned medium (2h Hpt-Y4 and 24h Hpt-Y4; Figure 6A);
- (2)
- The MLO-Y4 cells were normally cultured for 48 h, and the supernatant was collected. Through incubating this supernatant with various concentrations of heparin and adding with 5 ng/mL RANKL, heparin-incubated MLO-Y4 conditioned media (Hin-Y4) were obtained (Figure 6A). The RAW264.7 cells were cultured in Hin-Y4 conditioned medium for eight days, and osteoclast formation was assessed. Osteoclasts induced by empty MLO-Y4 supernatant with only the addition of 5 ng/mL RANKL served as the control;
- (3)
- To determine the role of OPG in heparin’s effect on osteoclast formation, we set up three major groups, and the conditioned medium was prepared as follows (Figure 7A):
- •
- NONE groups: adding recombinant RANKL to empty Y4 supernatant (final concentration is 5 ng/mL) and then incubating with or without 100 IU/mL heparin;
- •
- Anti-OPG groups: adding recombinant RANKL to empty Y4 supernatant (final concentration was 5 ng/mL), then incubating with OPG neutralizing antibody (final concentration was 5 µg/mL), and finally incubating with or without 100 IU/mL heparin;
- •
- Exogenous OPG groups: replenishing exogenous OPG (final concentration was 20 ng/mL) in the conditioned medium of the anti-OPG groups.
4.10. Statistical Analysis
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Jones, C.J.; Beni, S.; Limtiaco, J.F.; Langeslay, D.J.; Larive, C.K. Heparin characterization: Challenges and solutions. Annu. Rev. Anal. Chem. 2011, 4, 439–465. [Google Scholar] [CrossRef] [PubMed]
- Douketis, J.D.; Ginsberg, J.S.; Burrows, R.F.; Duku, E.K.; Webber, C.E.; Brill-Edwards, P. The effects of long-term heparin therapy during pregnancy on bone density. A prospective matched cohort study. Thromb. Haemost. 1996, 75, 254–257. [Google Scholar] [PubMed]
- Meng, Y.; Zhang, H.; Li, Y.; Li, Q.; Zuo, L. Effects of unfractionated heparin on renal osteodystrophy and vascular calcification in chronic kidney disease rats. Bone 2014, 58, 168–176. [Google Scholar] [CrossRef] [PubMed]
- Suda, T.; Takahashi, N.; Udagawa, N.; Jimi, E.; Gillespie, M.T.; Martin, T.J. Modulation of osteoclast differentiation and function by the new members of the tumor necrosis factor receptor and ligand families. Endocr. Rev. 1999, 20, 345–357. [Google Scholar] [CrossRef] [PubMed]
- Lacey, D.L.; Timms, E.; Tan, H.L.; Kelley, M.J.; Dunstan, C.R.; Burgess, T.; Elliott, R.; Colombero, A.; Elliott, G.; Scully, S.; et al. Osteoprotegerin ligand is a cytokine that regulates osteoclast differentiation and activation. Cell 1998, 93, 165–176. [Google Scholar] [CrossRef]
- Udagawa, N.; Takahashi, N.; Jimi, E.; Matsuzaki, K.; Tsurukai, T.; Itoh, K.; Nakagawa, N.; Yasuda, H.; Goto, M.; Tsuda, E.; et al. Osteoblasts/stromal cells stimulate osteoclast activation through expression of osteoclast differentiation factor/RANKL but not macrophage colony-stimulating factor: Receptor activator of NF-κB ligand. Bone 1999, 25, 517–523. [Google Scholar] [CrossRef]
- O’Brien, C.A.; Nakashima, T.; Takayanagi, H. Osteocyte control of osteoclastogenesis. Bone 2013, 54, 258–263. [Google Scholar] [CrossRef] [PubMed]
- Kostenuik, P.J.; Shalhoub, V. Osteoprotegerin: A physiological and pharmacological inhibitor of bone resorption. Curr. Pharm. Des. 2001, 7, 613–635. [Google Scholar] [CrossRef] [PubMed]
- Shandala, T.; Shen, N.Y.; Hopwood, B.; Yip, Y.C.; Foster, B.K.; Xian, C.J. The role of osteocyte apoptosis in cancer chemotherapy-induced bone loss. J. Cell. Physiol. 2012, 227, 2889–2897. [Google Scholar] [CrossRef] [PubMed]
- Cheng, F.; Hulley, P. The osteocyte—A novel endocrine regulator of body phosphate homeostasis. Maturitas 2010, 67, 327–338. [Google Scholar] [CrossRef] [PubMed]
- Bonewald, L.F. The amazing osteocyte. J. Bone Miner. Res. 2011, 26, 229–238. [Google Scholar] [CrossRef] [PubMed]
- Kramer, I.; Halleux, C.; Keller, H.; Pegurri, M.; Gooi, J.H.; Weber, P.B.; Feng, J.Q.; Bonewald, L.F.; Kneissel, M. Osteocyte Wnt/β-catenin signaling is required for normal bone homeostasis. Mol. Cell. Biol. 2010, 30, 3071–3085. [Google Scholar] [CrossRef] [PubMed]
- Sabbagh, Y.; Graciolli, F.G.; O’Brien, S.; Tang, W.; dos Reis, L.M.; Ryan, S.; Phillips, L.; Boulanger, J.; Song, W.; Bracken, C.; et al. Repression of osteocyte Wnt/β-catenin signaling is an early event in the progression of renal osteodystrophy. J. Bone Miner. Res. 2012, 27, 1757–1772. [Google Scholar] [CrossRef] [PubMed]
- Osorio, J. Bone. Osteocyte-specific activation of the canonical Wnt-β catenin pathway stimulates bone formation. Nat. Rev. Endocrinol. 2015, 11, 192. [Google Scholar] [CrossRef] [PubMed]
- Xiong, J.; Onal, M.; Jilka, R.L.; Weinstein, R.S.; Manolagas, S.C.; O’Brien, C.A. Matrix-embedded cells control osteoclast formation. Nat. Med. 2011, 17, 1235–1241. [Google Scholar] [CrossRef] [PubMed]
- Xiong, J.; Piemontese, M.; Onal, M.; Campbell, J.; Goellner, J.J.; Dusevich, V.; Bonewald, L.; Manolagas, S.C.; O’Brien, C.A. Osteocytes, not osteoblasts or lining cells, are the main source of the RANKL required for osteoclast formation in remodeling bone. PLoS ONE 2015, 10, e0138189. [Google Scholar] [CrossRef] [PubMed]
- Irie, A.; Takami, M.; Kubo, H.; Sekino-Suzuki, N.; Kasahara, K.; Sanai, Y. Heparin enhances osteoclastic bone resorption by inhibiting osteoprotegerin activity. Bone 2007, 41, 165–174. [Google Scholar] [CrossRef] [PubMed]
- Theoleyre, S.; Kwan, T.S.; Vusio, P.; Blanchard, F.; Gallagher, J.; Ricard-Blum, S.; Fortun, Y.; Padrines, M.; Redini, F.; Heymann, D. Characterization of osteoprotegerin binding to glycosaminoglycans by surface plasmon resonance: Role in the interactions with receptor activator of nuclear factor κB ligand (RANKL) and RANK. Biochem. Biophys. Res. Commun. 2006, 347, 460–467. [Google Scholar] [CrossRef] [PubMed]
- Kato, Y.; Windle, J.J.; Koop, B.A.; Mundy, G.R.; Bonewald, L.F. Establishment of an osteocyte-like cell line, MLO-Y4. J. Bone Miner. Res. 1997, 12, 2014–2023. [Google Scholar] [CrossRef] [PubMed]
- Zhao, S.; Zhang, Y.K.; Harris, S.; Ahuja, S.S.; Bonewald, L.F. MLO-Y4 osteocyte-like cells support osteoclast formation and activation. J. Bone Miner. Res. 2002, 17, 2068–2079. [Google Scholar] [CrossRef] [PubMed]
- Ren, Z.Y.; Machuca-Gayet, I.; Domenget, C.; Buchet, R.; Wu, Y.; Jurdic, P.; Mebarek, S. Azanitrile cathepsin K Inhibitors: Effects on cell toxicity, osteoblast-induced mineralization and osteoclast-mediated bone resorption. PLoS ONE 2015, 10, e0132513. [Google Scholar] [CrossRef] [PubMed]
- Conners, C.M.; Bhethanabotla, V.R.; Gupta, V.K. Concentration-dependent effects of alendronate and pamidronate functionalized gold nanoparticles on osteoclast and osteoblast viability. J. Biomed. Mater. Res. B Appl. Biomater. 2015. [Google Scholar] [CrossRef] [PubMed]
- Wei, Z.F.; Lv, Q.; Xia, Y.; Yue, M.F.; Shi, C.; Xia, Y.F.; Chou, G.X.; Wang, Z.T.; Dai, Y. Norisoboldine, an Anti-arthritis alkaloid isolated from radix linderae, attenuates osteoclast differentiation and inflammatory bone erosion in an aryl hydrocarbon receptor-dependent manner. Int. J. Biol. Sci. 2015, 11, 1113–1126. [Google Scholar] [CrossRef] [PubMed]
- Feng, R.; Anderson, G.; Xiao, G.; Elliott, G.; Leoni, L.; Mapara, M.Y.; Roodman, G.D.; Lentzsch, S. SDX-308, a nonsteroidal anti-inflammatory agent, inhibits NF-κB activity, resulting in strong inhibition of osteoclast formation/activity and multiple myeloma cell growth. Blood 2007, 109, 2130–2138. [Google Scholar] [CrossRef] [PubMed]
- Hong, S.; Huh, J.E.; Lee, S.Y.; Shim, J.K.; Rhee, S.G.; Jeong, W. TRP14 inhibits osteoclast differentiation via its catalytic activity. Mol. Cell. Biol. 2014, 34, 3515–3524. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.; Yamazaki, M.; Zella, L.A.; Shevde, N.K.; Pike, J.W. Activation of receptor activator of NF-κB ligand gene expression by 1,25-dihydroxyvitamin D3 is mediated through multiple long-range enhancers. Mol. Cell. Biol. 2006, 26, 6469–6486. [Google Scholar] [CrossRef] [PubMed]
- Griffin, A.C.; Kern, M.J.; Kirkwood, K.L. MKP-1 is essential for canonical vitamin D-induced signaling through nuclear import and regulates RANKL expression and function. Mol. Endocrinol. 2012, 26, 1682–1693. [Google Scholar] [CrossRef] [PubMed]
- Teitelbaum, S.L. Bone resorption by osteoclasts. Science 2000, 289, 1504–1508. [Google Scholar] [CrossRef] [PubMed]
- Zulueta, M.M.; Lin, S.Y.; Hu, Y.P.; Hung, S.C. Synthetic heparin and heparan sulfate oligosaccharides and their protein interactions. Curr. Opin. Chem. Biol. 2013, 17, 1023–1029. [Google Scholar] [CrossRef] [PubMed]
- Vik, A.; Brodin, E.; Sveinbjornsson, B.; Hansen, J.B. Heparin induces mobilization of osteoprotegerin into the circulation. Thromb. Haemost. 2007, 98, 148–154. [Google Scholar] [CrossRef] [PubMed]
- Klejna, K.; Naumnik, B.; Koc-Żórawska, E.; Myśliwiec, M. Effect of unfractionated and low-molecular-weight heparin on OPG, sRANKL, and von Willebrand factor concentrations during hemodialysis. Clin. Appl. Thromb. Hemost. 2014, 20, 433–441. [Google Scholar] [CrossRef] [PubMed]
- Cianciolo, G.; Manna, G.L.; Donati, G.; Dormi, A.; Cappuccilli, M.L.; Cuna, V.; Legnani, C.; Palareti, G.; Colì, L.; Stefoni, S. Effects of unfractioned heparin and low-molecular-weight heparin on osteoprotegerin and RANKL plasma levels in haemodialysis patients. Nephrol. Dial. Transplant. 2011, 26, 646–652. [Google Scholar] [CrossRef] [PubMed]
- Al-Dujaili, S.A.; Lau, E.; Al-Dujaili, H.; Tsang, K.; Guenther, A.; You, L. Apoptotic osteocytes regulate osteoclast precursor recruitment and differentiation in vitro. J. Cell. Biochem. 2011, 112, 2412–2423. [Google Scholar] [CrossRef] [PubMed]
- Kennedy, O.D.; Laudier, D.M.; Majeska, R.J.; Sun, H.B.; Schaffler, M.B. Osteocyte apoptosis is required for production of osteoclastogenic signals following bone fatigue in vivo. Bone 2014, 64, 132–137. [Google Scholar] [CrossRef] [PubMed]
- Maurel, D.B.; Pallu, S.; Jaffre, C.; Fazzalari, N.L.; Boisseau, N.; Uzbekov, R.; Benhamou, C.L.; Rochefort, G.Y. Osteocyte apoptosis and lipid infiltration as mechanisms of alcohol-induced bone loss. Alcohol Alcohol. 2012, 47, 413–422. [Google Scholar] [CrossRef] [PubMed]
- Cardoso, L.; Herman, B.C.; Verborgt, O.; Laudier, D.; Majeska, R.J.; Schaffler, M.B. Osteocyte apoptosis controls activation of intracortical resorption in response to bone fatigue. J. Bone Miner. Res. 2009, 24, 597–605. [Google Scholar] [CrossRef] [PubMed]
- Tatsumi, S.; Ishii, K.; Amizuka, N.; Li, M.; Kobayashi, T.; Kohno, K.; Ito, M.; Takeshita, S.; Ikeda, K. Targeted ablation of osteocytes induces osteoporosis with defective mechanotransduction. Cell Metab. 2007, 5, 464–475. [Google Scholar] [CrossRef] [PubMed]


© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, B.; Lu, D.; Chen, Y.; Zhao, M.; Zuo, L. Unfractionated Heparin Promotes Osteoclast Formation in Vitro by Inhibiting Osteoprotegerin Activity. Int. J. Mol. Sci. 2016, 17, 613. https://doi.org/10.3390/ijms17040613
Li B, Lu D, Chen Y, Zhao M, Zuo L. Unfractionated Heparin Promotes Osteoclast Formation in Vitro by Inhibiting Osteoprotegerin Activity. International Journal of Molecular Sciences. 2016; 17(4):613. https://doi.org/10.3390/ijms17040613
Chicago/Turabian StyleLi, Binghan, Dan Lu, Yuqing Chen, Minghui Zhao, and Li Zuo. 2016. "Unfractionated Heparin Promotes Osteoclast Formation in Vitro by Inhibiting Osteoprotegerin Activity" International Journal of Molecular Sciences 17, no. 4: 613. https://doi.org/10.3390/ijms17040613
APA StyleLi, B., Lu, D., Chen, Y., Zhao, M., & Zuo, L. (2016). Unfractionated Heparin Promotes Osteoclast Formation in Vitro by Inhibiting Osteoprotegerin Activity. International Journal of Molecular Sciences, 17(4), 613. https://doi.org/10.3390/ijms17040613





