Liver Fibrosis in HCV Monoinfected and HIV/HCV Coinfected Patients: Dysregulation of Matrix Metalloproteinases (MMPs) and Their Tissue Inhibitors TIMPs and Effect of HCV Protease Inhibitors
Abstract
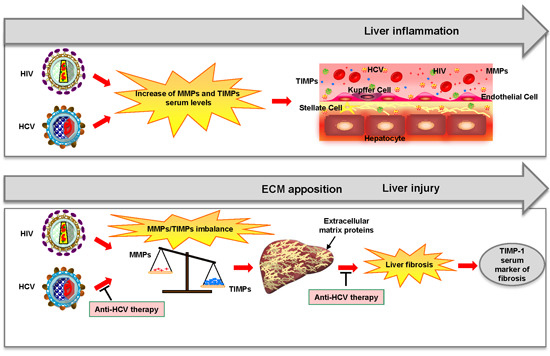
:1. Introduction
2. Results
2.1. Detection of Different Matrix Metalloproteinase (MMP) and Tissue Inhibitors of Metalloproteinase (TIMP) Levels in Plasma of Hepatitis C Virus (HCV) Monoinfected, HIV/HCV Coinfected Subjects, and Healthy Donors
2.2. Plasma Levels of MMP-2, MMP-9, TIMP-1, and TIMP-2 in the Different Groups Analyzed
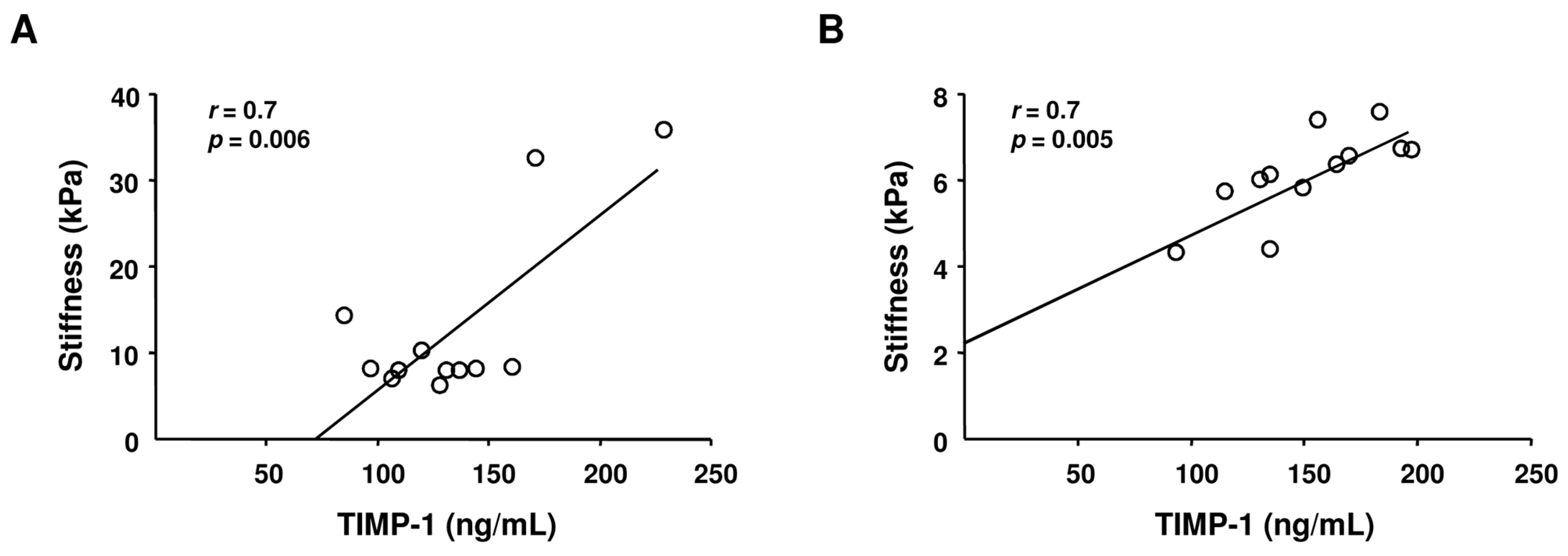
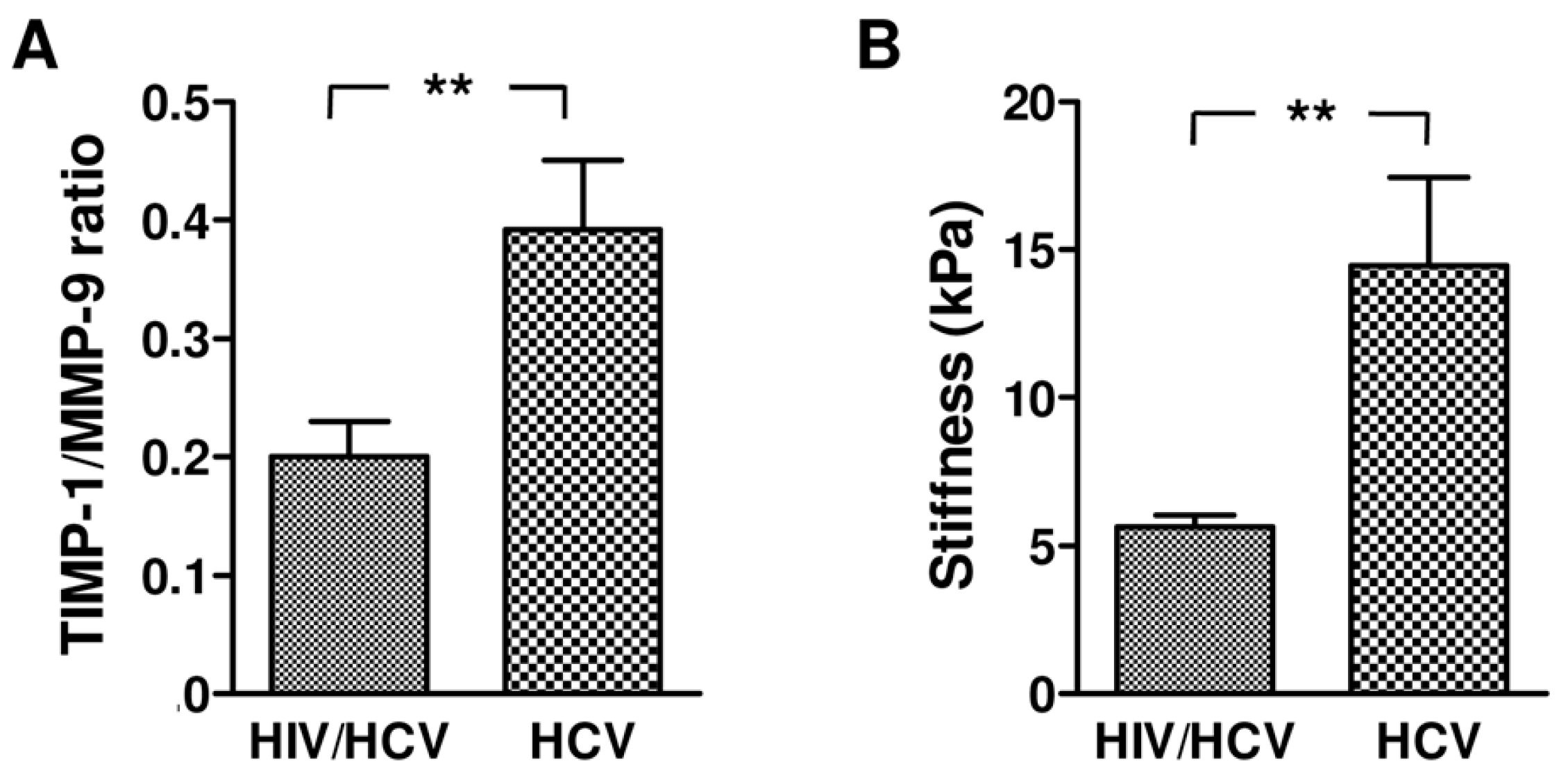
2.3. Correlation between Liver Stiffness and TIMP-1 Levels in HCV Monoinfected and HIV/HCV Coinfected Subjects
2.4. TIMP-1/MMP-9 Ratio between HCV Monoinfected and HIV/HCV Coinfected Subjects
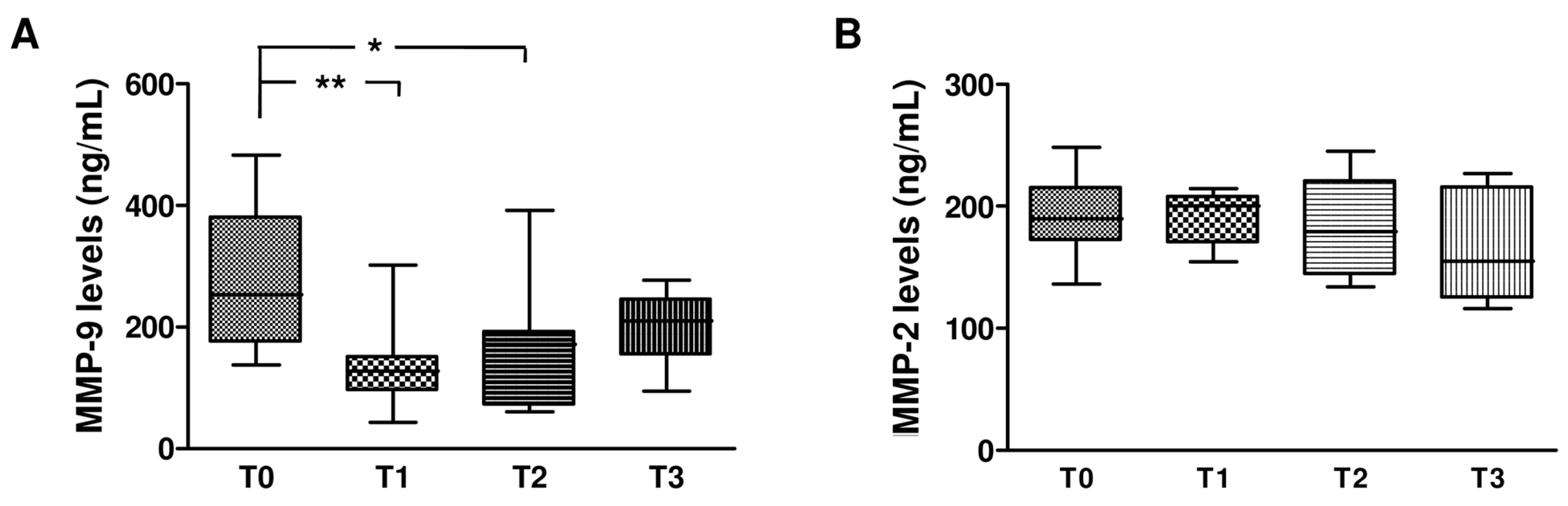
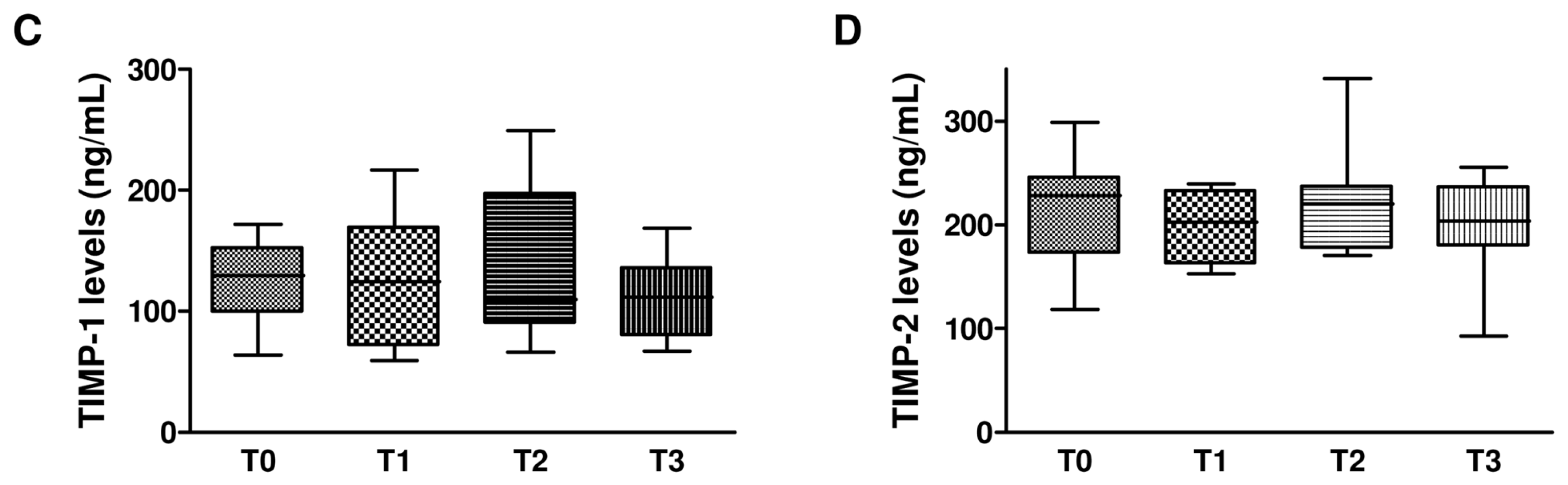
2.5. Effect of Dual and Triple Anti-HCV Therapy on MMPs and TIMPs in HCV Monoinfected Subjects
3. Discussion
4. Materials and Methods
4.1. Study Design
4.2. Detection of MMP and TIMP Levels in Plasma Samples
4.3. Statistical Analysis
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Guidotti, L.G.; Chisari, F.V. Immunobiology and pathogenesis of viral hepatitis. Annu. Rev. Pathol. 2006, 1, 23–61. [Google Scholar] [CrossRef] [PubMed]
- Iredale, J.P.; Thompson, A.; Henderson, N.C. Extracellular matrix degradation in liver fibrosis: Biochemistry and regulation. Biochim. Biophys. Acta 2013, 1832, 876–883. [Google Scholar] [CrossRef] [PubMed]
- Visse, R.; Nagase, H. Matrix metalloproteinases and tissue inhibitors of metalloproteinases: Structure, function, and biochemistry. Circ. Res. 2003, 92, 827–839. [Google Scholar] [CrossRef] [PubMed]
- Vandooren, J.; van den Steen, P.E.; Opdenakker, G. Biochemistry and molecular biology of gelatinase B or matrix metalloproteinase-9 (MMP-9): The next decade. Crit. Rev. Biochem. Mol. Biol. 2013, 48, 222–272. [Google Scholar] [CrossRef] [PubMed]
- Arpino, V.; Brock, M.; Gill, S.E. The role of TIMPs in regulation of extracellular matrix proteolysis. Matrix Biol. 2015, 44–46, 247–254. [Google Scholar] [CrossRef] [PubMed]
- Lichtinghagen, R.; Huegel, O.; Seifert, T.; Haberkorn, C.I.; Michels, D.; Flemming, P.; Bahr, M.; Boeker, K.H. Expression of matrix metalloproteinase-2 and -9 and their inhibitors in peripheral blood cells of patients with chronic hepatitis C. Clin. Chem. 2000, 46, 183–192. [Google Scholar] [PubMed]
- Bruno, C.M.; Valenti, M.; Bertino, G.; Ardiri, A.; Consolo, M.; Mazzarino, C.M.; Amoroso, A.; Neri, S. Altered pattern of circulating matrix metalloproteinases-2,- 9 and tissue inhibitor of metalloproteinase-2 in patients with HCV-related chronic hepatitis. Relationship to histological features. Panminerva Med. 2009, 51, 191–196. [Google Scholar] [PubMed]
- Lichtinghagen, R.; Bahr, M.J.; Wehmeier, M.; Michels, D.; Haberkorn, C.I.; Arndt, B.; Flemming, P.; Manns, M.P.; Boeker, K.H. Expression and coordinated regulation of matrix metalloproteinases in chronic hepatitis C and hepatitis C virus-induced liver cirrhosis. Clin. Sci. 2003, 105, 373–382. [Google Scholar] [CrossRef] [PubMed]
- Mastroianni, C.M.; Lichtner, M.; Mascia, C.; Zuccalà, P.; Vullo, V. Molecular mechanisms of liver fibrosis in HIV/HCV coinfection. Int. J. Mol. Sci. 2014, 15, 9184–9208. [Google Scholar] [CrossRef] [PubMed]
- Sulkowski, M.S.; Thomas, D.L. Hepatitis C in the HIV-infected person. Ann. Intern. Med. 2003, 138, 197–207. [Google Scholar] [CrossRef] [PubMed]
- Kim, A.Y.; Chung, R.T. Coinfection with HIV-1 and HCV—A one-two punch. Gastroenterology 2009, 137, 781–795. [Google Scholar] [CrossRef] [PubMed]
- Hajarizadeh, B.; Grebely, J.; Dore, G.J. Epidemiology and natural history of HCV infection. Nat. Rev. Gastroenterol. Hepatol. 2013, 10, 553–562. [Google Scholar] [CrossRef] [PubMed]
- Brau, N.; Salvatore, M.; Rios-Bedoya, C.F.; Fernandez-Carbia, A.; Paronetto, F.; Rodriguez-Orengo, J.F.; Rodríguez-Torres, M. Slower fibrosis progression in HIV/HCVcoinfected patients with successful HIV suppression using antiretroviral therapy. J. Hepatol. 2006, 44, 47–55. [Google Scholar] [CrossRef] [PubMed]
- Mastroianni, C.M.; Liuzzi, G.M.; D’Ettorre, G.; Lichtner, M.; Forcina, G.; di Campli, N.F.; Riccio, P.; Vullo, V. Matrix metalloproteinase-9 and tissue inhibitors of matrix metalloproteinase-1 in plasma of patients coinfected with HCV and HIV. HIV Clin. Trials 2002, 3, 310–315. [Google Scholar] [PubMed]
- Mastroianni, C.M.; Liuzzi, G.M. Matrix metalloproteinase dysregulation in HIV infection: Implications for therapeutic strategies. Trends Mol. Med. 2007, 13, 449–459. [Google Scholar] [CrossRef] [PubMed]
- Fried, M.W.; Peter, J.; Hoots, K.; Gaglio, P.J.; Talbut, D.; Davis, P.C.; Key, N.S.; White, G.C.; Lindblad, L.; Rickles, F.R.; et al. Hepatitis C in adults and adolescents with hemophilia: A randomized, controlled trial of interferon alfa-2b and ribavirin. Hepatology 2002, 36, 967–972. [Google Scholar] [CrossRef] [PubMed]
- Bacon, B.R.; Gordon, S.C.; Lawitz, E.; Marcellin, P.; Vierling, J.M.; Zeuzem, S.; Poordad, F.; Goodman, Z.D.; Sings, H.L.; Boparai, N.; et al. Boceprevir for previously treated chronic HCV genotype 1 infection. N. Engl. J. Med. 2011, 364, 1207–1217. [Google Scholar] [CrossRef] [PubMed]
- Leroy, V.; Monier, F.; Bottari, S.; Trocme, C.; Sturm, N.; Hilleret, M.N.; Morel, F.; Zarski, J.P. Circulating matrix metalloproteinases 1, 2, 9 and their inhibitors TIMP-1 and TIMP-2 as serum markers of liver fibrosis in patients with chronic hepatitis C: Comparison with PIIINP and hyaluronic acid. Am. J. Gastroenterol. 2004, 99, 271–279. [Google Scholar] [CrossRef] [PubMed]
- Reif, S.; Somech, R.; Brazovski, E.; Reich, R.; Belson, A.; Konikoff, F.M.; Kessler, A. Matrix metalloproteinases 2 and 9 are markers of inflammation but not of the degree of fibrosis in chronic hepatitis C. Digestion 2005, 71, 124–130. [Google Scholar] [CrossRef] [PubMed]
- Capone, F.; Guerriero, E.; Sorice, A.; Maio, P.; Colonna, G.; Castello, G.; Costantini, S. Characterization of metalloproteinases, oxidative status and inflammation levels in the different stages of fibrosis in HCV patients. Clin. Biochem. 2002, 45, 525–529. [Google Scholar] [CrossRef] [PubMed]
- Hemmann, S.; Graf, J.; Roderfeld, M.; Roeb, E. Expression of MMPs and TIMPs in liver fibrosis—A systematic review with special emphasis on anti-fibrotic strategies. J. Hepatol. 2007, 46, 955–975. [Google Scholar] [CrossRef] [PubMed]
- Duarte, S.; John Baber, J.; Fujii, T.; Coito, A.J. Matrix metalloproteinases in liver injury, repair and fibrosis. Matrix Biol. 2015, 44–46, 147–156. [Google Scholar] [CrossRef] [PubMed]
- Liuzzi, G.M.; Mastroianni, C.M.; Latronico, T.; Mengoni, F.; Fasano, A.; Lichtner, M.; Vullo, V.; Riccio, P. Anti-HIV drugs decrease the expression of matrix metalloproteinases in astrocytes and microglia. Brain 2004, 127, 398–407. [Google Scholar] [CrossRef] [PubMed]
- Latronico, T.; Liuzzi, G.M.; Riccio, P.; Lichtner, M.; Mengoni, F.; D’Agostino, C.; Vullo, V.; Mastroianni, C.M. Antiretroviral therapy inhibits matrix metalloproteinase-9 from blood mononuclear cells of HIV-infected patients. AIDS 2007, 21, 677–684. [Google Scholar] [CrossRef] [PubMed]
- Liu, T.; Wang, X.; Karsdal, M.A.; Leeming, D.J.; Genovese, F. Molecular serum markers of liver fibrosis. Biomark. Insights 2012, 7, 105–117. [Google Scholar] [PubMed]
- Larrousse, M.; Laguno, M.; Segarra, M.; de Lazzari, E.; Martinez, E.; Blanco, J.L.; León, A.; Deulofeu, R.; Miquel, R.; Milinkovic, A.; et al. Noninvasive diagnosis of hepatic fibrosis in HIV/HCV-coinfected patients. J. Acquir. Immune Defic. Syndr. 2007, 46, 304–311. [Google Scholar] [CrossRef] [PubMed]
- Lin, W.; Weinberg, E.M.; Chung, R.T. Pathogenesis of accelerated fibrosis in HIV/HCV co-infection. J. Infect. Dis. 2013, 207, S13–S18. [Google Scholar] [CrossRef] [PubMed]
- Deng, L.; Gui, X.; Zhang, Y.; Gao, S.C.; Yang, R.R. Impact of human immunodeficiency virus infection on the course of hepatitis C virus infection: A meta-analysis. World J. Gastroenterol. 2009, 15, 996–1003. [Google Scholar] [CrossRef] [PubMed]
- Monga, H.K.; Rodriguez-Barradas, M.C.; Breaux, K.; Khattak, K.; Troisi, C.L.; Velez, M.; Yoffe, B. Hepatitis C virus infection-related morbidity and mortality among patients with human immunodeficiency virus infection. Clin. Infect. Dis. 2001, 33, 240–247. [Google Scholar] [CrossRef] [PubMed]
- Martinez-Sierra, C.; Arizcorreta, A.; Díaz, F.; Roldán, R.; Martín-Herrera, L.; Pérez-Guzmán, E.; Girón-González, J.A. Progression of chronic hepatitis C to liver fibrosis and cirrhosis in patients coinfected with hepatitis C virus and human immunodeficiency virus. Clin. Infect. Dis. 2003, 36, 491–498. [Google Scholar] [CrossRef] [PubMed]
- Thein, H.; Yi, Q.; Dore, G.J.; Krahn, M.D. Natural history of hepatitis C virus infection in HIV-infected individuals and the impact of HIV in the era of highly active antiretroviral therapy: A meta-analysis. AIDS 2008, 22, 1979–1991. [Google Scholar] [CrossRef] [PubMed]
- Qurishi, N.; Kreuzberg, C.; Luchters, G.; Effenberger, W.; Kupfer, B.; Sauerbruch, T.; Rockstroh, J.K.; Spengler, U. Effect of antiretroviral therapy on liver-related mortality in patients with HIV and hepatitis C virus coinfection. Lancet 2003, 362, 1708–1713. [Google Scholar] [CrossRef]
- Li, Y.; Xie, J.; Han, Y.; Wang, H.; Lv, W.; Guo, F.; Qiu, Z.; Li, Y.; Du, S.; Song, X.; et al. Combination antiretroviral therapy is associated with reduction in liver fibrosis scores in HIV-1-infected subjects. Medicine 2016, 95, e2660. [Google Scholar] [CrossRef] [PubMed]
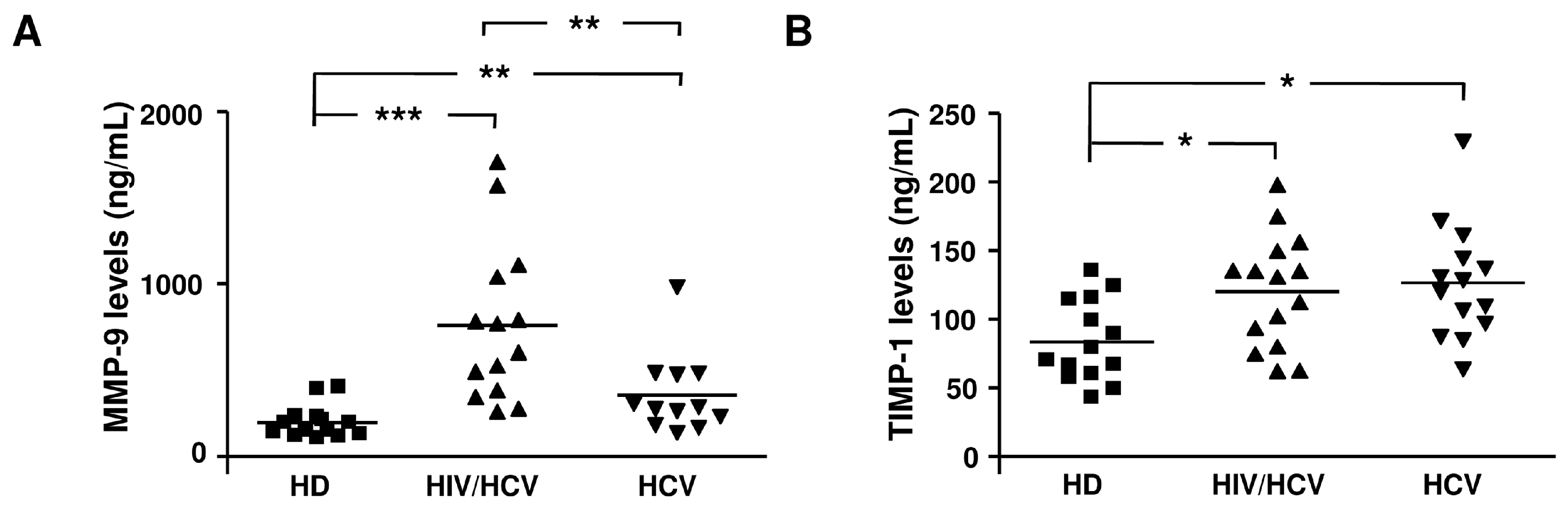
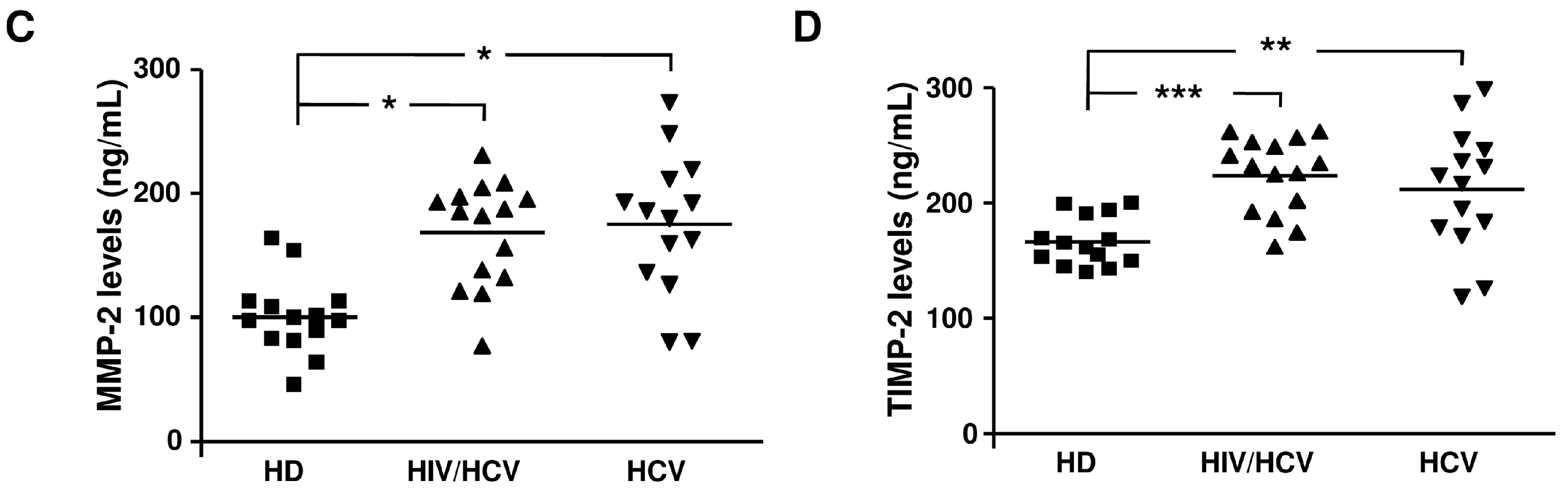

| Enzyme or Inhibitor | HD | HCV/HIV | HCV |
|---|---|---|---|
| MMP-1 (ng/mL) | 3.5 (0.2–11) | 26 (7.6–56) *** | 20.5 (0.8–37) *** |
| MMP-2 (ng/mL) | 96.7 (54–163.4) | 185 (77–231) ** | 183 (80–274) ** |
| MMP-3 (ng/mL) | 1 (1–3.5) | 1 (3.9–1.14) | 1 (1–20) |
| MMP-8 (ng/mL) | 8.3 (1.5–66) | 15.3 (4.6–147) | 18 (1.6–49.4) |
| MMP-9 (ng/mL) | 171 (107–403) | 685 (260–1707) *** | 295 (137–1000) * |
| MMP-10 (ng/mL) | 0.02 (0.2–2.9) | 1.1 (0.02–8) | 1.1 (0.02–4.4) |
| TIMP-1 (ng/mL) | 79 (43–135) | 130.6 (62–198) * | 124 (63.3–230) * |
| TIMP-2 (ng/mL) | 161 (139–200) | 232 (162–262) *** | 220 (119–300) ** |
| Parameters | HD (n = 12) | HCV (n = 16) | HIV/HCV (n = 15) | p Value |
|---|---|---|---|---|
| Age, Years | 46 (28–56) | 46 (24−66) | 53 (47−57) | 0.02 a |
| Male/Female (% male) | 3/9 (25%) | 11/5 (68%) | 7/8 (46%) | 0.07 b |
| Liver Stiffness (kPa) | – | 10 (6.1−35.8) | 6.1 (4.3−29.5) | 0.03 c |
| ALT (UI/L) | – | 98 (40−450) | 58 (24−115) | 0.09 c |
| AST (UI/L) | – | 65.5 (27−141) | 43 (24−79) | 0.24 c |
| GGT (UI/L) | – | 48.5 (17−134) | 44 (20−665) | 0.9 c |
| PLT (109/L) | – | 168 (89−385) | 167 (89−332) | 0.06 c |
| Tot BIL (mg/dL) | – | 0.64 (0.38−1.12) | 0.8 (0.39−4.3) | 0.39 c |
| HCV-RNA (copies × 106/mL) | NA | 2.72 (0.3−11) | 1.13 (0.06−16.5) | 0.090 c |
| HIV-RNA (copies/mL) | NA | NA | <20 | NA |
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons by Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Latronico, T.; Mascia, C.; Pati, I.; Zuccala, P.; Mengoni, F.; Marocco, R.; Tieghi, T.; Belvisi, V.; Lichtner, M.; Vullo, V.; et al. Liver Fibrosis in HCV Monoinfected and HIV/HCV Coinfected Patients: Dysregulation of Matrix Metalloproteinases (MMPs) and Their Tissue Inhibitors TIMPs and Effect of HCV Protease Inhibitors. Int. J. Mol. Sci. 2016, 17, 455. https://doi.org/10.3390/ijms17040455
Latronico T, Mascia C, Pati I, Zuccala P, Mengoni F, Marocco R, Tieghi T, Belvisi V, Lichtner M, Vullo V, et al. Liver Fibrosis in HCV Monoinfected and HIV/HCV Coinfected Patients: Dysregulation of Matrix Metalloproteinases (MMPs) and Their Tissue Inhibitors TIMPs and Effect of HCV Protease Inhibitors. International Journal of Molecular Sciences. 2016; 17(4):455. https://doi.org/10.3390/ijms17040455
Chicago/Turabian StyleLatronico, Tiziana, Claudia Mascia, Ilaria Pati, Paola Zuccala, Fabio Mengoni, Raffaella Marocco, Tiziana Tieghi, Valeria Belvisi, Miriam Lichtner, Vincenzo Vullo, and et al. 2016. "Liver Fibrosis in HCV Monoinfected and HIV/HCV Coinfected Patients: Dysregulation of Matrix Metalloproteinases (MMPs) and Their Tissue Inhibitors TIMPs and Effect of HCV Protease Inhibitors" International Journal of Molecular Sciences 17, no. 4: 455. https://doi.org/10.3390/ijms17040455
APA StyleLatronico, T., Mascia, C., Pati, I., Zuccala, P., Mengoni, F., Marocco, R., Tieghi, T., Belvisi, V., Lichtner, M., Vullo, V., Mastroianni, C. M., & Liuzzi, G. M. (2016). Liver Fibrosis in HCV Monoinfected and HIV/HCV Coinfected Patients: Dysregulation of Matrix Metalloproteinases (MMPs) and Their Tissue Inhibitors TIMPs and Effect of HCV Protease Inhibitors. International Journal of Molecular Sciences, 17(4), 455. https://doi.org/10.3390/ijms17040455