Changes in Immunogenicity during the Development of Urinary Bladder Cancer: A Preliminary Study
Abstract
:1. Introduction
2. Results
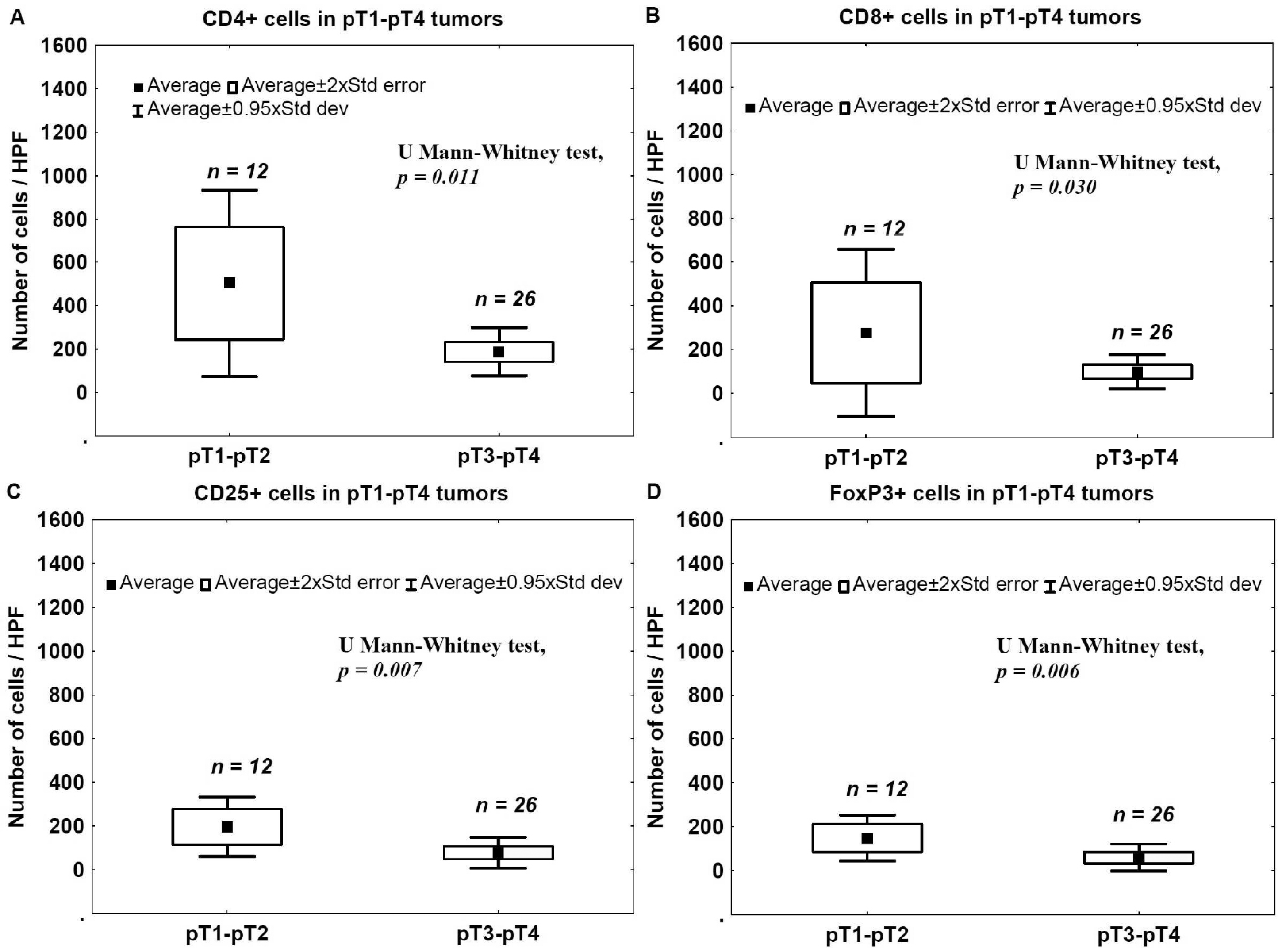
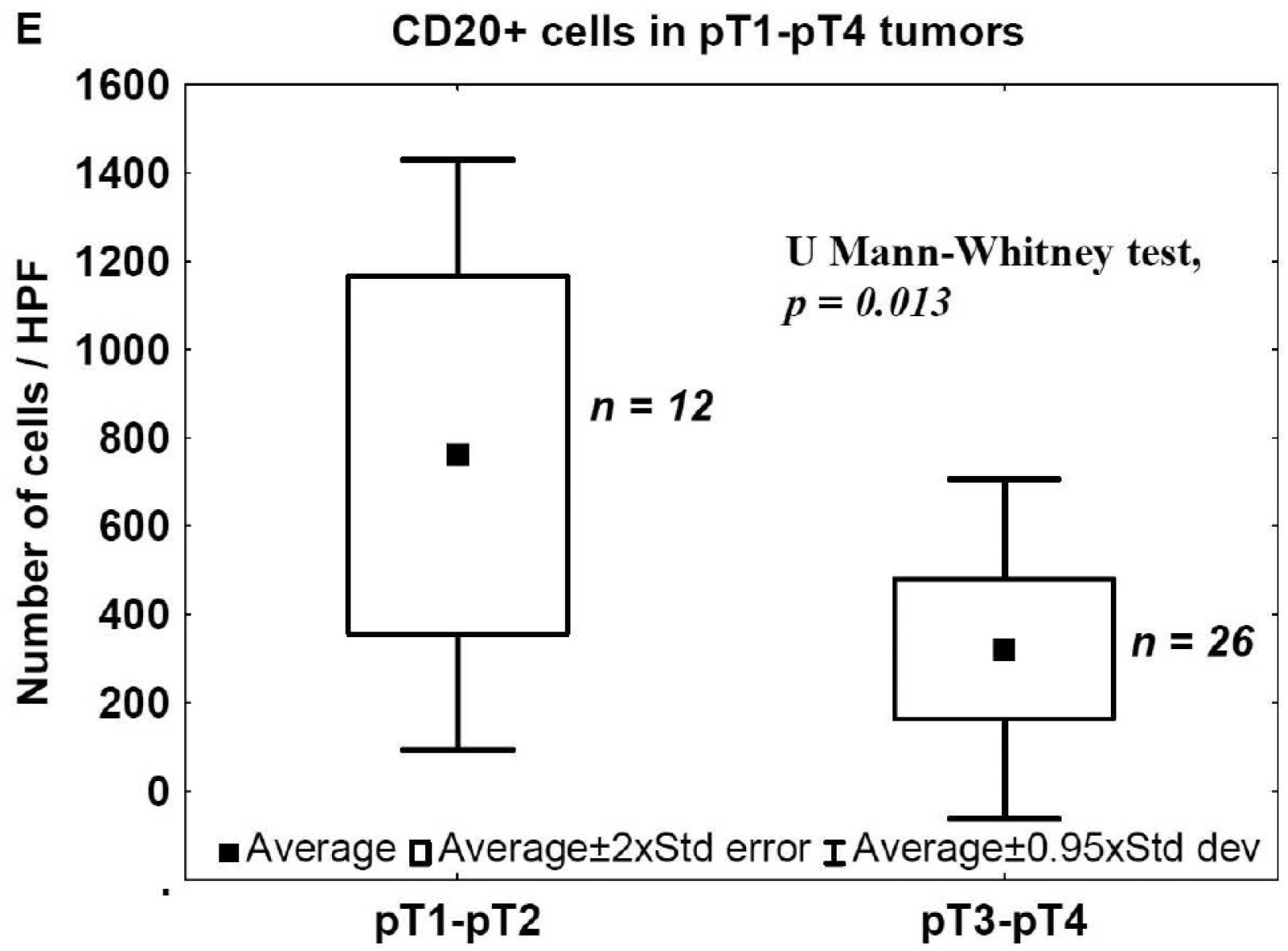
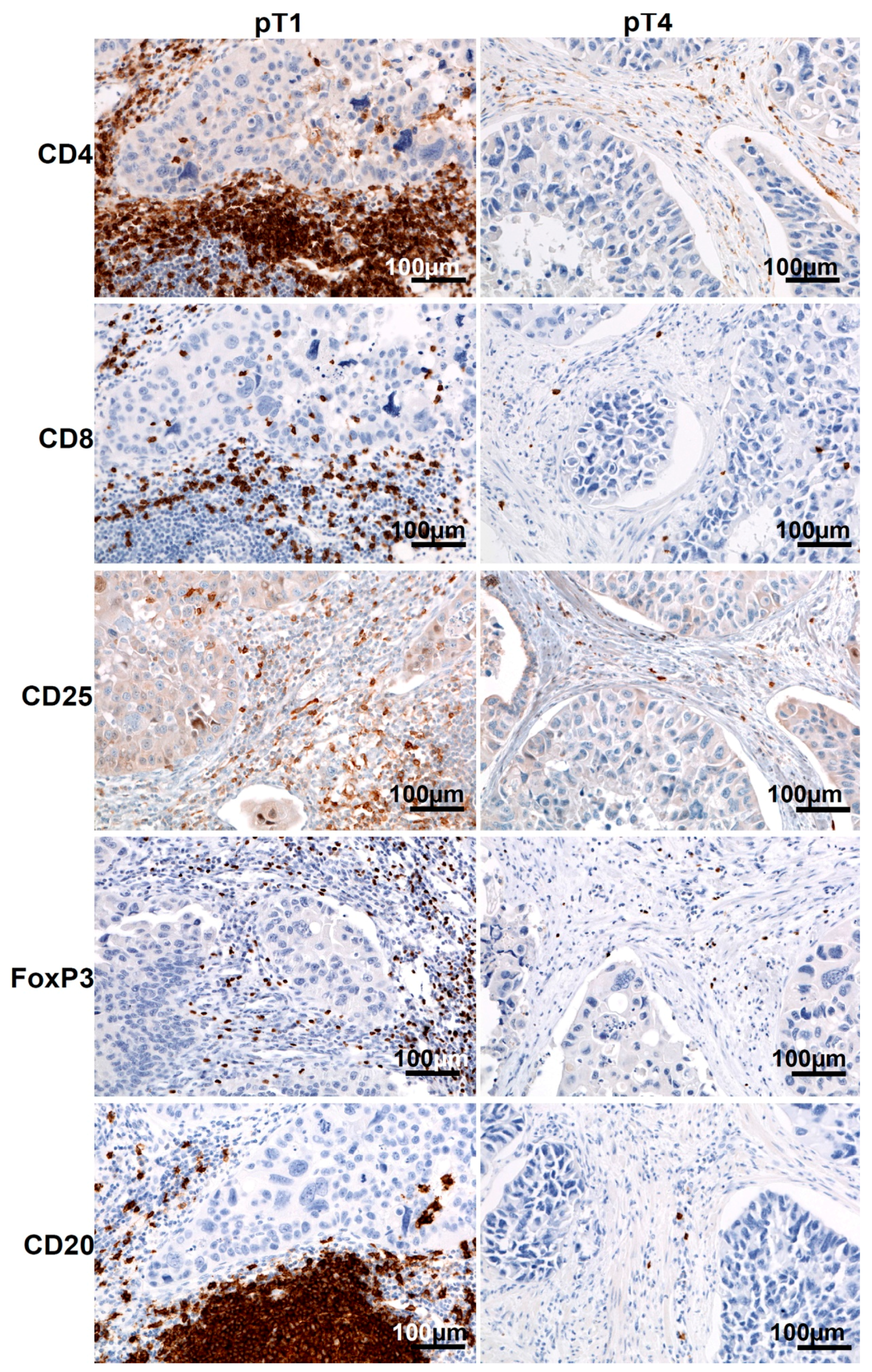
2.1. Staging and Tumor-Infiltrating Lymphocytes (TILs) in Urinary Bladder Cancer
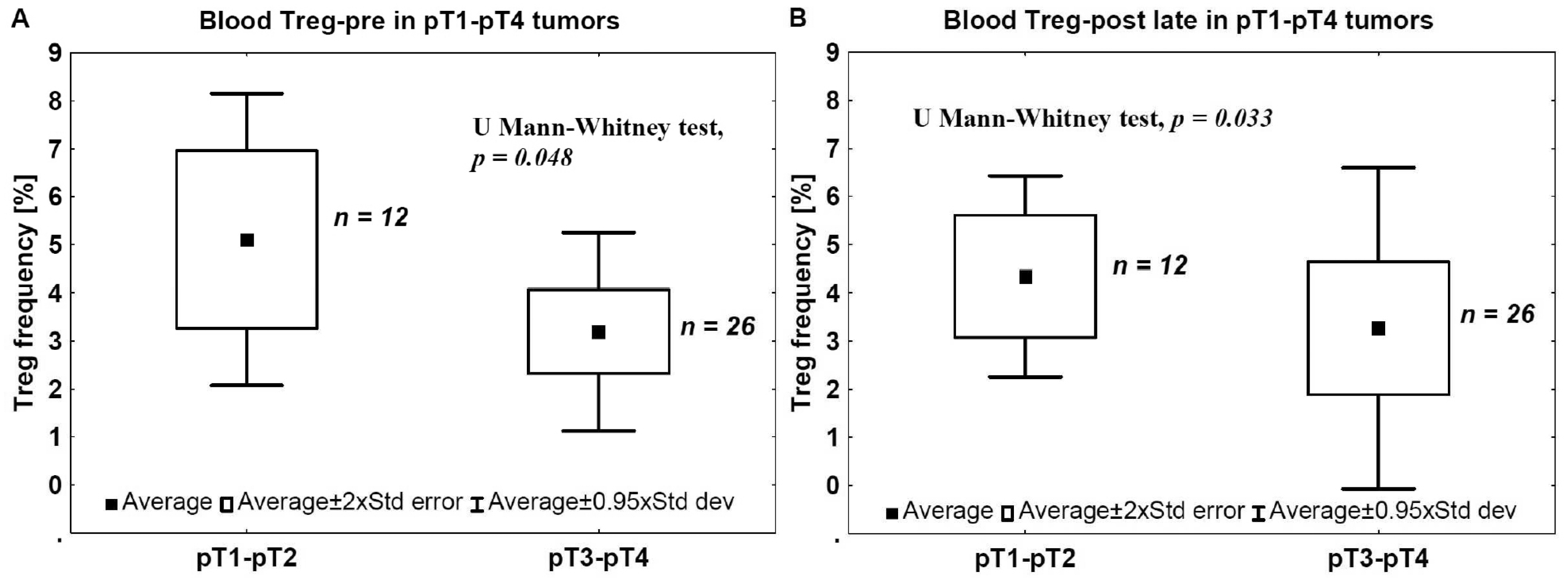
2.2. Staging and Blood Tregs
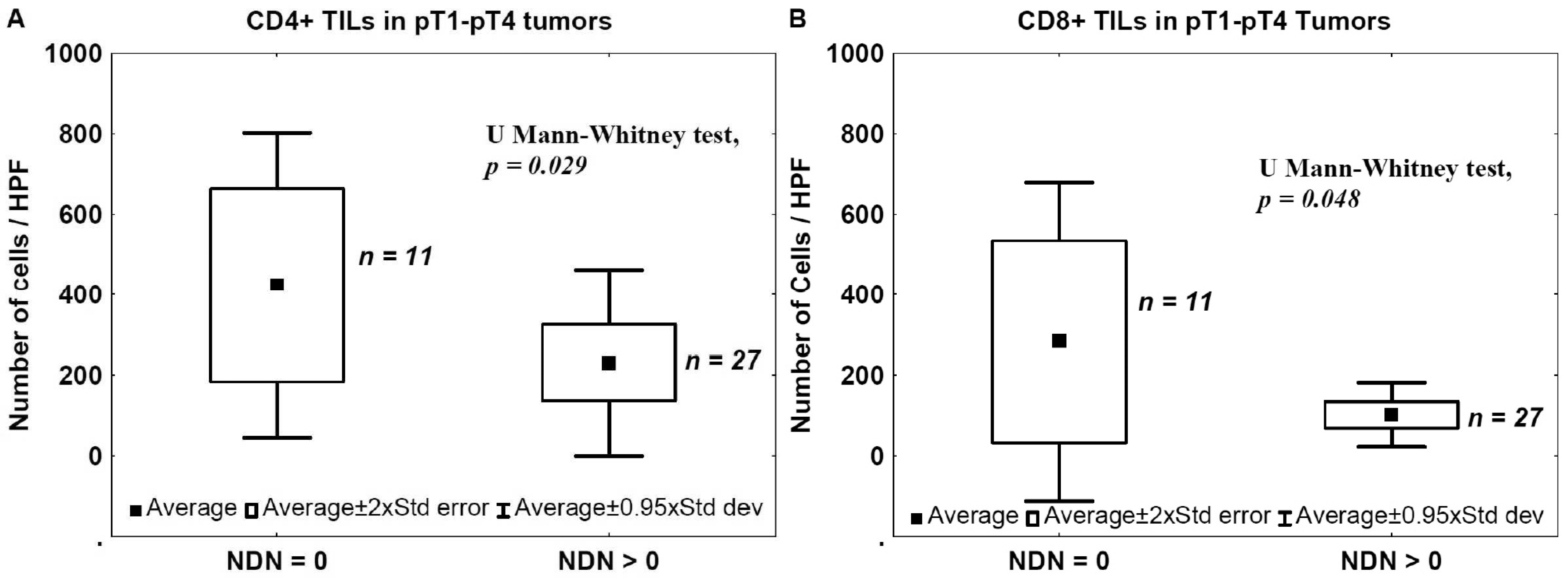
2.3. Nonclassic Differentiation Number (NDN) and TILs
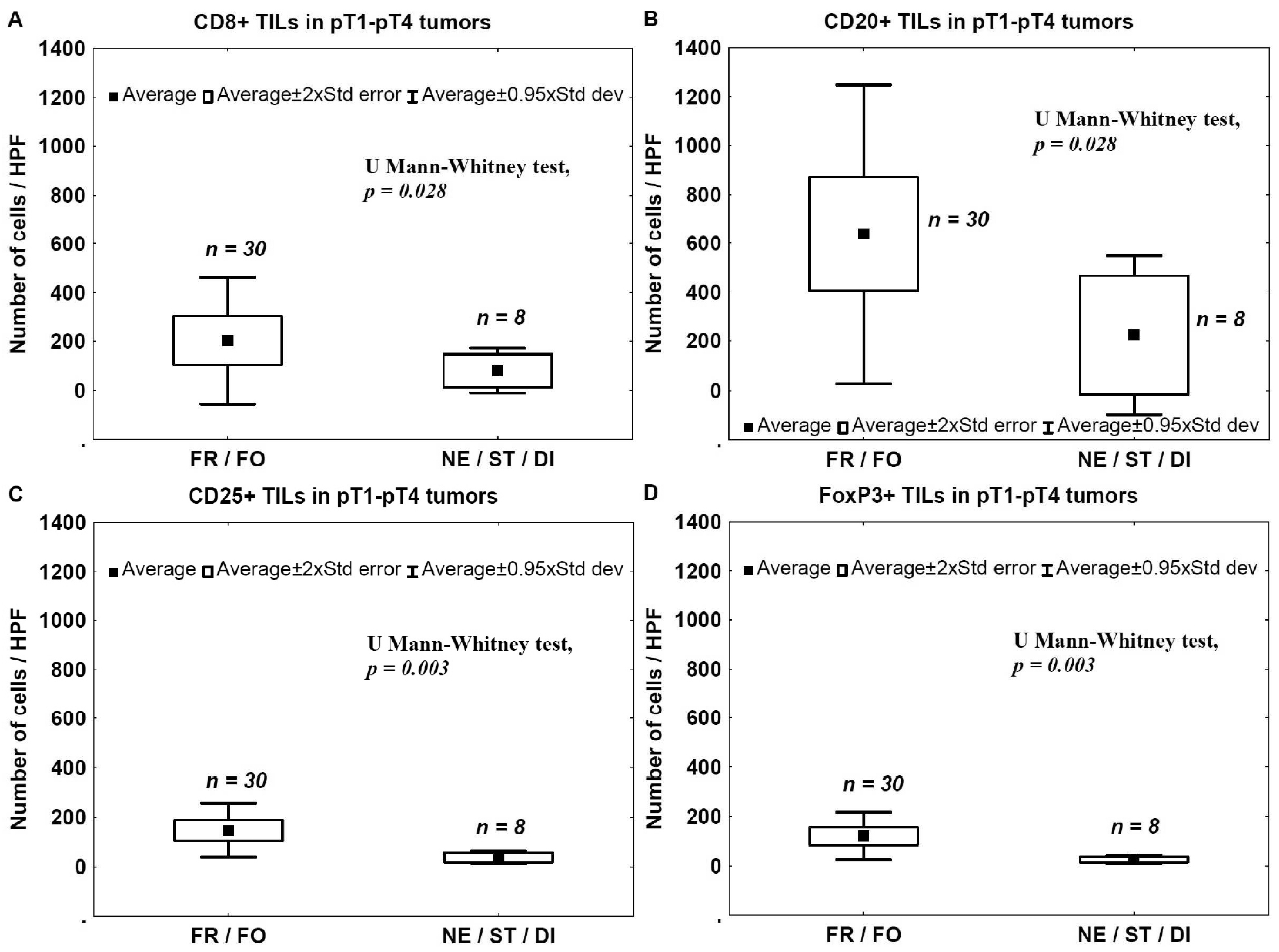
2.4. Tumor Invasion Type (TIT) and TILs
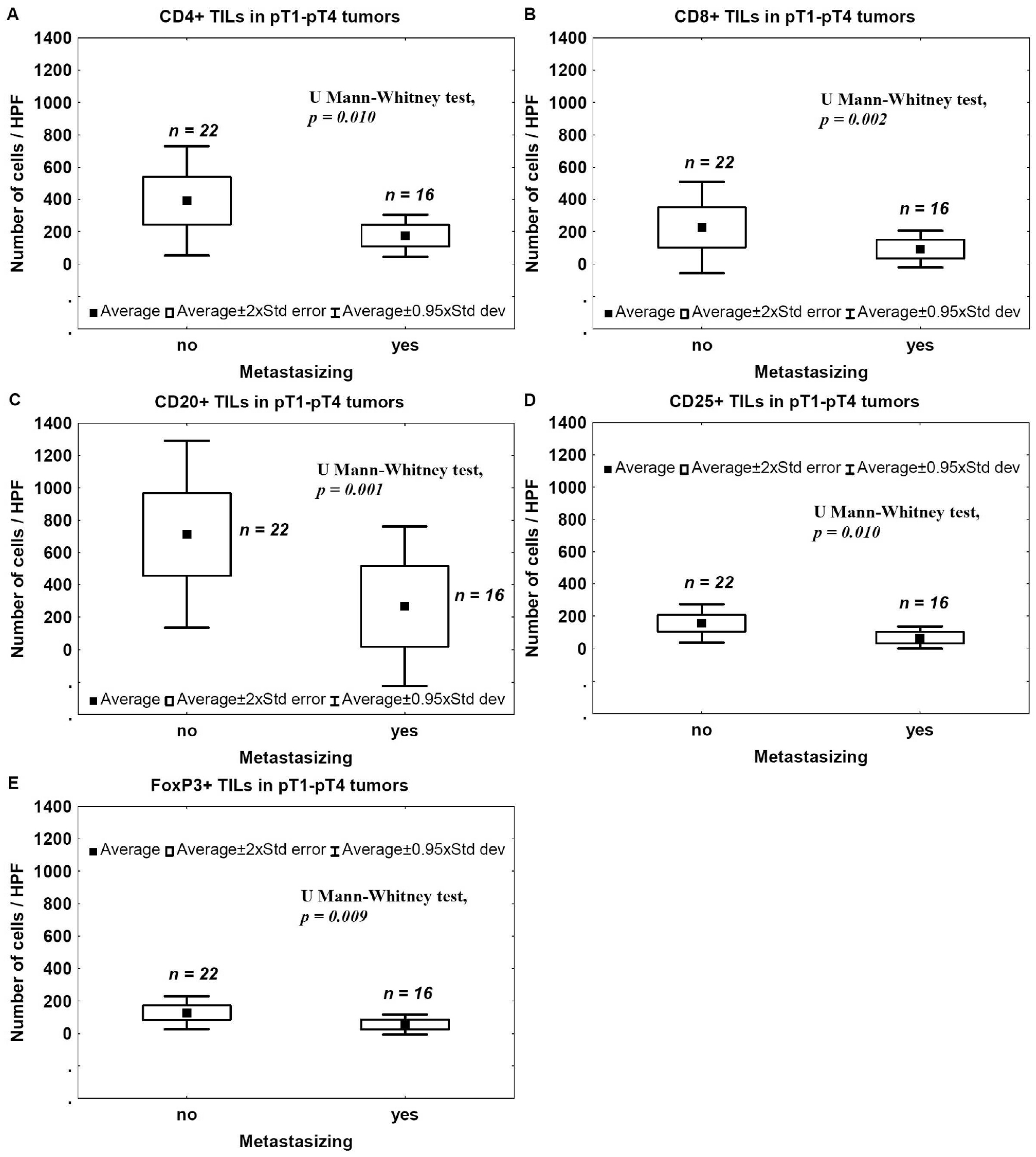
2.5. Metastasis and TILs
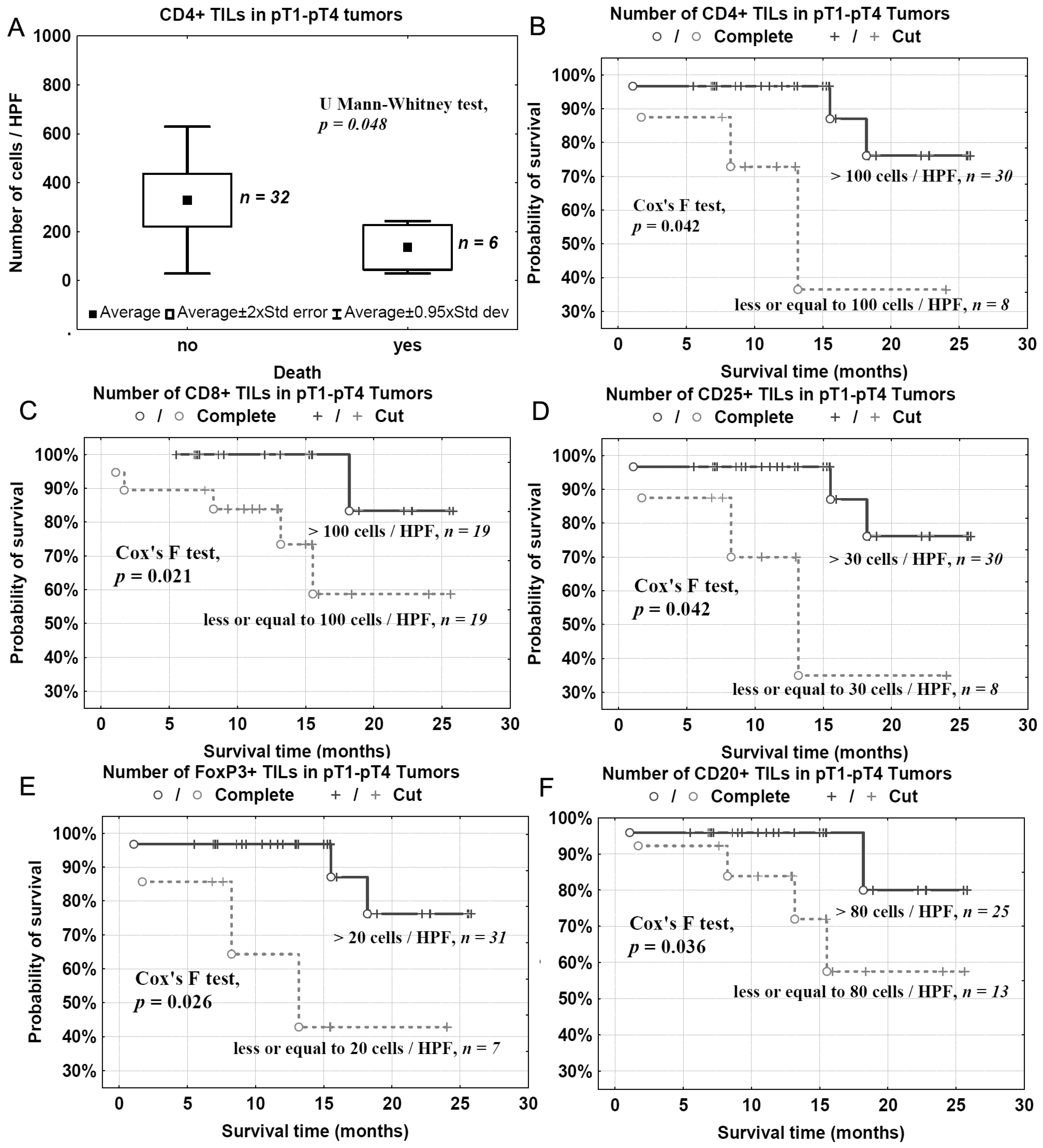
2.6. Survival and TILs
3. Discussion
4. Materials and Methods
4.1. Patients
4.2. Immunohistochemistry
4.3. Immunohistochemistry Assessment
4.4. Immunolabeling of Tregs in Peripheral Blood and Flow Cytometry
4.5. Histological Assessment
4.6. Statistical Analyses
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
Abbreviations
| APC | allophycocyanin |
| CAFs | cancer associated fibroblasts |
| DI | dispersive |
| FITC | fluorescein isothiocyanate |
| FO | focal |
| FR | frontal |
| ND | nonclassic differentiation |
| NDN | nonclassic differentiation number |
| NE | nested |
| PE | phycoerythrin |
| RT | room temperature |
| ST | styloid |
| TAMs | tumor-associated macrophages |
| TIL | tumor-infiltrating lymphocyte |
| TIT | tissue invasion type |
| Treg | blood regulatory T lymphocyte |
References
- Lopez-Beltran, A.; Sauter, G.; Gasser, T.; Hartmann, A.; Schmitz-Dräger, B.J.; Helpap, B.; Ayala, A.G.; Tamboli, P.; Knowles, M.A.; Sidransky, D.; et al. Tumours of the urinary system. Infiltrating urothelial carcinoma. In WHO Classification of Tumours. Pathology and Genetics of Tumors of the Urinary System and Male Genital Organs; Eble, J.N., Sauter, G., Epstein, J.I., Sesterhenn, I.A., Eds.; IARC Press: Lyon, France, 2004; pp. 93–109. [Google Scholar]
- Madersbacher, S.; Hochreiter, W.; Burkhard, F.; Thalmann, G.N.; Danuser, H.; Markwalder, R.; Studer, U.E. Radical cystectomy for bladder cancer today—A homogeneous series without neoadjuvant therapy. J. Clin. Oncol. 2003, 21, 690–696. [Google Scholar] [CrossRef] [PubMed]
- Kaufman, D.S.; Shipley, W.U.; Feldman, A.S. Bladder cancer. Lancet 2009, 374, 239–249. [Google Scholar] [CrossRef]
- Van den Bosch, S.; Witjes, J.A. Long-term cancer-specific survival in patients with high-risk non-muscle-invasive bladder cancer and tumour progression: A systematic review. Eur. Urol. 2011, 60, 493–500. [Google Scholar] [CrossRef] [PubMed]
- Jozwicki, W.; Domaniewski, J.; Skok, Z.; Wolski, Z.; Domanowska, E.; Jozwicka, G. Usefulness of histologic homogeneity estimation of muscle-invasive urinary bladder cancer in an individual prognosis: A mapping study. Urology 2005, 66, 1122–1126. [Google Scholar] [CrossRef] [PubMed]
- Domanowska, E.; Jozwicki, W.; Domaniewski, J.; Golda, R.; Skok, Z.; Wisniewska, H.; Sujkowska, R.; Wolski, Z.; Jozwicka, G. Muscle-invasive urothelial cell carcinoma of the human bladder: Multidirectional differentiation and ability to metastasize. Hum. Pathol. 2007, 38, 741–746. [Google Scholar] [CrossRef] [PubMed]
- Jóźwicki, W.; Brożyna, A.A.; Siekiera, J. Expression of OCT4a: The first step to the next stage of urothelial bladder cancer progression. Int. J. Mol. Sci. 2014, 15, 16069–16082. [Google Scholar] [CrossRef] [PubMed]
- Thanan, R.; Pairojkul, C.; Pinlaor, S.; Khuntikeo, N.; Wongkham, C.; Sripa, B.; Ma, N.; Vaeteewoottacharn, K.; Furukawa, A.; Kobayashi, H.; et al. Inflammation-related DNA damage and expression of CD133 and Oct3/4 in cholangiocarcinoma patients with poor prognosis. Free Radic. Biol. Med. 2013, 65, 1464–1472. [Google Scholar] [CrossRef] [PubMed]
- Yin, J.Y.; Tang, Q.; Zhai, L.L.; Zhou, L.Y.; Qian, J.; Lin, J.; Wen, X.M.; Zhou, J.D.; Zhang, Y.Y.; Zhu, X.W.; et al. High expression of OCT4 is frequent and may cause undesirable treatment outcomes in patients with acute myeloid leukemia. Tumour Biol. 2015, 36, 9711–9716. [Google Scholar] [CrossRef] [PubMed]
- Li, N.; Deng, W.; Ma, J.; Wei, B.; Guo, K.; Shen, W.; Zhang, Y.; Luo, S. Prognostic evaluation of Nanog, Oct4, Sox2, PCNA, Ki67 and E-cadherin expression in gastric cancer. Med. Oncol. 2015, 32, 433. [Google Scholar] [CrossRef] [PubMed]
- Jóźwicki, W.; Brożyna, A.A.; Siekiera, J.; Slominski, A.T. Expression of RCAS1 correlates with urothelial bladder cancer malignancy. Int. J. Mol. Sci. 2015, 16, 3783–3803. [Google Scholar] [CrossRef] [PubMed]
- Jóźwicki, W.; Windorbska, W.; Brożyna, A.A.; Jochymski, C.; Basta, P.; Sikora, J.; Stasienko, E.; Dutsch-Wicherek, M.; Koper, K.; Wicherek, L. The analysis of receptor-binding cancer antigen expressed on SiSo cells (RCAS1) immunoreactivity within the microenvironment of the ovarian cancer lesion relative to the applied therapeutic strategy. Cell Tissue Res. 2011, 345, 405–414. [Google Scholar] [CrossRef] [PubMed]
- Escors, D. Tumour immunogenicity, antigen presentation, and immunological barriers in cancer immunotherapy. New J. Sci. 2014, 2014, 734515. [Google Scholar] [CrossRef] [PubMed]
- Blankenstein, T.; Coulie, P.G.; Gilboa, E.; Jaffee, E.M. The determinants of tumour immunogenicity. Nat. Rev. Cancer 2012, 12, 307–313. [Google Scholar] [CrossRef] [PubMed]
- Pradeu, T.; Carosella, E.D. On the definition of a criterion of immunogenicity. Proc. Natl. Acad. Sci. USA 2006, 103, 17858–17861. [Google Scholar] [CrossRef] [PubMed]
- Drake, C.G.; Jaffee, E.M.; Pardoll, D.M. Mechanisms of immune evasion by tumors. Adv. Immunol. 2006, 90, 51–81. [Google Scholar] [PubMed]
- Laheru, D.A.; Jaffee, E.M. Immunotherapy for pancreatic cancer—Science driving clinical practice. Nat. Rev. Cancer 2005, 5, 459–467. [Google Scholar] [CrossRef] [PubMed]
- Tran, T.; Burt, D.; Eapen, L.; Keller, O.R. Spontaneous regression of metastatic melanoma after inoculation with tetanus-diphtheria-pertussis vaccine. Curr. Oncol. 2013, 20, e270–e273. [Google Scholar] [CrossRef] [PubMed]
- Dupage, M.; Mazumdar, C.; Schmidt, L.M.; Cheung, A.F.; Jacks, T. Expression of tumour-specific antigens underlies cancer immunoediting. Nature 2012, 482, 405–409. [Google Scholar] [CrossRef] [PubMed]
- Jóźwicki, W.; Brożyna, A.A.; Siekiera, J.; Slominski, A.T. Expression of vitamin D receptor (VDR) positively correlates with survival of urothelial bladder cancer patients. Int. J. Mol. Sci. 2015, 16, 24369–24386. [Google Scholar] [CrossRef] [PubMed]
- Santegoets, S.J.; Dijkgraaf, E.M.; Battaglia, A.; Beckhove, P.; Britten, C.M.; Gallimore, A.; Godkin, A.; Gouttefangeas, C.; de Gruijl, T.D.; Koenen, H.J. Monitoring regulatory T cells in clinical samples: Consensus on an essential marker set and gating strategy for regulatory T cell analysis by flow cytometry. Cancer Immunol. Immunother. 2015, 64, 1271–1286. [Google Scholar] [CrossRef] [PubMed]
- Wicherek, L.; Jozwicki, W.; Windorbska, W.; Roszkowski, K.; Lukaszewska, E.; Wisniewski, M.; Brozyna, A.A.; Basta, P.; Skret-Magierlo, J.; Koper, K.; et al. Analysis of Treg cell population alterations in the peripheral blood of patients treated surgically for ovarian cancer—A preliminary report. Am. J. Reprod. Immunol. 2011, 66, 444–450. [Google Scholar] [CrossRef] [PubMed]
- Baecher-Allan, C.; Brown, J.A.; Freeman, G.J.; Hafler, D.A. CD4+CD25high regulatory cells in human peripheral blood. J. Immunol. 2001, 167, 1245–1253. [Google Scholar] [CrossRef] [PubMed]
- Zhang, D.; Tu, E.; Kasagi, S.; Zanvit, P.; Chen, Q.; Chen, W. Manipulating regulatory T cells: A promising strategy to treat autoimmunity. Immunotherapy 2015, 7, 1201–1211. [Google Scholar] [CrossRef] [PubMed]
- Jóźwicki, W.; Skok, Z.; Brożyna, A.; Siekiera, J.; Wolski, Z.; Domaniewski, J. Prognostic and diagnostic implications of histological differentiation in invasive urothelial cell carcinoma of the bladder: Variant or non-classic differentiation number. Cent. Eur. J. Urol. 2010, 63, 112–116. [Google Scholar] [CrossRef]
| Feature | Number |
|---|---|
| pT1–pT2 cohort | 12 |
| Gender | |
| F | 1 |
| M | 11 |
| pT | |
| pT1 | 4 |
| pT2 | 8 |
| NDN | |
| NDN 0 | 5 |
| NDN 1 | 3 |
| NDN 2 | 4 |
| NDN ≥ 3 | 0 |
| TIT | |
| FR/FO | 12 |
| NE/ST/DI | 0 |
| Metastasis | |
| No | 10 |
| Yes | 2 |
| Death | |
| No | 12 |
| Yes | 0 |
| pT3–pT4 cohort | 26 |
| Sex | |
| F | 1 |
| M | 25 |
| pT | |
| pT3 | 18 |
| pT4 | 8 |
| NDN | |
| NDN 0 | 6 |
| NDN 1 | 8 |
| NDN 2 | 8 |
| NDN ≥ 3 | 4 |
| TIT | |
| FR/FO | 18 |
| NE/ST/DI | 8 |
| Metastasis | |
| No | 12 |
| Yes | 14 |
| Death | |
| No | 20 |
| Yes | 6 |
| Antibody | Vendor | Epitope Retrieval | Primary Antibody (Dilution; Incubation Time) | Method of Detection | Positive Control |
|---|---|---|---|---|---|
| anti-CD4 | Dako (Carpinteria, CA, USA) | PT-Link, High pH | RTU; 20 min | Mouse Linker/EnVision/DAB (Dako) | lymph node |
| anti-CD8 | Dako | PT-Link, High pH | RTU; 20 min | EnVision/DAB (Dako) | lymph node |
| anti-CD20 | Dako | PT-Link, High pH | RTU; 20 min | EnVision/DAB (Dako) | lymph node |
| anti-CD25 | Ventana Medical Systems, Inc. (Tucson, AZ, USA) | manufacturer’s protocol | RTU; manufacturer’s protocol | UltraView Universal DAB (Ventana) | lymph node |
| anti-FoxP3 | Abcam (Cambridge, UK) | PT-Link, Low pH (Dako) | 1:100; O/N 4 °C | EnVision/DAB (Dako) | lymph node |
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons by Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jóźwicki, W.; Brożyna, A.A.; Siekiera, J.; Slominski, A.T. Changes in Immunogenicity during the Development of Urinary Bladder Cancer: A Preliminary Study. Int. J. Mol. Sci. 2016, 17, 285. https://doi.org/10.3390/ijms17030285
Jóźwicki W, Brożyna AA, Siekiera J, Slominski AT. Changes in Immunogenicity during the Development of Urinary Bladder Cancer: A Preliminary Study. International Journal of Molecular Sciences. 2016; 17(3):285. https://doi.org/10.3390/ijms17030285
Chicago/Turabian StyleJóźwicki, Wojciech, Anna A. Brożyna, Jerzy Siekiera, and Andrzej T. Slominski. 2016. "Changes in Immunogenicity during the Development of Urinary Bladder Cancer: A Preliminary Study" International Journal of Molecular Sciences 17, no. 3: 285. https://doi.org/10.3390/ijms17030285
APA StyleJóźwicki, W., Brożyna, A. A., Siekiera, J., & Slominski, A. T. (2016). Changes in Immunogenicity during the Development of Urinary Bladder Cancer: A Preliminary Study. International Journal of Molecular Sciences, 17(3), 285. https://doi.org/10.3390/ijms17030285









