Trans-Stilbenes in Commercial Grape Juices: Quantification Using HPLC Approaches
Abstract
:1. Introduction
2. Results and Discussion
2.1. Chromatographic Method and Performance Characteristics
2.2. Determination of Stilbenes in Commercial Grape Juices
3. Materials and Methods
3.1. Samples
3.2. Reagents and Standard Solutions
3.3. Identification and Quantification
3.4. Preparation of Samples
3.5. Instrumentation and Method Conditions
3.5.1. High Performance Liquid Chromatography (HPLC) Method
3.5.2. HPLC-PDA-MS/MS as a Confirmatory Technique
3.5.3. Statistical Analysis
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Palomino, O.; Gómez-Serranillos, M.P.; Slowing, K.; Carretero, E.; Villar, A. Study of polyphenols in grape berries by reversed-phase high-performance liquid chromatography. J. Chromatogr. A 2000, 870, 449–451. [Google Scholar] [CrossRef]
- Acuña-Avila, P.E.; Vázquez-Murrieta, M.S.; Franco-Hernández, M.O.; López-Cortez, M.S. Relatioship between the elemental composition of grapeyards and bioactive compounds in the Cabernet Sauvignon grapes Vitis vinifera harvested in Mexico. Food Chem. 2016, 203, 79–85. [Google Scholar] [CrossRef] [PubMed]
- Huang, X.; Mazza, G. Simultaneous analysis of serotonin, melatonin, piceid and resveratrol in fruits using liquid chromatography tandem mass spectrometry. J. Chromatogr. A 2011, 1218, 3890–3899. [Google Scholar] [CrossRef] [PubMed]
- Rimando, A.M.; Kalt, W.; Magee, J.B.; Dewey, J.; Ballington, J.R. Resveratrol, pterostilbene, and piceatannol in Vaccinium berries. J. Agric. Food Chem. 2004, 52, 4713–4719. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.Y.; Chen, C.T.; Wang, C.Y.; Chen, A.P. Resveratrol content in strawberry fruit is affected by preharvest conditions. J. Agric. Food Chem. 2007, 55, 8269–8274. [Google Scholar] [CrossRef] [PubMed]
- Reinisalo, M.; Kårlund, A.; Koskela, A.; Kaarniranta, K.; Karjalainen, R.O. Polyphenol stilbenes: Molecular mechanisms of defense against oxidative stress and aging-related diseases. Oxid. Med. Cell. Longev. 2015, 2015. [Google Scholar] [CrossRef] [PubMed]
- Carter, L.G.; D’Orazio, J.A.; Pearson, K.J. Resveratrol and cancer: Focus on in vivo evidence. Endocr. Relat. Cancer 2014, 21, R209–R225. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Bian, Y.; Shi, Y.; Zheng, S.; Gu, X.; Zhang, D.; Zhu, X.; Wang, X.; Jiang, D.; Xiong, Q. An economical and efficient technology for the extraction of resveratrol from peanut (Arachis hypogaea) sprouts by multi-stage countercurrent extraction. Food Chem. 2015, 179, 15–25. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez-Cabo, T.; Rodríguez, I.; Cela, R. Determination of hydroxylated stilbenes in wine by dispersive liquid-liquid microextraction followed by gas chromatography mass spectrometry. J. Chromatogr. A 2012, 1258, 21–29. [Google Scholar] [CrossRef] [PubMed]
- Kukreja, A.; Tandon, S.; Mishra, A.; Tiwari, A. Piceatannol: A potential futuristic natural stilbene as fetal haemoglobin inducer. J. Clin. Diagn. Res. 2013, 7, 3028–3031. [Google Scholar] [CrossRef] [PubMed]
- Rosa, M.; Lamuela-Raventos, R.M.; Romero-Pérez, A.I.; Waterhouse, A.L.; de la Torre-Boronatt, M.C. Direct HPLC analysis of cis- and trans-resveratrol and piceid isomers in Spanish red Vitis vinifera wines. J. Agric. Food Chem. 1995, 41, 281–283. [Google Scholar]
- Domínguez, C.; Guillén, D.A.; Barroso, C.G. Automated solid-phase extraction for sample preparation followed by high-performance liquid chromatography with diode array and mass spectrometric detection for the analysis of resveratrol derivatives in wine. J. Chromatogr. A 2001, 918, 303–310. [Google Scholar] [CrossRef]
- Fan, E.; Lin, S.; Du, D.; Jia, Y.; Kang, L.; Zhang, K. Current separative strategies used for resveratrol determination from natural sources. Anal. Methods 2011, 3, 2454–2462. [Google Scholar] [CrossRef]
- Kolouchová-Hanzlíková, I.; Melzoch, K.; Filip, V.; Smidrkal, J. Rapid method for resveratrol determination by HPLC with electrochemical and UV detections in wines. Food Chem. 2004, 87, 151–158. [Google Scholar] [CrossRef]
- Rodríguez-Bernaldo de Quirós, A.; López-Hernández, J.; Ferraces-Casais, P.; Lage-Yusty, M.A. Analysis of non-anthocyanin phenolic compounds in wine samples using high performance liquid chromatography with ultraviolet and fluorescence detection. J. Sep. Sci. 2007, 30, 1262–1266. [Google Scholar] [CrossRef] [PubMed]
- Zhou, J.; Cui, H.; Wan, G.; Xu, H.; Pang, Y.; Duan, C. Direct analysis of trans-resveratrol in red wine by high performance liquid chromatography with chemiluminescent detection. Food Chem. 2004, 88, 613–620. [Google Scholar] [CrossRef]
- Paulo, L.; Domingues, F.; Queiroz, J.A.; Gallardo, E. Development and validation of an analytical method for the determination of trans- and cis-resveratrol in wine: Analysis of its contents in 186 portuguese red wines. J. Agric. Food Chem. 2011, 59, 2157–2168. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Z.; Li, J.; Gao, X.; Amponsem, E.; Kang, L.; Hu, L.; Zhang, B.; Chang, Y. Simultaneous determination of stilbenes, phenolic acids, flavonoids and anthraquinones in Radix polygoni multiflori by LC-MS/MS. J. Pharm. Biomed. Anal. 2012, 62, 162–166. [Google Scholar] [CrossRef] [PubMed]
- Moss, R.; Mao, Q.; Taylor, D.; Saucier, C. Investigation of monomeric and oligomeric wine stilbenoids in red wines by ultra-high-performance liquid chromatography/electrospray ionization quadrupole time-of-flight mass spectrometry. Rapid Commun. Mass Spectrom. 2013, 27, 1815–1827. [Google Scholar]
- Rodríguez-Cabo, T.; Rodríguez, I.; López, P.; Ramil, M.; Cela, R. Investigation of liquid chromatography quadrupole time-of-flightmass spectrometry performance for identification and determination of hydroxylated stilbene antioxidants in wine. J. Chromatogr. A 2014, 1337, 162–170. [Google Scholar] [CrossRef] [PubMed]
- MacDougall, D.; Crummett, W.B. Guidelines for data acquisition and data quality evaluation in environmental chemistry. Anal. Chem. 1980, 52, 2242–2249. [Google Scholar] [CrossRef]
- Buiarelli, F.; Coccioli, F.; Jasionowska, R.; Merolle, M.; Terracciano, A. Analysis of some stilbenes in Italian wines by liquid chromatography/tandem mass spectrometry. Rapid Commun. Mass Spectrom. 2007, 2, 2955–2964. [Google Scholar] [CrossRef] [PubMed]
- Boue, S.M.; Shih, B.Y.; Burow, M.E.; Eggleston, G.; Lingle, S.; Pan, Y.B.; Daigle, K.; Bhatnagar, D. Postharvest accumulation of resveratrol and piceatannol in sugarcane with enhanced antioxidant activity. J. Agric. Food Chem. 2013, 61, 8412–8419. [Google Scholar] [CrossRef] [PubMed]
- Stecher, G.; Huck, C.W.; Popp, M.; Bonn, G.K. Determination of flavonoids and stilbenes in red wine and related biological products by HPLC and HPLC-ESI-MS-MS. Fresenius J. Anal. Chem. 2001, 371, 73–80. [Google Scholar] [CrossRef] [PubMed]
- Baptista, J.A.B.; da P Tavares, J.F.; Carvalho, R.C.B. Comparison of polyphenols and aroma in red wines from Portuguese mainland versus Azores Islands. Food Res. Int. 2001, 34, 345–355. [Google Scholar] [CrossRef]
- Gerogiannaki-Christopoulou, M.; Athanasopoulos, P.; Kyriakidis, N.; Gerogiannaki, I.A.; Spanos, M. Trans-resveratrol in wines from the major Greek red and white grape varieties. Food Control 2006, 17, 700–706. [Google Scholar] [CrossRef]
- Rodríguez-Bernaldo de Quirós, A.; Lage-Yusty, M.A.; López-Hernández, J. HPLC-analysis of polyphenolic compounds in Spanish white wines and determination of their antioxidant activity by radical scavenging assay. Food Res. Int. 2009, 42, 1018–1022. [Google Scholar] [CrossRef]
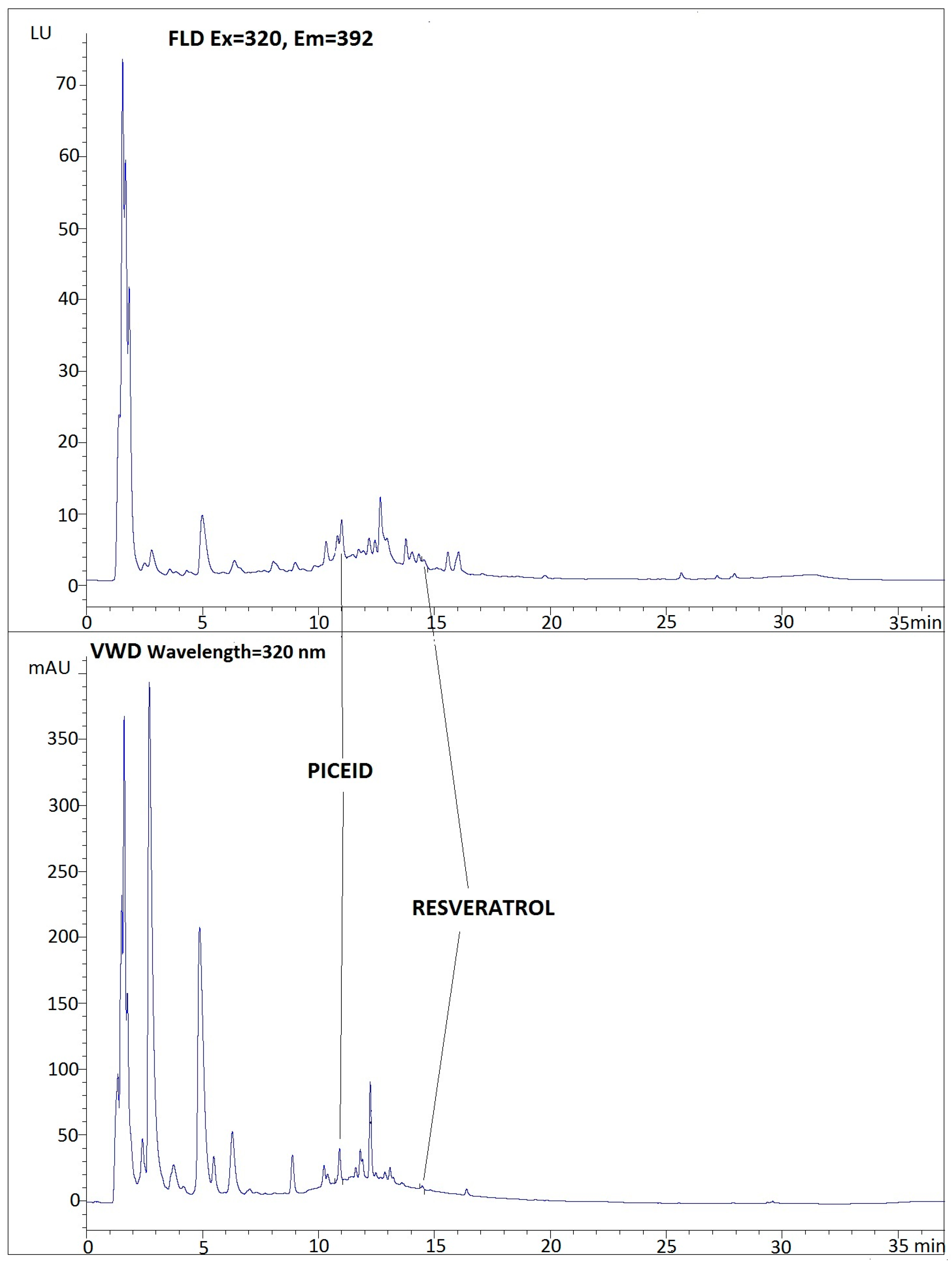
| Compound | Retention Time (min) | AreaVW AreaFL | Intercept | Slope | r2 | Range (µg/mL) | LOD (µg/mL) | LOQ (µg/mL) | RSD% |
|---|---|---|---|---|---|---|---|---|---|
| Trans-piceid (HPLC-FL) | 10.9 | 3.35 ± 0.13 | 0.8219 ± 0.1191 | 50.30 ± 0.45 | 0.9993 | 0.12–4.8 | 0.0226 | 0.0752 | 3.2 |
| Trans-piceid (HPLC-VW) | 10.8 | 3.2061 ± 0.7037 | 169.90 ± 0.068 | 0.9994 | 0.12–4.8 | 0.007 | 0.0238 | 0.42 | |
| Trans-piceatannol (HPLC-FL) | 12.1 | 1.25 ± 0.055 | −22.768 ± 4.8633 | 214.84 ± 1.08 | 0.9995 | 0.1–4.0 | 0.009 | 0.0300 | 2.9 |
| Trans-piceatannol (HPLC-VW) | 12.0 | −30.090 ± 1.2111 | 270.99 ± 0.88 | 0.9993 | 0.1–4.0 | 0.007 | 0.0223 | 2.1 | |
| Trans-resveratrol (HPLC-FL) | 14.6 | 3.24 ± 0.061 | −6.0551 ± 1.4745 | 127.00 ± 2.30 | 0.9995 | 0.02–5.0 | 0.010 | 0.0330 | 0.75 |
| Trans-resv (HPLC-VW) | 14.5 | −14.880 ± 0.6842 | 406.85 ± 0.63 | 0.9996 | 0.02–5.0 | 0.003 | 0.0101 | 0.15 | |
| Trans-pterostilbene (HPLC-FL) | 24.8 | 3.21 ± 0.14 | −10.463 ± 0.0388 | 100.59 ± 0.24 | 0.9991 | 0.08–8.0 | 0.012 | 0.0406 | 2.4 |
| Trans-pterostilbene (HPLC-VW) | 24.7 | −25.0530 ± 2.6570 | 328.96 ± 2.25 | 0.9995 | 0.08–8.0 | 0.005 | 0.0161 | 0.8 |
| Samples | Trans-Piceid | Trans-Piceatannol | Trans-Resveratrol | Trans-Pterostilbene | ||||
|---|---|---|---|---|---|---|---|---|
| FL | VW | FL | VW | FL | VW | FL | VW | |
| 1 | 0.29 ± 0.01 | 0.29 ± 0.02 | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. |
| 2 | 6.56 ± 0.22 | 6.59 ± 0.45 | 0.25 ± 0.01 | 0.26 ± 0.01 | 0.27 ± 0.01 | 0.27 ± 0.02 | n.d. | n.d. |
| 3 | 1.24 ± 0.10 | 1.24 ± 0.10 | n.d. | n.d. | 0.83 ± 0.07 | 0.79 ± 0.05 | n.d. | n.d. |
| 4 | 1.42 ± 0.06 | 1.35 ± 0.06 | n.d. | n.d. | 0.16 ± 0.01 | 0.15 ± 0.01 | n.d. | n.d. |
| 5 | 1.31 ± 0.11 | 1.27 ± 0.10 | n.d. | n.d. | 0.16 ± 0.08 | 0.15 ± 0.01 | n.d. | n.d. |
| 6 | 0.85 ± 0.06 | 0.81 ± 0.05 | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. |
| 7 | 1.25 ± 0.04 | 1.24 ± 0.04 | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. |
| 8 | 1.47 ± 0.09 | 1.42 ± 0.06 | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. |
| 9 | 0.52 ± 0.02 | 0.51 ± 0.02 | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. |
| 10 | n.d. | n.d. | n.d. | n.d. | 0.36 ± 0.02 | 0.36 ± 0.02. | n.d. | n.d. |
| 11 | 0.46 ± 0.02 | 0.41 ± 0.025 | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. |
| Molecule | PDA λ max. | Precursor Ion | Product Ions | Collision Energy | Tube Lens |
|---|---|---|---|---|---|
| Trans-piceid | 308, 319 | 389 | 227 | 22 | −67.08 |
| 185 | 40 | ||||
| 143 | 51 | ||||
| Trans-piceatannol | 306, 322 | 243 | 225 | 21 | −68.83 |
| 201 | 22 | ||||
| 159 | 28 | ||||
| Trans-resveratrol | 306, 317 | 227 | 185 | 21 | −65.83 |
| 183 | 20 | ||||
| 143 | 29 | ||||
| Trans-pterostilbene | 307, 319 | 255 | 240 | 22 | −59.32 |
| 197 | 32 | ||||
| 169 | 35 |
| Samples | Composition |
|---|---|
| 1 | Red grapes juice 100% |
| 2 | Grape juice made from concentrate 99% |
| 3 | Grape juice from concentrate |
| 4 | Red grape Merlot juice 100% |
| 5 | Red grape Merlot juice 100% |
| 6 | Organic grape juice made from concentrate |
| 7 | Red grape juice 65%, pomegranate juice 25%, blackcurrant juice 10% |
| 8 | Red grape juice 65%, pomegranate juice 25%, blackcurrant juice 10% |
| 9 | Red grape juice 65%, pomegranate juice 25%, blackcurrant juice 10% |
| 10 | Grape juice 50%, pomegranate juice 50% |
| 11 | Red grapes squeezed juice 92%, strawberry pure 4% and cranberry juice squeezed 4% |
| Chemical Structure | Name | CAS | MW | Log P (octanol-water) |
|---|---|---|---|---|
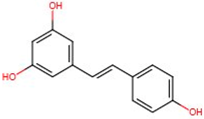 | Trans-resveratrol | 501-36-0 | 228.246 | 3.080 |
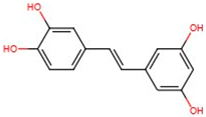 | Trans-piceatannol | 10083-24-6 | 244.2448 | n.a. |
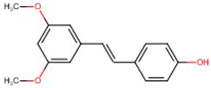 | Trans-pterostilbene | 537-42-8 | 256.299 | n.a. |
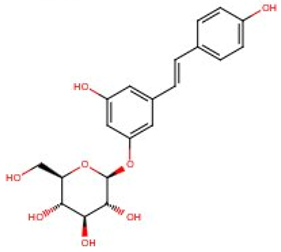 | Trans-piceid | 65914-17-2 | 390.386 | n.a. |
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
López-Hernández, J.; Rodríguez-Bernaldo de Quirós, A. Trans-Stilbenes in Commercial Grape Juices: Quantification Using HPLC Approaches. Int. J. Mol. Sci. 2016, 17, 1769. https://doi.org/10.3390/ijms17101769
López-Hernández J, Rodríguez-Bernaldo de Quirós A. Trans-Stilbenes in Commercial Grape Juices: Quantification Using HPLC Approaches. International Journal of Molecular Sciences. 2016; 17(10):1769. https://doi.org/10.3390/ijms17101769
Chicago/Turabian StyleLópez-Hernández, Julia, and Ana Rodríguez-Bernaldo de Quirós. 2016. "Trans-Stilbenes in Commercial Grape Juices: Quantification Using HPLC Approaches" International Journal of Molecular Sciences 17, no. 10: 1769. https://doi.org/10.3390/ijms17101769
APA StyleLópez-Hernández, J., & Rodríguez-Bernaldo de Quirós, A. (2016). Trans-Stilbenes in Commercial Grape Juices: Quantification Using HPLC Approaches. International Journal of Molecular Sciences, 17(10), 1769. https://doi.org/10.3390/ijms17101769





