Discovery of Potent Carbonic Anhydrase and Acetylcholinesterase Inhibitors: 2-Aminoindan β-Lactam Derivatives
Abstract
:1. Introduction
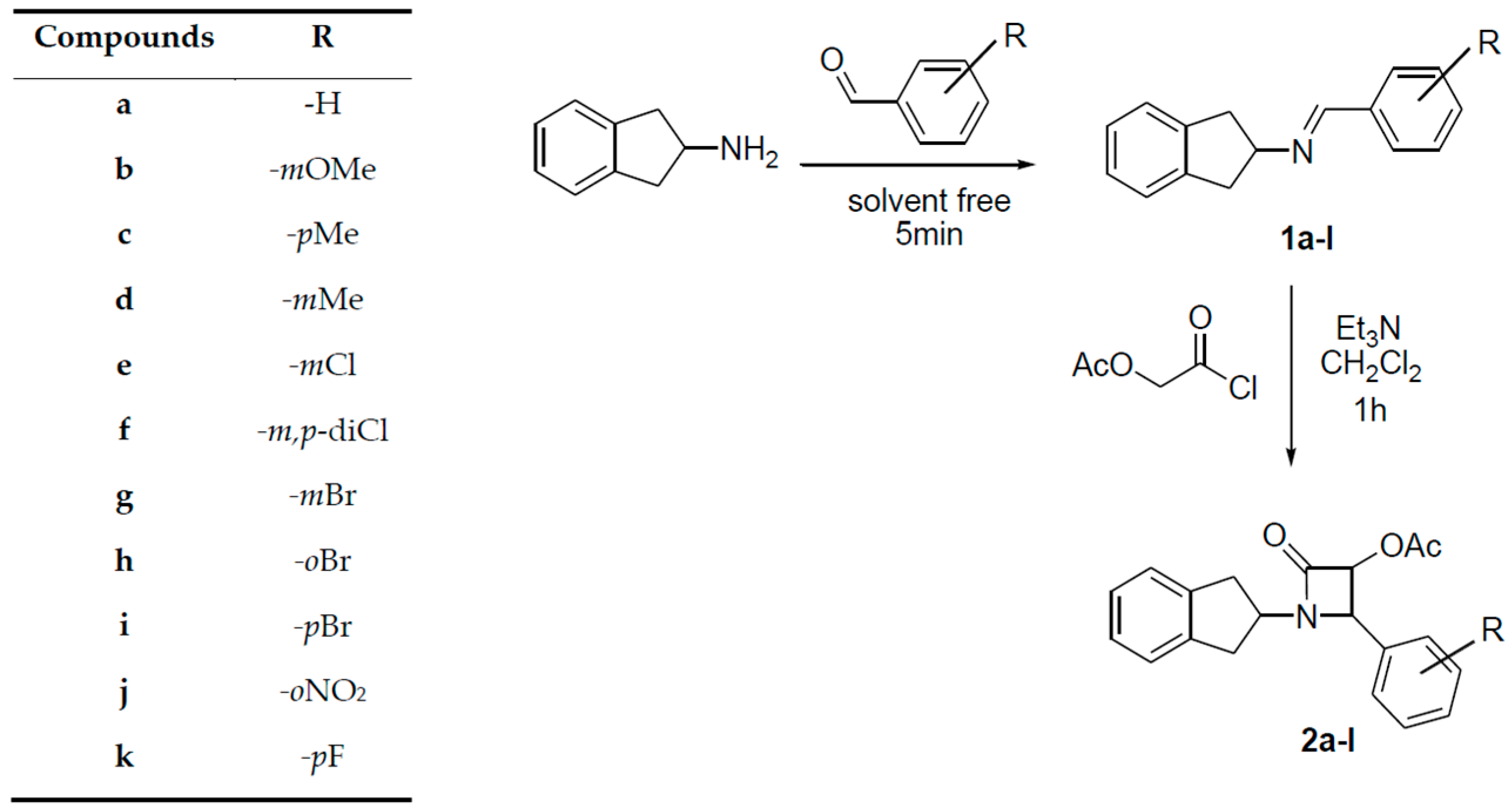
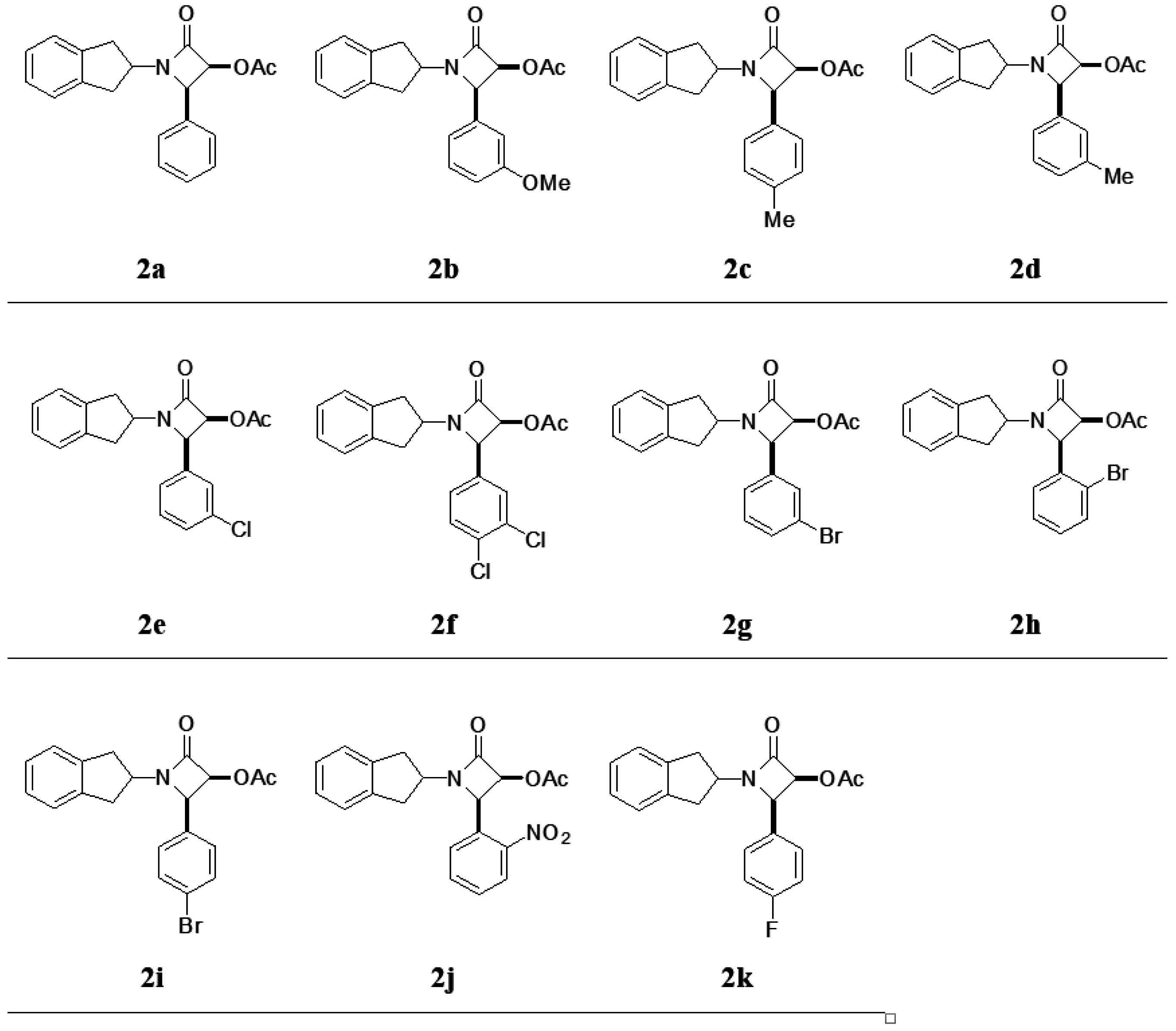
2. Results and Discussion
3. Materials and Methods
3.1. Chemicals
3.2. General Procedure for the Synthesis of Imines
3.3. General Procedure for the Synthesis of β-Lactams
3.4. Spectral Data
3.5. Biochemical Assays
4. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Huang, Z.; Pan, X.D.; Huang, B.; Xu, J.J.; Wang, M.L.; Ren, Y.P. Determination of 15 β-lactam antibiotics in pork muscle by matrix solid-phase dispersion extraction (MSPD) and ultra-high pressure liquid chromatography tandem mass spectrometry. Food Control 2016, 66, 145–150. [Google Scholar] [CrossRef]
- Zhang, L.; Jin, Y.; Mao, H.; Zheng, L.; Zhao, J.; Peng, Y.; Du, S.; Zhang, Z. Structure-selective hot-spot Raman enhancement for direct identification and detection of trace penicilloic acid allergen in penicillin. Biosens. Bioelectron. 2014, 58, 165–171. [Google Scholar] [CrossRef] [PubMed]
- Vijay Kumar, M.M.J.; Yogananda, R.; Snehalatha Shameer, H.; Jayachandran, E.; Sreenivasa, G.M. Synthesis and characterization of novel N-substituted-3-chloro-2-azetidinones as potential anticonvulsant agents. J. Biomed. Sci. Res. 2009, 1, 1–10. [Google Scholar]
- Banik, I.; Becker, F.F.; Banik, B.K. Stereoselective synthesis of β-lactams with polyaromatic imines: Entry to new and novel anticancer agents. J. Med. Chem. 2003, 46, 12–15. [Google Scholar] [CrossRef] [PubMed]
- Clemente, A.; Domingos, A.; Grancho, A.P.; Iley, J.; Moreira, R.; Neres, J.; Palma, N.; Santana, A.B.; Valente, E. Design, synthesis and stability of N-acyloxymethyl- and N-aminocarbonyloxymethyl-2-azetidinones as human leukocyte elastase inhibitors. Bioorg. Med. Chem. Lett. 2001, 11, 1065–1068. [Google Scholar] [CrossRef]
- Bytyqi-Damoni, A.; Genç, H.; Zengin, M.; Beyaztas, S.; Gençer, N.; Arslan, O. In vitro effect of novel β-lactam compounds on xanthine oxidase enzyme activity. Artif. Cells Blood Substit. Immobil. Biotechnol. 2012, 40, 369–377. [Google Scholar] [CrossRef] [PubMed]
- Küçük, M.; Gulcin, I. Purification and characterization of carbonic anhydrase enzyme from black sea trout (Salmo trutta Labrax Coruhensis) kidney and inhibition effects of some metal ions on the enzyme activity. Environ. Toxicol. Pharmacol. 2016, 44, 134–139. [Google Scholar] [CrossRef] [PubMed]
- Troisi, L.; Granito, C.; Pindinelli, E. Novel and recent synthesis and applications of β-lactams. Top. Heterocycl. Chem. 2010, 22, 101–209. [Google Scholar]
- Taslimi, P.; Gulcin, I.; Ozgeris, B.; Goksu, S.; Tumer, F.; Alwasel, S.H.; Supuran, C.T. The human carbonic anhydrase isoenzymes I and II (hCA I and II) inhibition effects of trimethoxyindane derivatives. J. Enzym. Inhib. Med. Chem. 2016, 31, 152–157. [Google Scholar] [CrossRef] [PubMed]
- Scozzafava, A.; Kalın, P.; Supuran, C.T.; Gülçin, I.; Alwasel, S. The impact of hydroquinone on acetylcholine esterase and certain human carbonic anhydrase isoenzymes (hCA I, II, IX, and XII). J. Enzym. Inhib. Med. Chem. 2015, 30, 941–946. [Google Scholar] [CrossRef] [PubMed]
- Artunç, T.; Çetinkaya, Y.; Göçer, H.; Gulcin, I.; Menzek, A.; Sahin, E.; Supuran, C.T. Synthesis of 4-[2-(3,4-dimethoxybenzyl)cyclopentyl]-1,2-dimethoxybenzene derivatives and evaluations of their carbonic anhydrase isoenzymes inhibitory effects. Chem. Biol. Drug Des. 2016, 87, 594–607. [Google Scholar] [CrossRef] [PubMed]
- Akıncıoğlu, A.; Akıncıoğlu, H.; Gülçin, I.; Durdağı, S.; Supuran, C.T.; Göksu, S. Discovery of potent carbonic anhydrase and acetylcholine esterase inhibitors: Novel sulfamoylcarbamates and sulfamides derived from acetophenones. Bioorg. Med. Chem. 2015, 23, 3592–3602. [Google Scholar] [CrossRef] [PubMed]
- Yıldırım, A.; Atmaca, U.; Keskin, A.; Topal, M.; Çelik, M.; Gülçin, I.; Supuran, C.T. N-Acylsulfonamides strongly inhibit human carbonic anhydrase isoenzymes I and II. Bioorg. Med. Chem. 2015, 23, 2598–2605. [Google Scholar] [CrossRef] [PubMed]
- Boztaş, M.; Çetinkaya, Y.; Topal, M.; Gülçin, İ.; Menzek, A.; Şahin, E.; Tanc, M.; Supuran, C.T. Synthesis and carbonic anhydrase isoenzymes I, II, IX, and XII inhibitory effects of dimethoxy-bromophenol derivatives incorporating cyclopropane moieties. J. Med. Chem. 2015, 58, 640–650. [Google Scholar] [CrossRef] [PubMed]
- Scozzafava, A.; Passaponti, M.; Supuran, C.T.; Gülçin, İ. Carbonic anhydrase inhibitors: Guaiacol and catechol derivatives effectively inhibit certain human carbonic anhydrase isoenzymes (hCA I, II, IX, and XII). J. Enzym. Inhib. Med. Chem. 2015, 30, 586–591. [Google Scholar] [CrossRef] [PubMed]
- Göçer, H.; Akıncıoğlu, A.; Göksu, S.; Gülçin, İ.; Supuran, C.T. Carbonic anhydrase and acetylcholine esterase inhibitory effects of carbamates and sulfamoylcarbamates. J. Enzym. Inhib. Med. Chem. 2015, 30, 316–320. [Google Scholar] [CrossRef] [PubMed]
- Arabaci, B.; Gülçin, İ.; Alwasel, S. Capsaicin: A potent inhibitor of carbonic anhydrase isoenzymes. Molecules 2014, 19, 10103–10114. [Google Scholar] [CrossRef] [PubMed]
- Akbaba, Y.; Bastem, E.; Topal, F.; Gülçin, İ.; Maraş, A.; Göksu, S. Synthesis and carbonic anhydrase inhibitory effects of novel sulfamides derived from 1-aminoindanes and anilines. Arch. Pharm. 2014, 347, 950–957. [Google Scholar] [CrossRef] [PubMed]
- Göksu, S.; Naderi, A.; Akbaba, Y.; Kalın, P.; Akıncıoğlu, A.; Gulcin, İ.; Durdaği, S.; Salmas, R.E. Carbonic anhydrase inhibitory properties of novel benzylsulfamides using molecular modeling and experimental studies. Bioorg. Chem. 2014, 56, 75–82. [Google Scholar] [CrossRef] [PubMed]
- Fujikawa-Adachi, K.; Nishimori, I.; Taguchi, T.; Onishi, S. Human mitochondrial carbonic anhydrase VB. cDNA cloning, mRNA expression, subcellular localization, and mapping to chromosome x. J. Biol. Chem. 1999, 274, 21228–21233. [Google Scholar] [CrossRef] [PubMed]
- Nagao, Y.; Platero, J.S.; Waheed, A.; Sly, W.S. Human mitochondrial carbonic-anhydrase-cDNA cloning, expression, subcellular-localization, and mapping to chromosome-16. Am. J. Hum. Genet. 1993, 53, 7623–7627. [Google Scholar] [CrossRef]
- Topal, M.; Gülçin, I. Rosmarinic acid: a potent carbonic anhydrase isoenzymes inhibitor. Turk. J. Chem. 2014, 38, 894–902. [Google Scholar] [CrossRef]
- Çetinkaya, Y.; Göçer, H.; Gülçin, I.; Menzek, A. Synthesis and carbonic anhydrase isoenzymes inhibitory effects of brominated diphenylmethanone and its derivatives. Arch. Pharm. 2014, 347, 354–359. [Google Scholar] [CrossRef] [PubMed]
- Çetinkaya, Y.; Göçer, H.; Göksu, S.; Gülçin, I. Synthesis and carbonic anhydrase isoenzymes inhibitory effects of novel benzylamine derivatives. J. Enzym. Inhib. Med. Chem. 2014, 29, 168–174. [Google Scholar] [CrossRef] [PubMed]
- Akbaba, Y.; Akıncıoğlu, A.; Göçer, H.; Göksu, S.; Gülçin, I.; Supuran, C.T. Carbonic anhydrase inhibitory properties of novel sulfonamide derivatives of aminoindanes and aminotetralins. J. Enzym. Inhib. Med. Chem. 2014, 29, 35–42. [Google Scholar] [CrossRef] [PubMed]
- Özgeriş, B.; Göksu, S.; Köse Polat, L.; Gülçin, I.; Salmas, R.E.; Durdagi, S.; Tümer, F.; Supuran, C.T. Acetylcholinesterase and carbonic anhydrase inhibitory properties of novel urea and sulfamide derivatives incorporating dopaminergic 2-aminotetralin scaffolds. Bioorg. Med. Chem. 2016, 24, 2318–2329. [Google Scholar] [CrossRef] [PubMed]
- Aksu, K.; Nar, M.; Tanç, M.; Vullo, D.; Gülçin, I.; Göksu, S.; Tümer, F.; Supuran, C.T. The synthesis of sulfamide analogues of dopamine related compounds and their carbonic anhydrase inhibitory properties. Bioorg. Med. Chem. 2013, 21, 2925–2931. [Google Scholar] [CrossRef] [PubMed]
- Akıncıoğlu, A.; Akbaba, Y.; Göçer, H.; Göksu, S.; Gülçin, I.; Supuran, C.T. Novel sulfamides as potential carbonic anhydrase isoenzymes inhibitors. Bioorg. Med. Chem. 2013, 21, 1379–1385. [Google Scholar] [CrossRef] [PubMed]
- Gülçin, I.; Beydemir, S. Phenolic compounds as antioxidants: Carbonic anhydrase isoenzymes inhibitors. Mini Rev. Med. Chem. 2013, 13, 408–430. [Google Scholar] [PubMed]
- Nar, M.; Çetinkaya, Y.; Gülçin, I.; Menzek, A. (3,4-Dihydroxyphenyl) (2,3,4-trihydroxyphenyl) methanone and its derivatives as carbonic anhydrase isoenzymes inhibitors. J. Enzym. Inhib. Med. Chem. 2013, 28, 402–406. [Google Scholar] [CrossRef] [PubMed]
- Öztürk Sarıkaya, S.B.; Topal, F.; Şentürk, M.; Gülçin, I.; Supuran, C.T. In vitro inhibition of α-carbonic anhydrase isozymes by some phenolic compounds. Bioorg. Med. Chem. Lett. 2011, 21, 4259–4262. [Google Scholar] [CrossRef] [PubMed]
- Şentürk, M.; Gülçin, I.; Beydemir, Ş.; Küfrevioğlu, Ö.I.; Supuran, C.T. In vitro inhibition of human carbonic anhydrase I and II isozymes with natural phenolic compounds. Chem. Biol. Drug Des. 2011, 77, 494–499. [Google Scholar] [CrossRef] [PubMed]
- Lomelino, C.L.; Supuran, C.T.; McKenna, R. Non-classical inhibition of carbonic anhydrase. Int. J. Mol. Sci. 2016, 17, 1150. [Google Scholar] [CrossRef] [PubMed]
- Innocenti, A.; Gülçin, I.; Scozzafava, A.; Supuran, C.T. Carbonic anhydrase inhibitors. Antioxidant polyphenol natural products effectively inhibit mammalian isoforms I-XV. Bioorg. Med. Chem. Lett. 2010, 20, 5050–5053. [Google Scholar] [CrossRef] [PubMed]
- Innocenti, A.; Öztürk Sarıkaya, S.B.; Gülçin, I.; Supuran, C.T. Carbonic anhydrase inhibitors. Inhibition of mammalian isoforms I-XIV with a series of natural product polyphenols and phenolic acids. Bioorg. Med. Chem. 2010, 18, 2159–2164. [Google Scholar] [CrossRef] [PubMed]
- Polat Köse, L.; Gülçin, I.; Gören, A.C.; Namiesnik, J.; Martinez-Ayala, A.L.; Gorinstein, S. LC-MS/MS analysis, antioxidant and anticholinergic properties of galanga (Alpinia officinarum Hance) rhizomes. Ind. Crops Prod. 2015, 74, 712–721. [Google Scholar] [CrossRef]
- Aksu, K.; Topal, F.; Gülçin, I.; Tümer, F.; Göksu, S. Acetylcholinesterase inhibitory and antioxidant activities of novel symmetric sulfamides derived from phenethylamines. Arch. Pharm. 2015, 348, 446–455. [Google Scholar] [CrossRef] [PubMed]
- Akıncıoğlu, A.; Topal, M.; Gülçin, I.; Göksu, S. Novel sulfamides and sulfonamides incorporating tetralin scaffold as carbonic anhydrase and acetylcholine esterase inhibitors. Arch. Pharm. 2014, 347, 68–76. [Google Scholar] [CrossRef] [PubMed]
- Göçer, H.; Akıncıoğlu, A.; Öztaşkın, N.; Göksu, S.; Gülçin, I. Synthesis, antioxidant and antiacetylcholinesterase activities of sulfonamide derivatives of dopamine related compounds. Arch. Pharm. 2013, 346, 783–792. [Google Scholar] [CrossRef] [PubMed]
- Gocer, H.; Topal, F.; Topal, M.; Küçük, M.; Teke, D.; Gulcin, I.; Alwasel, S.H.; Supuran, C.T. Acetylcholinesterase and carbonic anhydrase isoenzymes I and II inhibition profiles of taxifolin. J. Enzym. Inhib. Med. Chem. 2016, 31, 441–447. [Google Scholar] [CrossRef] [PubMed]
- Van der Zee, E.A.; Platt, B.; Riedel, G. Localization of pre- and postsynaptic cholinergic markers in rodent forebrain: A brief history and comparison of rat and mouse. Behav. Brain Res. 2011, 221, 583–586. [Google Scholar] [CrossRef] [PubMed]
- Achilles, K.; Schirmeister, T.; Otto, H.H. β-Lactam derivatives as enzyme inhibitors: 1-Peptidyl derivatives of 4-phenylazetidin-2-one as inhibitors of elastase and papain. Arch. Pharm. 2000, 333, 243–253. [Google Scholar] [CrossRef]
- Mollet, K. Transformation of trans-4-aryl-3-chloro-1-(2-chloroethyl)azetidin-2-ones into 3-aryl-2-(ethylamino)propan-1-ols via intermediate 1-(1-aryl-2-chloro-3-hydroxypropyl)aziridines and trans-2-aryl-3-(hydroxymethyl)aziridines. J. Org. Chem. 2010, 76, 264–269. [Google Scholar] [CrossRef] [PubMed]
- Zhao, L. Functionalization of the C–H Bond Adjacent to a Secondary Nitrogen Atom. Ph.D. Thesis, McGill University, Montréal, QC, Canada, August 2009. [Google Scholar]
- France, S.; Shah, M.H.; Weatherwax, A.; Wack, H.; Roth, J.P.; Lectka, T. Bifunctional Lewis acid-nucleophile-based asymmetric catalysis: Mechanistic evidence for imine activation working in tandem with chiral enolate formation in the synthesis of β-lactams. J. Am. Chem. Soc. 2005, 127, 1206–1215. [Google Scholar] [CrossRef] [PubMed]
- Shapiro, R.; Vallee, B.L. Interaction of human placental ribonuclease with placental ribonuclease inhibitor. Biochemistry 1991, 30, 2246–2255. [Google Scholar] [CrossRef] [PubMed]
- Supuran, C.T. Carbonic anhydrase inhibitors. Bioorg. Med. Chem. Lett. 2010, 20, 3467–3474. [Google Scholar] [CrossRef] [PubMed]
- Gülçin, I.; Beydemir, Ş.; Büyükokuroglu, M.E. In vitro and in vivo effects of dantrolene on carbonic anhydrase enzyme activities. Biol. Pharm. Bull. 2004, 27, 613–616. [Google Scholar] [CrossRef] [PubMed]
- Innocenti, A.; Maresca, A.; Scozzafava, A.; Supuran, C.T. Carbonic anhydrase inhibitors: thioxolone versus sulfonamides for obtaining isozyme-selective inhibitors? Bioorg. Med. Chem. Lett. 2008, 18, 3938–3941. [Google Scholar] [CrossRef] [PubMed]
- Hochster, R.M.; Kates, M.; Quaste, J.H. Metabolic Inhibitors: A Comprehensive Treatise; Academic Press: New York, NY, USA, 1973; Volume 4, pp. 66–82. [Google Scholar]
- Christensen, G.M.; Olson, D.; Riedel, B. Chemical effects on the activity of eight enzymes: A review and discussion relevant to environmental monitoring. Environ. Res. 1982, 29, 247–255. [Google Scholar] [CrossRef]
- Turan, B.; Sendil, K.; Sengul, E.; Gultekin, M.S.; Taslimi, P.; Gulcin, I.; Supuran, C.T. The synthesis of some β-lactams and investigation of their metal chelating activity, carbonic anhydrase and achetylcholinesterase inhibition profiles. J. Enzym. Inhib. Med. Chem. 2016. [Google Scholar] [CrossRef] [PubMed]
- Berber, N.; Arslan, M.; Bilen, C.; Sackes, Z.; Gencer, N.; Arslan, O. Synthesis and evaluation of new phthalazine substituted β-lactam derivatives as carbonic anhydrase inhibitors. Russ. J. Bioorg. Chem. 2015, 41, 414–420. [Google Scholar] [CrossRef]
- Şişecioğlu, M.; Gülçin, I.; Çankaya, M.; Özdemir, H. The inhibitory effects of L-Adrenaline on lactoperoxidase enzyme (LPO) purified from buffalo milk. Int. J. Food Propert. 2012, 15, 1182–1189. [Google Scholar] [CrossRef]
- Polat Kose, L.; Gülçin, I.; Özdemir, H.; Atasever, A.; Alwasel, S.H.; Supuran, C.T. The effects of some avermectins on bovine carbonic anhydrase enzyme. J. Enzym. Inhib. Med. Chem. 2016, 31, 773–778. [Google Scholar] [CrossRef] [PubMed]
- Lineweaver, H.; Burk, D. The determination of enzyme dissociation constants. J. Am. Chem. Soc. 1934, 56, 658–666. [Google Scholar] [CrossRef]
- Atasever, A.; Özdemir, H.; Gülçin, I.; Küfrevioğlu, Ö.İ. One-step purification of lactoperoxidase from bovine milk by affinity chromatography. Food Chem. 2013, 136, 864–870. [Google Scholar] [CrossRef] [PubMed]
- Öztürk Sarıkaya, S.B.; Gülçin, I.; Supuran, C.T. Carbonic anhydrase inhibitors. Inhibition of human erythrocyte isozymes I and II with a series of phenolic acids. Chem. Biol. Drug Des. 2010, 75, 515–520. [Google Scholar] [CrossRef] [PubMed]
- Şentürk, M.; Gülçin, I.; Daştan, A.; Küfrevioğlu, Ö.İ.; Supuran, C.T. Carbonic anhydrase inhibitors. Inhibition of human erythrocyte isozymes I and II with a series of antioxidant phenols. Bioorg. Med. Chem. 2009, 17, 3207–3211. [Google Scholar] [CrossRef] [PubMed]
- Çoban, T.A.; Beydemir, Ş.; Gülçin, I.; Ekinci, D. The inhibitory effect of ethanol on carbonic anhydrase isoenzymes: In vivo and in vitro studies. J. Enzym. Inhib. Med. Chem. 2008, 23, 266–270. [Google Scholar] [CrossRef] [PubMed]
- Çoban, T.A.; Beydemir, Ş.; Gülçin, I.; Ekinci, D. Morphine inhibits erythrocyte carbonic anhydrase in vitro and in vivo. Biol. Pharm. Bull. 2007, 30, 2257–2261. [Google Scholar] [CrossRef] [PubMed]
- Hisar, O.; Beydemir, Ş.; Gülçin, I.; ArasHisar, Ş.; Yanık, T.; Küfrevioğlu, Ö.İ. The effect of melatonin hormone on carbonic anhydrase enzyme activity in rainbow trout (Oncorhynchus mykiss) erythrocytes in vitro and in vivo. Turk. J. Vet. Anim. Sci. 2005, 29, 841–845. [Google Scholar]
- Bradford, M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
- Gülçin, I.; Küfrevioğlu, Ö.İ.; Oktay, M. Purification and characterization of polyphenol oxidase from nettle (Urtica dioica L.) and inhibition effects of some chemicals on the enzyme activity. J. Enzym. Inhib. Med. Chem. 2005, 20, 297–302. [Google Scholar] [CrossRef] [PubMed]
- Köksal, E.; Gülçin, İ. Purification and characterization of peroxidase from cauliflower (Brassica oleracea L.) buds. Protein Pept. Lett. 2008, 15, 320–326. [Google Scholar] [CrossRef] [PubMed]
- Şentürk, M.; Gülçin, I.; Çiftci, M.; Küfrevioğlu, Ö.İ. Dantrolene inhibits human erythrocyte glutathione reductase. Biol. Pharmacol. Bull. 2008, 31, 2036–2039. [Google Scholar] [CrossRef]
- Laemmli, D.K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 1970, 227, 680–683. [Google Scholar] [CrossRef] [PubMed]
- Gülçin, I.; Beydemir, Ş.; Çoban, T.A.; Ekinci, D. The inhibitory effect of dantrolene sodium and propofol on 6-phosphogluconate dehydrogenase from rat erythrocyte. Fresenius Environ. Bull. 2008, 17, 1283–1287. [Google Scholar]
- Şişecioğlu, M.; Çankaya, M.; Gülçin, I.; Özdemir, M. The Inhibitory effect of propofol on lactoperoxidase. Protein Pept. Lett. 2009, 16, 46–49. [Google Scholar] [CrossRef] [PubMed]
- Şişecioğlu, M.; Çankaya, M.; Gülçin, I.; Özdemir, H. Interactions of melatonin and serotonin to lactoperoxidase enzyme. J. Enzym. Inhib. Med. Chem. 2010, 25, 779–783. [Google Scholar] [CrossRef] [PubMed]
- Verpoorte, J.A.; Mehta, S.; Edsall, J.T. Esterase activities of human carbonic anhydrases B and C. J. Biol. Chem. 1967, 242, 4221–4229. [Google Scholar] [PubMed]
- Şişecioğlu, M.; Gülçin, I.; Çankaya, M.; Atasever, A.; Şehitoğlu, M.H.; Budak Kaya, H.; Özdemir, H. Purification and characterization of peroxidase from Turkish black radish (Raphanus sativus L.). J. Med. Plants Res. 2010, 4, 1187–1196. [Google Scholar]
- Köksal, E.; Ağgül, A.G.; Bursal, E.; Gülçin, I. Purification and characterization of peroxidase from sweet gourd (Cucurbita Moschata Lam. Poiret). Int. J. Food Propert. 2012, 15, 1110–1119. [Google Scholar] [CrossRef]
- Şişecioğlu, M.; Kireçci, E.; Çankaya, M.; Özdemir, H.; Gülçin, I.; Atasever, A. The prohibitive effect of lactoperoxidase system (LPS) on some pathogen fungi and bacteria. Afr. J. Pharm. Pharmacol. 2010, 4, 671–677. [Google Scholar]
- Ellman, G.L.; Courtney, K.D.; Andres, V.; Featherston, R.M. A new and rapid colorimetric determination of acetylcholinesterase activity. Biochem. Pharmacol. 1961, 7, 88–95. [Google Scholar] [CrossRef]
- Gül, H.İ.; Kucukoglu, K.; Yamali, C.; Bilginer, S.; Yuca, H.; Ozturk, İ.; Taslimi, P.; Gülçin, I.; Supuran, C.T. Synthesis of 4-(2-substitutedhydrazinyl)benzenesulfonamides and their carbonic anhydrase inhibitory effects. J. Enzym. Inhib. Med. Chem. 2016, 31, 568–573. [Google Scholar]
- Goksu, H.; Topal, M.; Keskin, A.; Gultekin, M.S.; Çelik, M.; Gülçin, I.; Tanc, M.; Supuran, C.T. 9,10-Dibromo-N-aryl-9,10-dihydro-9,10-[3,4]epipyrroloanthracene-12,14-diones: Synthesis and investigation of their effects on carbonic anhydrase isozymes I, II, IX, and XII. Arch. Pharm. 2016, 349, 466–474. [Google Scholar] [CrossRef] [PubMed]
| Purification Steps | Volume (mL) | Total Enzyme Activity (EU) | Total Protein (mg) | Specific Activity (EU/mg) | Yield (%) | Purification Fold | |
|---|---|---|---|---|---|---|---|
| Hemolysate | 50 | 6300 | 700 | 9.0 | 100 | 1 | |
| Sepharose-4B-l-tyrosine-sulphanilamide affinity chromatography | hCA I | 10 | 4030 | 3.5 | 1151.4 | 63.9 | 127.9 |
| hCA II | 5 | 3550 | 0.50 | 7100.0 | 56.4 | 788.9 | |
| Compounds | IC50 (nM) | Ki (nM) | |||||||
|---|---|---|---|---|---|---|---|---|---|
| hCA I | R2 | hCA II | R2 | AChE | R2 | hCA I | hCA II | AChE | |
| 2a | 0.612 | 0.9578 | 5.212 | 0.9099 | 0.885 | 0.9960 | 0.44 ± 0.115 | 3.54 ± 0.405 | 1.13 ± 0.472 |
| 2b | 2.303 | 0.9906 | 6.418 | 0.9009 | 0.913 | 0.9868 | 1.41 ± 0.547 | 3.15 ± 1.139 | 0.39 ± 0.069 |
| 2c | 2.107 | 0.9400 | 3.707 | 0.9012 | 0.783 | 0.9913 | 1.50 ± 0.657 | 2.96 ± 0.157 | 0.55 ± 0.136 |
| 2d | 0.231 | 0.9704 | 4.176 | 0.9029 | 0.634 | 0.9926 | 1.49 ± 0.290 | 3.26 ± 0.708 | 0.77 ± 0.041 |
| 2e | 3.984 | 0.9107 | 5.825 | 0.9136 | 0.668 | 0.9724 | 6.29 ± 2.068 | 8.34 ± 3.530 | 0.42 ± 0.020 |
| 2f | 1.627 | 0.9936 | 5.975 | 0.9230 | 0.602 | 0.9791 | 1.16 ± 0.514 | 1.50 ± 0.421 | 0.46 ± 0.045 |
| 2g | 0.652 | 0.9000 | 3.809 | 0.9295 | 0.734 | 0.9803 | 0.35 ± 0.105 | 3.39 ± 1.158 | 0.44 ± 0.057 |
| 2h | 2.646 | 0.9264 | 5.212 | 0.9662 | 0.450 | 0.9947 | 0.91 ± 0.143 | 2.19 ± 0.921 | 0.25 ± 0.019 |
| 2ı | 2.548 | 0.9359 | 4.030 | 0.9604 | 0.705 | 0.9984 | 0.97 ± 0.245 | 0.93 ± 0.295 | 0.36 ± 0.045 |
| 2j | 4.814 | 0.9284 | 5.023 | 0.9057 | 0.704 | 0.9746 | 1.09 ± 0.136 | 2.88 ± 1.168 | 0.56 ± 0.073 |
| 2k | 2.502 | 0.9886 | 6.863 | 0.9132 | 0.859 | 0.9939 | 0.94 ± 0.430 | 1.34 ± 0.539 | 0.68 ± 0.117 |
| AZA Ψ,* | 101.19 | 0.9509 | 113.75 | 0.9791 | - | - | 170.34 ± 2.48 | 115.43 ± 1.63 | - |
| TAC ⌘ | - | - | - | - | 4.101 | 0.9951 | - | - | 3.90 ± 0.792 |
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Genç, H.; Kalin, R.; Köksal, Z.; Sadeghian, N.; Kocyigit, U.M.; Zengin, M.; Gülçin, İ.; Özdemir, H. Discovery of Potent Carbonic Anhydrase and Acetylcholinesterase Inhibitors: 2-Aminoindan β-Lactam Derivatives. Int. J. Mol. Sci. 2016, 17, 1736. https://doi.org/10.3390/ijms17101736
Genç H, Kalin R, Köksal Z, Sadeghian N, Kocyigit UM, Zengin M, Gülçin İ, Özdemir H. Discovery of Potent Carbonic Anhydrase and Acetylcholinesterase Inhibitors: 2-Aminoindan β-Lactam Derivatives. International Journal of Molecular Sciences. 2016; 17(10):1736. https://doi.org/10.3390/ijms17101736
Chicago/Turabian StyleGenç, Hayriye, Ramazan Kalin, Zeynep Köksal, Nastaran Sadeghian, Umit M. Kocyigit, Mustafa Zengin, İlhami Gülçin, and Hasan Özdemir. 2016. "Discovery of Potent Carbonic Anhydrase and Acetylcholinesterase Inhibitors: 2-Aminoindan β-Lactam Derivatives" International Journal of Molecular Sciences 17, no. 10: 1736. https://doi.org/10.3390/ijms17101736
APA StyleGenç, H., Kalin, R., Köksal, Z., Sadeghian, N., Kocyigit, U. M., Zengin, M., Gülçin, İ., & Özdemir, H. (2016). Discovery of Potent Carbonic Anhydrase and Acetylcholinesterase Inhibitors: 2-Aminoindan β-Lactam Derivatives. International Journal of Molecular Sciences, 17(10), 1736. https://doi.org/10.3390/ijms17101736







