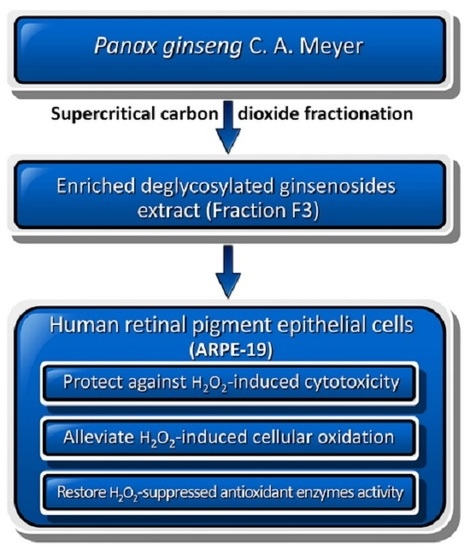
Panax ginseng Fraction F3 Extracted by Supercritical Carbon Dioxide Protects against Oxidative Stress in ARPE-19 Cells
Abstract
:1. Introduction
2. Results
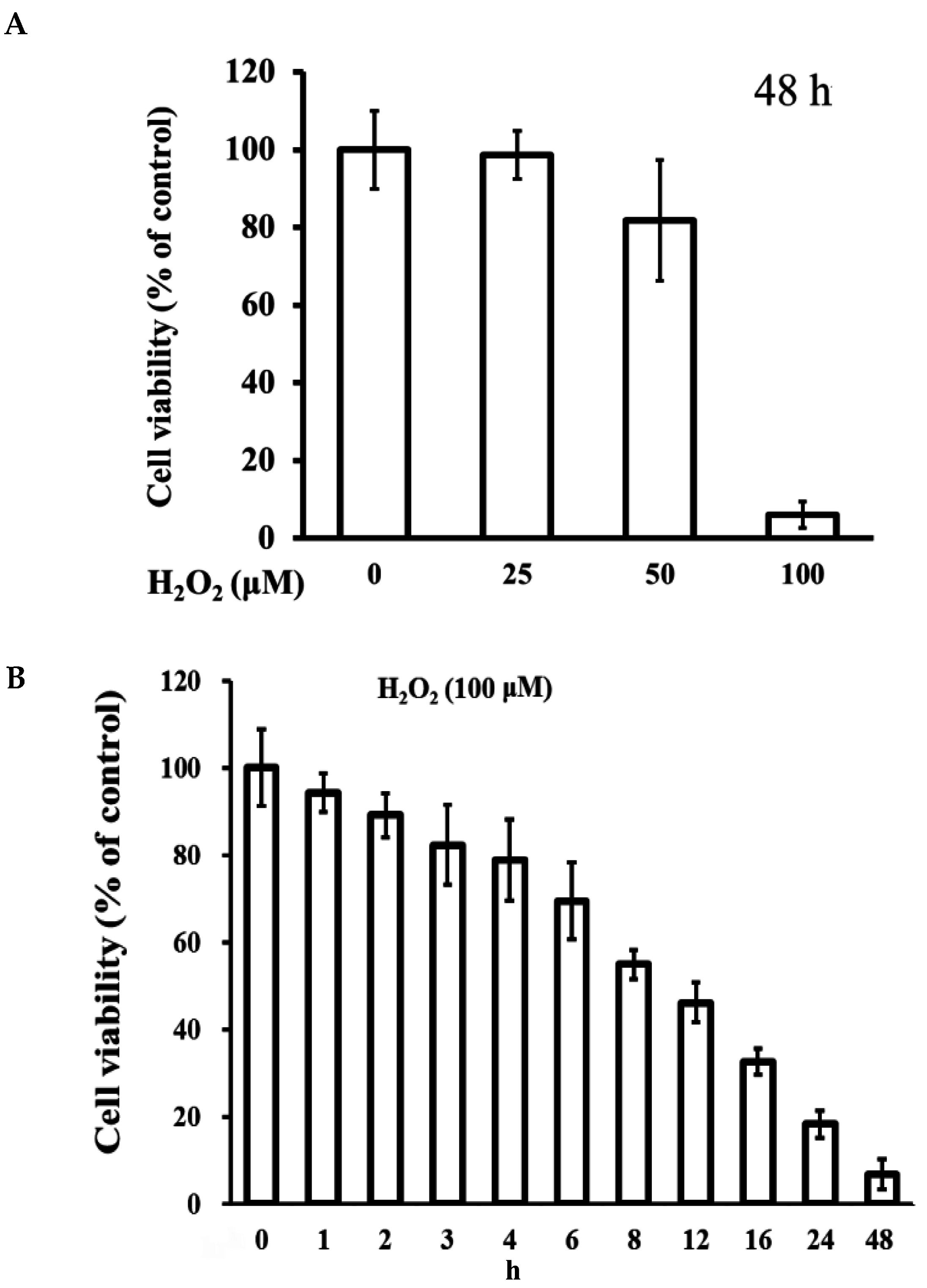
2.1. Cytotoxicity of H2O2 on ARPE-19 Cells
2.2. Effects of P. ginseng Supercritical CO2 Fractions on Cell Viability of H2O2-Induced Cytotoxicity in ARPE-19 Cells
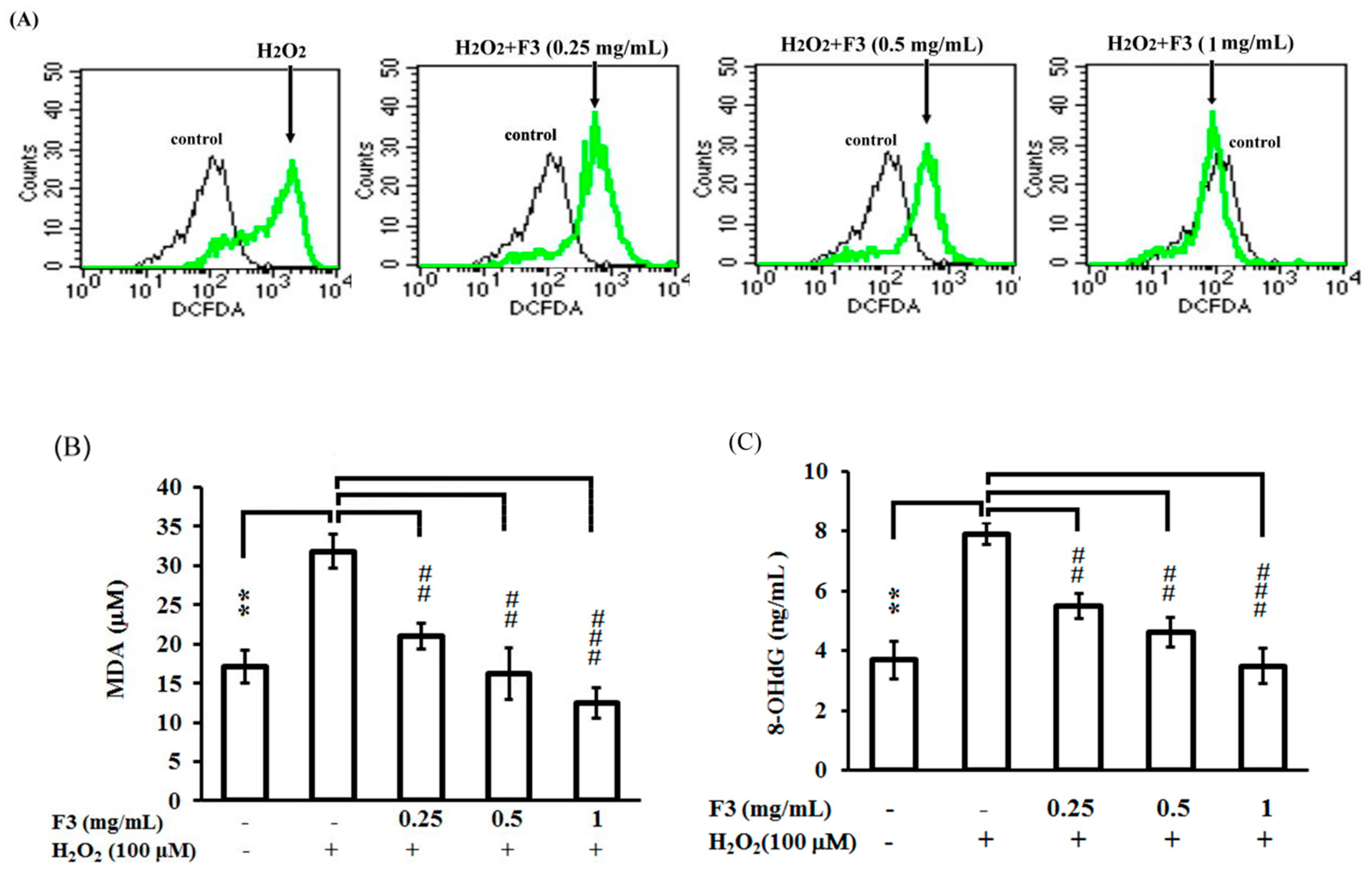
2.3. Effects of P. ginseng F3 Fraction on Cellular Oxidation Induced by H2O2 in ARPE-19 Cells
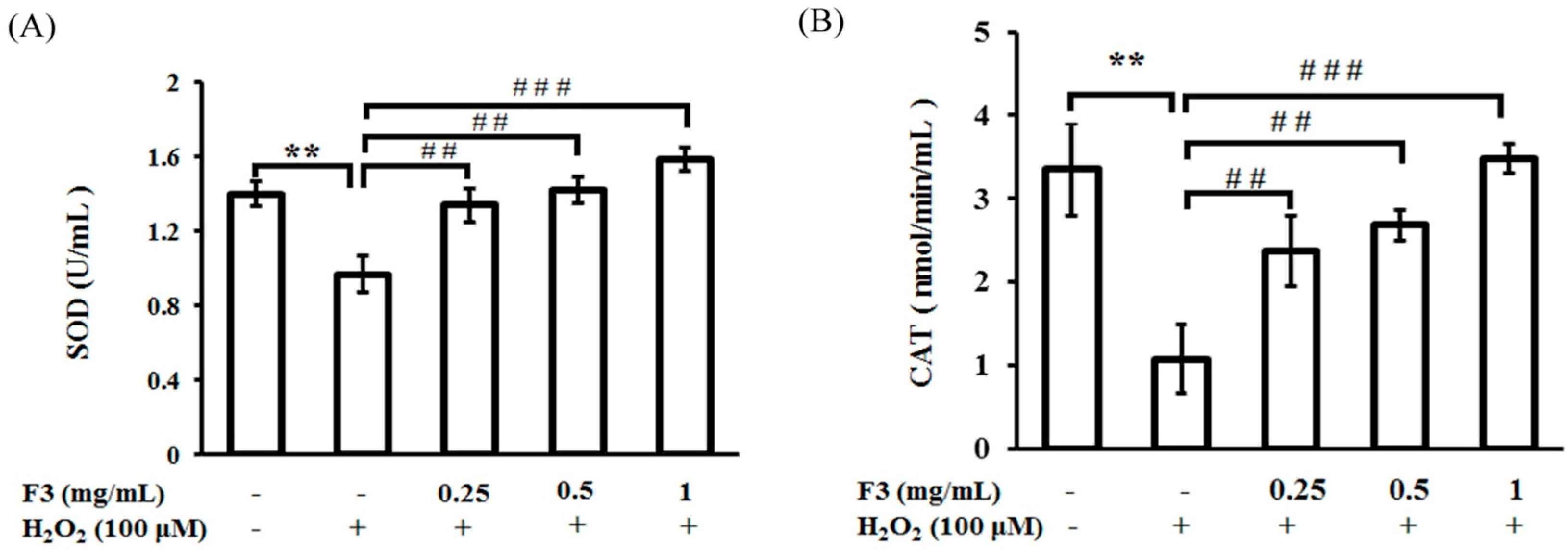
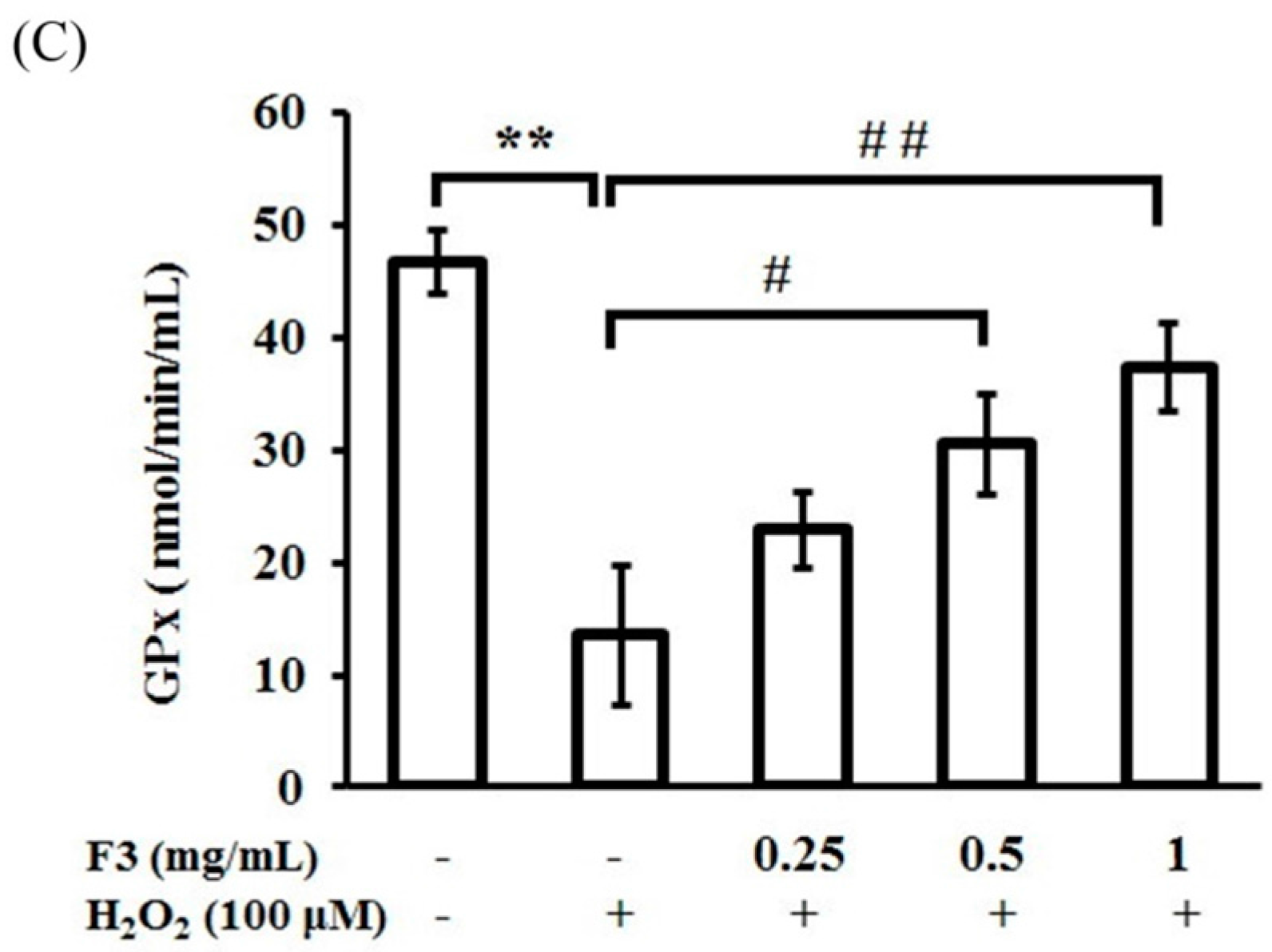
2.4. Effects of P. ginseng F3 Fraction on Antioxidant Enzymes Activities Induced by H2O2 in ARPE-19 Cells
3. Discussion
4. Materials and Methods
4.1. Materials
4.2. Extraction and Fractionation
4.3. Cell Culture and Viability Assay
4.4. Hoechst 33342 Staining
4.5. Measurement of Intracellular Reactive Oxygen Species
4.6. Determination of Superoxide Dismutase, Catalase, and Glutathione Peroxidase Activities
4.7. Statistical Analysis
5. Conclusions
Author Contributions
Conflicts of Interest
Abbreviations
| 8-OHdG | 8-hydroxydeoxyguanosine |
| ARPE-19 | adult retinal pigment epithelium-19 |
| CAT | catalase |
| CO2 | carbon dioxide |
| H2DCFDA | 2′,7′-dichlorodihydrofluorescein diacetate |
| DNA | deoxyribonucleic acid |
| ELISA | enzyme-linked immunosorbent assay |
| GPx | glutathione peroxidase |
| H2O2 | hydrogen peroxide |
| MDA | malondialdehyde |
| ROS | reactive oxygen species |
| SD | standard deviation |
| SOD | superoxide dismutase |
| TBA | thiobarbituric acid |
| TBARS | thiobarbituric acid reactive substances |
References
- Nowak, J.Z. Age-related macular degeneration (AMD): Pathogenesis and therapy. Pharmacol. Rep. 2006, 58, 353–363. [Google Scholar] [PubMed]
- Wang, C.Z.; Anderson, S.; Du, W.; He, T.C.; Yuan, C.S. Red ginseng and cancer treatment. Chin. J. Nat. Med. 2016, 14, 7–16. [Google Scholar] [PubMed]
- Jeon, W.J.; Oh, J.S.; Park, M.S.; Ji, G.E. Anti-hyperglycemic effect of fermented ginseng in type 2 diabetes mellitus mouse model. Phytother. Res. 2013, 27, 166–172. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Ichikawa, T.; Jin, Y.; Hofseth, L.J.; Nagarkatti, P.; Nagarkatti, M.; Windust, A.; Cui, T. An essential role of Nrf2 in American ginseng-mediated anti-oxidative actions in cardiomyocytes. J. Ethnopharmacol. 2010, 130, 222–230. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Xia, Z.Y.; Dou, J.; Zhang, L.; Xu, J.J.; Zhao, B.; Lei, S.; Liu, H.M. Protective effect of ginsenoside Rb1 against myocardial ischemia/reperfusion injury in streptozotocin-induced diabetic rats. Mol. Biol. Rep. 2011, 38, 4327–4335. [Google Scholar] [CrossRef] [PubMed]
- Kitts, D.D.; Wijewickreme, A.N.; Hu, C. Antioxidant properties of a North American ginseng extract. Mol. Cell. Biochem. 2000, 203, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Qi, L.W.; Wang, C.Z.; Yuan, C.S. American ginseng: Potential structure-function relationship in cancer chemoprevention. Biochem. Pharmacol. 2010, 80, 947–954. [Google Scholar] [CrossRef] [PubMed]
- Park, H.M.; Kim, S.J.; Kim, J.S.; Kang, H.S. Reactive oxygen species mediated ginsenoside Rg3- and Rh2-induced apoptosis in hepatoma cells through mitochondrial signaling pathways. Food Chem. Toxicol. 2012, 50, 2736–2741. [Google Scholar] [CrossRef] [PubMed]
- Kim, B.H.; Lee, Y.G.; Park, T.Y.; Kim, H.B.; Rhee, M.H.; Cho, J.Y. Ginsenoside Rp1, a ginsenoside derivative, blocks lipopolysaccharide-induced interleukin-1β production via suppression of the NF-κB pathway. Planta Med. 2009, 75, 321–326. [Google Scholar] [CrossRef] [PubMed]
- Leung, K.W.; Wong, A.S. Pharmacology of ginsenosides: A literature review. Chin. Med. 2010, 5. [Google Scholar] [CrossRef] [PubMed]
- Rozanowska, M.B. Light-induced damage to the retina: Current understanding of the mechanisms and unresolved questions: A symposium-in-print. Photochem. Photobiol. 2012, 88, 1303–1308. [Google Scholar] [CrossRef] [PubMed]
- Odani, T.; Tanizawa, H.; Takino, Y. Studies on the absorption, distribution, excretion and metabolism of ginseng saponins. IV. Decomposition of ginsenoside-Rg1 and -Rb1 in the digestive tract of rats. Chem. Pharm. Bull. 1983, 31, 3691–3697. [Google Scholar] [CrossRef] [PubMed]
- Bae, E.A.; Kim, E.J.; Park, J.S.; Kim, H.S.; Ryu, J.H.; Kim, D.H. Ginsenosides Rg3 and Rh2 inhibit the activation of AP-1 and protein kinase A pathway in lipopolysaccharide/interferon-γ-stimulated BV-2 microglial cells. Planta Med. 2006, 72, 627–633. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Li, Z.; Zhou, Z.; Yang, H.; Zhong, Z.; Lou, C. Antidepressant-like effects of ginsenosides: A comparison of ginsenoside Rb3 and its four deglycosylated derivatives, Rg3, Rh2, compound K, and 20(S)-protopanaxadiol in mice models of despair. Pharmacol. Biochem. Behav. 2016, 140, 17–26. [Google Scholar] [CrossRef] [PubMed]
- Xu, F.Y.; Shang, W.Q.; Yu, J.J.; Sun, Q.; Li, M.Q.; Sun, J.S. The antitumor activity study of ginsenosides and metabolites in lung cancer cell. Am. J. Transl. Res. 2016, 8, 1708–1718. [Google Scholar] [PubMed]
- Bailey, T.A.; Kanuga, N.; Romero, I.A.; Greenwood, J.; Luthert, P.J.; Cheetham, M.E. Oxidative stress affects the junctional integrity of retinal pigment epithelial cells. Investig. Ophthalmol. Vis. Sci. 2004, 45, 675–684. [Google Scholar] [CrossRef]
- Kauppinen, A.; Niskanen, H.; Suuronen, T.; Kinnunen, K.; Salminen, A.; Kaarniranta, K. Oxidative stress activates NLRP3 inflammasomes in ARPE-19 cells—Implications for age-related macular degeneration (AMD). Immunol. Lett. 2012, 147, 29–33. [Google Scholar] [CrossRef] [PubMed]
- Beatty, S.; Koh, H.; Phil, M.; Henson, D.; Boulton, M. The role of oxidative stress in the pathogenesis of age-related macular degeneration. Surv. Ophthalmol. 2000, 45, 115–134. [Google Scholar] [CrossRef]
- Choi, H.S.; Kim, K.H.; Sohn, E.; Park, J.D.; Kim, B.O.; Moon, E.Y.; Rhee, D.K.; Pyo, S. Red ginseng acidic polysaccharide (RGAP) in combination with IFN-gamma results in enhanced macrophage function through activation of the NF-κB pathway. Biosci. Biotechnol. Biochem. 2008, 72, 1817–1825. [Google Scholar] [CrossRef] [PubMed]
- Chien, Y.S.; Yu, Z.R.; Koo, M.; Wang, B.J. Supercritical fluid extractive fractionation: Study of the antioxidant activities of Panax ginseng. Sep. Sci. Technol. 2016, 51, 954–960. [Google Scholar] [CrossRef]
- Woo, J.M.; Shin, D.Y.; Lee, S.J.; Joe, Y.; Zheng, M.; Yim, J.H.; Callaway, Z.; Chung, H.T. Curcumin protects retinal pigment epithelial cells against oxidative stress via induction of heme oxygenase-1 expression and reduction of reactive oxygen. Mol. Vis. 2012, 18, 901–908. [Google Scholar] [PubMed]
- Wankun, X.; Wenzhen, Y.; Min, Z.; Weiyan, Z.; Huan, C.; Wei, D.; Lvzhen, H.; Xu, Y.; Xiaoxin, L. Protective effect of paeoniflorin against oxidative stress in human retinal pigment epithelium in vitro. Mol. Vis. 2011, 17, 3512–3522. [Google Scholar] [PubMed]
- Li, Z.; Dong, X.; Liu, H.; Chen, X.; Shi, H.; Fan, Y.; Hou, D.; Zhang, X. Astaxanthin protects ARPE-19 cells from oxidative stress via upregulation of Nrf2-regulated phase II enzymes through activation of PI3K/Akt. Mol. Vis. 2013, 19, 1656–1666. [Google Scholar] [PubMed]
- Zhou, N.; Tang, Y.; Keep, R.F.; Ma, X.; Xiang, J. Antioxidative effects of Panax notoginseng saponins in brain cells. Phytomedicine 2014, 21, 1189–1195. [Google Scholar] [CrossRef] [PubMed]
- Betts, B.S.; Parvathaneni, K.; Yendluri, B.B.; Grigsby, J.; Tsin, A.T. Ginsenoside-Rb1 induces ARPE-19 proliferation and reduces VEGF release. ISRN Ophthalmol. 2011, 2011. [Google Scholar] [CrossRef] [PubMed]
- Li, K.R.; Zhang, Z.Q.; Yao, J.; Zhao, Y.X.; Duan, J.; Cao, C.; Jiang, Q. Ginsenoside Rg-1 protects retinal pigment epithelium (RPE) cells from cobalt chloride (CoCl2) and hypoxia assaults. PLoS ONE 2013, 8, e84171. [Google Scholar] [CrossRef] [PubMed]

© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, C.-C.; Chen, C.-Y.; Wu, C.-C.; Koo, M.; Yu, Z.-R.; Wang, B.-J. Panax ginseng Fraction F3 Extracted by Supercritical Carbon Dioxide Protects against Oxidative Stress in ARPE-19 Cells. Int. J. Mol. Sci. 2016, 17, 1717. https://doi.org/10.3390/ijms17101717
Yang C-C, Chen C-Y, Wu C-C, Koo M, Yu Z-R, Wang B-J. Panax ginseng Fraction F3 Extracted by Supercritical Carbon Dioxide Protects against Oxidative Stress in ARPE-19 Cells. International Journal of Molecular Sciences. 2016; 17(10):1717. https://doi.org/10.3390/ijms17101717
Chicago/Turabian StyleYang, Chao-Chin, Chiu-Yuan Chen, Chun-Chi Wu, Malcolm Koo, Zer-Ran Yu, and Be-Jen Wang. 2016. "Panax ginseng Fraction F3 Extracted by Supercritical Carbon Dioxide Protects against Oxidative Stress in ARPE-19 Cells" International Journal of Molecular Sciences 17, no. 10: 1717. https://doi.org/10.3390/ijms17101717
APA StyleYang, C.-C., Chen, C.-Y., Wu, C.-C., Koo, M., Yu, Z.-R., & Wang, B.-J. (2016). Panax ginseng Fraction F3 Extracted by Supercritical Carbon Dioxide Protects against Oxidative Stress in ARPE-19 Cells. International Journal of Molecular Sciences, 17(10), 1717. https://doi.org/10.3390/ijms17101717