Critical Issues in the Study of Magnesium Transport Systems and Magnesium Deficiency Symptoms in Plants
Abstract
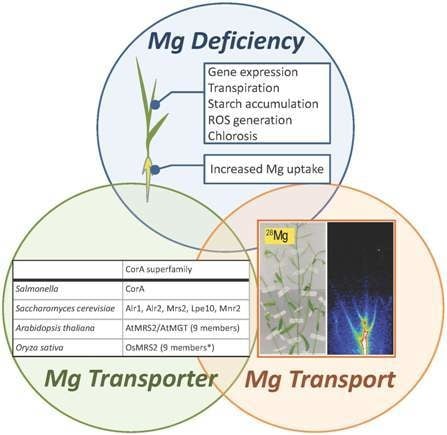
:1. Introduction
2. Physiological Features of Mg Deficiency
3. Mg2+ Transporters
3.1. Mg2+ Transporters in Microorganisms
3.2. Mg2+ Transporters in Plants: The Overview
3.3. Plant MRS2 Family Proteins
| Clade | Plant | Name (Number) | Transport Assay | Subcellular Localization | Reference | ||
|---|---|---|---|---|---|---|---|
| MRS2 | MGT | MM281 | CM66 | ||||
| A | Arabidopsis | 11 | 10 | ○ | ○ | Chloroplast | [67,69,71,72] |
| Oryza sativa | 6 | − | − | ○ | Chloroplast | [77] | |
| B | Arabidopsis | 1 | 2 | − | ○ | Vacuole | [29,66,67,71,72] |
| 5 | 3 | − | − | Vacuole | [29,66,67,72] | ||
| 10 | 1 | ○ | ○ | Plasma membrane | [66,67,71,75] | ||
| Oryza sativa | 1 | − | − | ○ | − | [77] | |
| 9 | − | − | ○ | − | [77] | ||
| C | Arabidopsis | 3 | 4 | − | − | − | [66,67,72] |
| Oryza sativa | 2 | 1 | − | × | Plasma membrane | [77,80] | |
| 3 | − | − | ○ | ER | [77] | ||
| 8 | − | − | × | − | [77] | ||
| D | Arabidopsis | 4 | 6 | ○ | − | Plasma membrane * | [30,66,67,72,76] |
| 6 | 5 | ○ | × | Mitochondria | [66,67,69,72] | ||
| Oryza sativa | 4 | − | − | × | − | [77] | |
| 5 | − | − | × | Chloroplast | [77] | ||
| E | Arabidopsis | 2 | 9 | ○ | × | − | [66,67,68,72] |
| 7 | 7 | ○ | − | ER | [30,66,67,70,72] | ||
| 8 | 8 | − | × | (pseudo gene) | [66,67] | ||
| 9 | − | − | − | (pseudo gene) | [66] | ||
| Oryza sativa | 7 | − | − | × | − | [77] | |
4. Mg2+ Uptake and Transport in Plants
5. Potential Regulation of Mg2+ Uptake and Transport under Mg Deficiency
6. Conclusions and Perspectives
Author Contributions
Conflicts of Interest
References
- Marschner, H. Mineral Nutrition of Higher Plants, 2nd ed.; Academic Press: San Diego, CA, USA, 1995; pp. 277–285. [Google Scholar]
- Gerendás, J.; Führs, H. The significance of magnesium for crop quality. Plant Soil 2013, 368, 101–128. [Google Scholar]
- Hermans, C.; Conn, S.J.; Chen, J.; Xiao, Q.; Verbruggen, N. An update on magnesium homeostasis mechanisms in plants. Metallomics 2013, 5, 1170–1183. [Google Scholar] [PubMed]
- Fischer, E.S.; Bremer, E. Influence of magnesium deficiency on rates of leaf expansion, starch and sucrose accumulation, and net assimilation in Phaseolus vulgaris. Physiol. Plant 1993, 89, 271–276. [Google Scholar] [CrossRef]
- Fischer, E.S.; Lohaus, G.; Heineke, D.; Heldt, H.W. Magnesium deficiency results in accumulation of carbohydrates and amino acids in source and sink leaves of spinach. Physiol. Plant 1998, 102, 16–20. [Google Scholar] [CrossRef]
- Hermans, C.; Johnson, G.N.; Strasser, R.J.; Verbruggen, N. Physiological characterisation of magnesium deficiency in sugar beet: Acclimation to low magnesium differentially affects photosystems I and II. Planta 2004, 220, 344–355. [Google Scholar] [CrossRef] [PubMed]
- Hermans, C.; Verbruggen, N. Physiological characterization of Mg deficiency in Arabidopsis thaliana. J. Exp. Bot. 2005, 56, 2153–2161. [Google Scholar] [CrossRef] [PubMed]
- Kobayashi, N.I.; Saito, T.; Iwata, N.; Ohmae, Y.; Iwata, R.; Tanoi, K.; Nakanishi, T.M. Leaf senescence in rice due to magnesium deficiency mediated defect in transpiration rate before sugar accumulation and chlorosis. Physiol. Plant 2013, 148, 490–501. [Google Scholar] [CrossRef] [PubMed]
- Ding, Y.; Luo, W.; Xu, G. Characterisation of magnesium nutrition and interaction of magnesium and potassium in rice. Ann. Appl. Biol. 2006, 149, 111–123. [Google Scholar] [CrossRef]
- Hermans, C.; Vuylsteke, M.; Coppens, F.; Cristescu, S.M.; Harren, F.J.M.; Inzé, D.; Verbruggen, N. Systems analysis of the responses to long-term magnesium deficiency and restoration in Arabidopsis thaliana. New Phytol. 2010, 187, 132–144. [Google Scholar] [CrossRef] [PubMed]
- Fischer, E.S. Photosynthetic irradiance response curves of Phaseolus vulgaris under moderate or severe magnesium deficiency. Photosynthetica. 1997. Available online: http://agris.fao.org/agris-search/search.do?recordID=CZ1997001001 (accessed on 11 September 2015).
- Sun, O.J.; Payn, T.W. Magnesium nutrition and photosynthesis in Pinus radiata: Clonal variation and influence of potassium. Tree Physiol. 1999, 19, 535–540. [Google Scholar] [CrossRef] [PubMed]
- Cakmak, I.; Hengeler, C.; Marschner, H. Partitioning of shoot and root dry matter and carbohydrates in bean plants suffering from phosphorus, potassium and magnesium deficiency. J. Exp. Bot. 1994, 45, 1245–1250. [Google Scholar] [CrossRef]
- Cakmak, I.; Hengeler, C.; Marschner, H. Changes in phloem export of sucrose in leaves in response to phosphorus, potassium and magnesium deficiency in bean plants. J. Exp. Bot. 1994, 45, 1251–1257. [Google Scholar] [CrossRef]
- Hermans, C.C.; Bourgis, F.F.; Faucher, M.M.; Strasser, R.J.R.; Delrot, S.S.; Verbruggen, N.N. Magnesium deficiency in sugar beets alters sugar partitioning and phloem loading in young mature leaves. Planta 2005, 220, 541–549. [Google Scholar] [CrossRef] [PubMed]
- Marschner, H.; Cakmak, I. High light intensity enhances chlorosis and necrosis in leaves of zinc, potassium, and magnesium deficient bean (Phaseolus vulgaris) plants. J. Plant Physiol. 1989, 134, 308–315. [Google Scholar] [CrossRef]
- Cakmak, I.; Yazici, A.M. Magnesium: A forgotten element in crop production. Better Crops 2010, 94, 23–25. [Google Scholar]
- Hermans, C.; Vuylsteke, M.; Coppens, F.; Craciun, A.; Inzé, D.; Verbruggen, N. Early transcriptomic changes induced by magnesium deficiency in Arabidopsis thaliana reveal the alteration of circadian clock gene expression in roots and the triggering of abscisic acid-responsive genes. New Phytol. 2010, 187, 119–131. [Google Scholar] [CrossRef] [PubMed]
- Jezek, M.; Geilfus, C.-M.; Bayer, A.; Mühling, K.-H. Photosynthetic capacity, nutrient status, and growth of maize (Zea mays L.) upon MgSO4 leaf-application. Front. Plant Sci. 2014, 5, 781. [Google Scholar] [CrossRef] [PubMed]
- Pei, Z.-M.; Murata, Y.; Benning, G.; Thomine, S.; Klüsener, B.; Allen, G.J.; Grill, E.; Schroeder, J.I. Calcium channels activated by hydrogen peroxide mediate abscisic acid signalling in guard cells. Nature 2000, 406, 731–734. [Google Scholar] [CrossRef] [PubMed]
- Tewari, R.K.; Kumar, P.; Sharma, P.N. Magnesium deficiency induced oxidative stress and antioxidant responses in mulberry plants. Sci. Hortic. 2006, 108, 7–14. [Google Scholar] [CrossRef]
- Cakmak, I.; Marschner, H. Magnesium deficiency and high light intensity enhance activities of superoxide dismutase, ascorbate peroxidase, and glutathione reductase in bean leaves. Plant Physiol. 1992, 98, 1222–1227. [Google Scholar] [CrossRef] [PubMed]
- Chou, T.-S.; Chao, Y.-Y.; Huang, W.-D.; Hong, C.-Y.; Kao, C.H. Effect of magnesium deficiency on antioxidant status and cadmium toxicity in rice seedlings. J. Plant Physiol. 2011, 168, 1021–1030. [Google Scholar] [CrossRef] [PubMed]
- Hermans, C.; Chen, J.; Coppens, F.; Inzé, D.; Verbruggen, N. Low magnesium status in plants enhances tolerance to cadmium exposure. New Phytol. 2011, 192, 428–436. [Google Scholar] [CrossRef] [PubMed]
- Yang, G.-H.; Yang, L.-T.; Jiang, H.-X.; Li, Y.; Wang, P.; Chen, L.-S. Physiological impacts of magnesium-deficiency in Citrus seedlings: Photosynthesis, antioxidant system and carbohydrates. Trees 2012, 26, 1237–1250. [Google Scholar] [CrossRef]
- Wang, H.; Ma, F.; Cheng, L. Metabolism of organic acids, nitrogen and amino acids in chlorotic leaves of “Honeycrisp” apple (Malus domestica Borkh) with excessive accumulation of carbohydrates. Planta 2010, 232, 511–522. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.T.; Yang, G.H.; You, X.; Zhou, C.P.; Lu, Y.B.; Chen, L.S. Magnesium deficiency-induced changes in organic acid metabolism of Citrus sinensis roots and leaves. Biol. Plant 2013, 57, 481–486. [Google Scholar] [CrossRef]
- Li, Z.; Philip, D.; Neuhaeuser, B.; Schulze, W.X.; Ludewig, U. Protein dynamics in young maize root hairs in response to macro- and micro-nutrient deprivation. J. Proteome Res. 2015, 14, 3362–3371. [Google Scholar] [CrossRef] [PubMed]
- Conn, S.J.; Conn, V.; Tyerman, S.D.; Kaiser, B.N.; Leigh, R.A.; Gilliham, M. Magnesium transporters, MGT2/MRS2-1 and MGT3/MRS2-5, are important for magnesium partitioning within Arabidopsis thaliana mesophyll vacuoles. New Phytol. 2011, 190, 583–594. [Google Scholar] [CrossRef] [PubMed]
- Kamiya, T.; Yamagami, M.; Hirai, M.Y.; Fujiwara, T. Establishment of an in planta magnesium monitoring system using CAX3 promoter-luciferase in Arabidopsis. J. Exp. Bot. 2012, 63, 355–363. [Google Scholar] [CrossRef] [PubMed]
- Lenz, H.; Dombinov, V.; Dreistein, J.; Reinhard, M.R.; Gebert, M.; Knoop, V. Magnesium deficiency phenotypes upon multiple knockout of Arabidopsis thaliana MRS2 Clade B genes can be ameliorated by concomitantly reduced calcium supply. Plant Cell Physiol. 2013, 54, 1118–1131. [Google Scholar] [CrossRef] [PubMed]
- Hmiel, S.P.; Snavely, M.D.; Florer, J.B.; Maguire, M.E.; Miller, C.G. Magnesium transport in Salmonella typhimurium: Genetic characterization and cloning of three magnesium transport loci. J. Bacteriol. 1989, 171, 4742–4751. [Google Scholar] [PubMed]
- Eshaghi, S.; Niegowski, D.; Kohl, A.; Martinez Molina, D.; Lesley, S.A.; Nordlund, P. Crystal structure of a divalent metal ion transporter CorA at 2.9 angstrom resolution. Science 2006, 313, 354–357. [Google Scholar] [CrossRef]
- Maguire, M.E. MgtA and MgtB: Prokaryotic P-type ATPases that mediate Mg2+ influx. J. Bioenerg. Biomembr. 1992, 24, 319–328. [Google Scholar] [PubMed]
- Tao, T.; Snavely, M.D.; Farr, S.G.; Maguire, M.E. Magnesium transport in Salmonella typhimurium: mgtA encodes a P-type ATPase and is regulated by Mg2+ in a manner similar to that of the mgtB P-type ATPase. J. Bacteriol. 1995, 177, 2654–2662. [Google Scholar] [PubMed]
- Snavely, M.D.; Miller, C.G.; Maguire, M.E. The mgtB Mg2+ transport locus of Salmonella typhimurium encodes a P-type ATPase. J. Biol. Chem. 1991, 266, 815–823. [Google Scholar] [PubMed]
- Snavely, M.D.; Florer, J.B.; Miller, C.G.; Maguire, M.E. Magnesium transport in Salmonella typhimurium: Expression of cloned genes for three distinct Mg2+ transport systems. J. Bacteriol. 1989, 171, 4752–4760. [Google Scholar] [PubMed]
- Smith, R.L.; Thompson, L.J.; Maguire, M.E. Cloning and characterization of MgtE, a putative new class of Mg2+ transporter from Bacillus firmus OF4. J. Bacteriol. 1995, 177, 1233–1238. [Google Scholar]
- Chamnongpol, S.; Groisman, E.A. Mg2+ homeostasis and avoidance of metal toxicity. Mol. Microbiol. 2002, 44, 561–571. [Google Scholar] [CrossRef] [PubMed]
- Dann, C.E.; Wakeman, C.A.; Sieling, C.L.; Baker, S.C.; Irnov, I.; Winkler, W.C. Structure and mechanism of a metal-sensing regulatory RNA. Cell 2007, 130, 878–892. [Google Scholar] [CrossRef] [PubMed]
- Wabakken, T.; Rian, E.; Kveine, M.; Aasheim, H.-C. The human solute carrier SLC41A1 belongs to a novel eukaryotic subfamily with homology to prokaryotic MgtE Mg2+ transporters. Biochem. Biophys. Res. Commun. 2003, 306, 718–724. [Google Scholar] [CrossRef]
- Snavely, M.D.; Florer, J.B.; Miller, C.G.; Maguire, M.E. Magnesium transport in Salmonella typhimurium: 28Mg2+ transport by the CorA, MgtA, and MgtB systems. J. Bacteriol. 1989, 171, 4761–4766. [Google Scholar] [PubMed]
- MacDiarmid, C.W.; Gardner, R.C. Overexpression of the Saccharomyces cerevisiae magnesium transport system confers resistance to aluminum ion. J. Biol. Chem. 1998, 273, 1727–1732. [Google Scholar] [CrossRef] [PubMed]
- Bui, D.M.; Gregan, J.; Jarosch, E.; Ragnini, A.; Schweyen, R.J. The bacterial magnesium transporter CorA can functionally substitute for its putative homologue Mrs2p in the yeast inner mitochondrial membrane. J. Biol. Chem. 1999, 274, 20438–20443. [Google Scholar] [CrossRef] [PubMed]
- Pisat, N.P.; Pandey, A.; MacDiarmid, C.W. MNR2 regulates intracellular magnesium storage in Saccharomyces cerevisiae. Genetics 2009, 183, 873–884. [Google Scholar] [CrossRef] [PubMed]
- Knoop, V.; Groth-Malonek, M.; Gebert, M.; Eifler, K.; Weyand, K. Transport of magnesium and other divalent cations: Evolution of the 2-TM-GxN proteins in the MIT superfamily. Mol. Genet. Genom. 2005, 274, 205–216. [Google Scholar] [CrossRef] [PubMed]
- Szegedy, M.A.M.; Maguire, M.E.M. The CorA Mg2+ transport protein of Salmonella typhimurium. Mutagenesis of conserved residues in the second membrane domain. J. Biol. Chem. 1999, 274, 36973–36979. [Google Scholar] [CrossRef]
- Sponder, G.; Svidovà, S.; Khan, M.B.; Kolisek, M.; Schweyen, R.J.; Carugo, O.; Djinović-Carugo, K. The G-M-N motif determines ion selectivity in the yeast magnesium channel Mrs2p. Metallomics 2013, 5, 745–752. [Google Scholar] [CrossRef] [PubMed]
- Dalmas, O.; Sandtner, W.; Medovoy, D.; Frezza, L.; Bezanilla, F.; Perozo, E. A repulsion mechanism explains magnesium permeation and selectivity in CorA. Proc. Natl. Acad. Sci. USA 2014, 111, 3002–3007. [Google Scholar] [CrossRef]
- Dalmas, O.; Sompornpisut, P.; Bezanilla, F.; Perozo, E. Molecular mechanism of Mg2+-dependent gating in CorA. Nat. Commun. 2014, 5. [Google Scholar] [CrossRef] [PubMed]
- Guskov, A.; Nordin, N.; Reynaud, A.; Engman, H.; Lundbäck, A.-K.; Jong, A.J.O.; Cornvik, T.; Phua, T.; Eshaghi, S. Structural insights into the mechanisms of Mg2+ uptake, transport, and gating by CorA. Proc. Natl. Acad. Sci. USA 2012, 109, 18459–18464. [Google Scholar] [CrossRef] [PubMed]
- Pfoh, R.; Li, A.; Chakrabarti, N.; Payandeh, J.; Pomès, R.; Pai, E.F. Structural asymmetry in the magnesium channel CorA points to sequential allosteric regulation. Proc. Natl. Acad. Sci. USA 2012, 109, 18809–18814. [Google Scholar] [CrossRef] [PubMed]
- Neale, C.; Chakrabarti, N.; Pomorski, P.; Pai, E.F.; Pomès, R. Hydrophobic gating of ion permeation in magnesium channel CorA. PLoS Comput. Biol. 2015, 11, e1004303. [Google Scholar] [CrossRef] [PubMed]
- Hattori, M.; Tanaka, Y.; Fukai, S.; Ishitani, R.; Nureki, O. Crystal structure of the MgtE Mg2+ transporter. Nature 2007, 448, 1072–1075. [Google Scholar] [CrossRef] [PubMed]
- Hattori, M.; Iwase, N.; Furuya, N.; Tanaka, Y.; Tsukazaki, T.; Ishitani, R.; Maguire, M.E.; Ito, K.; Maturana, A.; Nureki, O. Mg2+-dependent gating of bacterial MgtE channel underlies Mg2+ homeostasis. EMBO J. 2009, 28, 3602–3612. [Google Scholar] [CrossRef] [PubMed]
- Graschopf, A.; Stadler, J.A.; Hoellerer, M.K.; Eder, S.; Sieghardt, M.; Kohlwein, S.D.; Schweyen, R.J. The yeast plasma membrane protein Alr1 controls Mg2+ homeostasis and is subject to Mg2+-dependent control of its synthesis and degradation. J. Biol. Chem. 2001, 276, 16216–16222. [Google Scholar] [CrossRef] [PubMed]
- Lim, P.H.; Pisat, N.P.; Gadhia, N.; Pandey, A.; Donovan, F.X.; Stein, L.; Salt, D.E.; Eide, D.J.; MacDiarmid, C.W. Regulation of Alr1 Mg transporter activity by intracellular magnesium. PLoS ONE 2011, 6, e20896. [Google Scholar] [CrossRef] [PubMed]
- Horie, T.; Brodsky, D.E.; Costa, A.; Kaneko, T.; Schiavo, F.L.; Katsuhara, M.; Schroeder, J.I. K+ transport by the OsHKT2;4 transporter from rice with atypical Na+ transport properties and competition in permeation of K+ over Mg2+ and Ca2+ ions. Plant Physiol. 2011, 156, 1493–1507. [Google Scholar] [CrossRef]
- Pottosin, I.I.; Tikhonova, L.I.; Hedrich, R.; Schönknecht, G. Slowly activating vacuolar channels can not mediate Ca2+-induced Ca2+ release. Plant J. 1997, 12, 1387–1398. [Google Scholar] [CrossRef]
- Guo, K.M.; Babourina, O.; Christopher, D.A.; Borsic, T.; Rengel, Z. The cyclic nucleotide-gated channel AtCNGC10 transports Ca2+ and Mg2+ in Arabidopsis. Physiol. Plant 2010, 139, 303–312. [Google Scholar] [PubMed]
- Demidchik, V.; Maathuis, F.J.M. Physiological roles of nonselective cation channels in plants: From salt stress to signalling and development. New Phytol. 2007, 175, 387–404. [Google Scholar] [CrossRef] [PubMed]
- Shaul, O.; Hilgemann, D.W.; de-Almeida-Engler, J.; van Montagu, M.; Inz, D.; Galili, G. Cloning and characterization of a novel Mg2+/H+ exchanger. EMBO J. 1999, 18, 3973–3980. [Google Scholar] [CrossRef] [PubMed]
- David-Assael, O.; Saul, H.; Saul, V.; Mizrachy-Dagri, T.; Berezin, I.; Brook, E.; Shaul, O. Expression of AtMHX, an Arabidopsis vacuolar metal transporter, is repressed by the 5′ untranslated region of its gene. J. Exp. Bot. 2005, 56, 1039–1047. [Google Scholar] [CrossRef] [PubMed]
- Gaash, R.; Elazar, M.; Mizrahi, K.; Avramov-Mor, M.; Berezin, I.; Shaul, O. Phylogeny and a structural model of plant MHX transporters. BMC Plant Biol. 2013, 13, 75. [Google Scholar] [CrossRef] [PubMed]
- Borrelly, G.; Boyer, J.C.; Touraine, B.; Szponarski, W.; Rambier, M.; Gibrat, R. The yeast mutant vps5Δ affected in the recycling of Golgi membrane proteins displays an enhanced vacuolar Mg2+/H+ exchange activity. Proc. Natl. Acad. Sci. USA 2001, 98, 9660–9665. [Google Scholar] [PubMed]
- Schock, I.; Gregan, J.; Steinhauser, S.; Schweyen, R.; Brennicke, A.; Knoop, V. A member of a novel Arabidopsis thaliana gene family of candidate Mg2+ ion transporters complements a yeast mitochondrial group II intron-splicing mutant. Plant J. 2000, 24, 489–501. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Tutone, A.F.; Drummond, R.S.; Gardner, R.C.; Luan, S. A novel family of magnesium transport genes in Arabidopsis. Plant Cell 2001, 13, 2761–2775. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Li, L.-G.; Liu, Z.-H.; Yuan, Y.-J.; Guo, L.-L.; Mao, D.-D.; Tian, L.-F.; Chen, L.-B.; Luan, S.; Li, D.-P. Magnesium transporter AtMGT9 is essential for pollen development in Arabidopsis. Cell Res. 2009, 19, 887–898. [Google Scholar] [CrossRef] [PubMed]
- Li, L.-G.; Sokolov, L.N.; Yang, Y.-H.; Li, D.-P.; Ting, J.; Pandy, G.K.; Luan, S. A mitochondrial magnesium transporter functions in Arabidopsis pollen development. Mol. Plant 2008, 1, 675–685. [Google Scholar] [CrossRef] [PubMed]
- Mao, D.-D.; Tian, L.-F.; Li, L.-G.; Chen, J.; Deng, P.-Y.; Li, D.-P.; Luan, S. AtMGT7: An Arabidopsis gene encoding a low-affinity magnesium transporter. J. Integr. Plant Biol. 2008, 50, 1530–1538. [Google Scholar] [CrossRef] [PubMed]
- Drummond, R.S.M.; Tutone, A.; Li, Y.-C.; Gardner, R.C. A putative magnesium transporter AtMRS2-11 is localized to the plant chloroplast envelope membrane system. Plant Sci. 2006, 170, 78–89. [Google Scholar] [CrossRef]
- Gebert, M.; Meschenmoser, K.; Svidovà, S.; Weghuber, J.; Schweyen, R.; Eifler, K.; Lenz, H.; Weyand, K.; Knoop, V. A root-expressed magnesium transporter of the MRS2/MGT gene family in Arabidopsis thaliana allows for growth in low-Mg2+ environments. Plant Cell 2009, 21, 4018–4030. [Google Scholar] [CrossRef] [PubMed]
- Ishijima, S.; Shigemi, Z.; Adachi, H.; Makinouchi, N.; Sagami, I. Functional reconstitution and characterization of the Arabidopsis Mg2+ transporter AtMRS2-10 in proteoliposomes. Biochim. Biophys. Acta 2012, 1818, 2202–2208. [Google Scholar] [CrossRef] [PubMed]
- Deng, W.; Luo, K.; Li, D.; Zheng, X.; Wei, X.; Smith, W.; Thammina, C.; Lu, L.; Li, Y.; Pei, Y. Overexpression of an Arabidopsis magnesium transport gene, AtMGT1, in Nicotiana benthamiana confers Al tolerance. J. Exp. Bot. 2006, 57, 4235–4243. [Google Scholar] [CrossRef] [PubMed]
- Visscher, A.M.; Paul, A.-L.; Kirst, M.; Guy, C.L.; Schuerger, A.C.; Ferl, R.J. Growth performance and root transcriptome remodeling of Arabidopsis in response to mars-like levels of magnesium sulfate. PLoS ONE 2010, 5, e12348. [Google Scholar] [CrossRef] [PubMed]
- Mao, D.; Chen, J.; Tian, L.; Liu, Z.; Yang, L.; Tang, R.; Li, J.; Lu, C.; Yang, Y.; Shi, J.; et al. Arabidopsis transporter MGT6 mediates magnesium uptake and is required for growth under magnesium limitation. Plant Cell 2014, 26, 2234–2248. [Google Scholar] [CrossRef] [PubMed]
- Saito, T.; Kobayashi, N.I.; Tanoi, K.; Iwata, N.; Suzuki, H.; Iwata, R.; Nakanishi, T.M. Expression and functional analysis of the CorA-MRS2-ALR-type magnesium transporter family in rice. Plant Cell Physiol. 2013, 54, 1673–1683. [Google Scholar] [CrossRef] [PubMed]
- Kolisek, M.; Zsurka, G.; Samaj, J.; Weghuber, J.; Schweyen, R.J.; Schweigel, M. Mrs2p is an essential component of the major electrophoretic Mg2+ influx system in mitochondria. EMBO J. 2003, 22, 1235–1244. [Google Scholar] [CrossRef] [PubMed]
- Wan, Q.; Ahmad, M.F.; Fairman, J.; Gorzelle, B.; La Fuente, M.D.; Dealwis, C.; Maguire, M.E. X-ray crystallography and isothermal titration calorimetry studies of the Salmonella zinc transporter ZntB. Structure 2011, 19, 700–710. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.C.; Yamaji, N.; Motoyama, R.; Nagamura, Y.; Ma, J.F. Up-regulation of a magnesium transporter gene OsMGT1 is required for conferring aluminum tolerance in rice. Plant Physiol. 2012, 159, 1624–1633. [Google Scholar] [CrossRef] [PubMed]
- Yamaji, N.; Huang, C.F.; Nagao, S.; Yano, M.; Sato, Y.; Nagamura, Y.; Ma, J.F. A Zinc finger transcription factor ART1 regulates multiple genes implicated in aluminum tolerance in rice. Plant Cell 2009, 21, 3339–3349. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.C.; Ma, J.F. Magnesium transporters and their role in Al tolerance in plants. Plant Soil. 2013, 368, 51–56. [Google Scholar] [CrossRef]
- Werner, T.; Nehnevajova, E.; Köllmer, I.; Novák, O.; Strnad, M.; Krämer, U.; Schmülling, T. Root-specific reduction of cytokinin causes enhanced root growth, drought tolerance, and leaf mineral enrichment in Arabidopsis and tobacco. Plant Cell 2010, 22, 3905–3920. [Google Scholar] [CrossRef] [PubMed]
- Zhong, W.; Schobert, C.; Komor, E. Transport of magnesium ions in the phloem of Ricinus communis L. seedlings. Planta 1993, 190, 114–119. [Google Scholar] [CrossRef]
- Hayashi, H.; Chino, M. Collection of pure phloem sap from wheat and its chemical composition. Plant Cell Physiol. 1986, 27, 1387–1393. [Google Scholar]
- Kobayashi, N.I.; Iwata, N.; Saito, T.; Suzuki, H.; Iwata, R.; Tanoi, K.; Nakanishi, T.M. Application of 28Mg for characterization of Mg uptake in rice seedling under different pH conditions. J. Radioanal. Nucl. Chem. 2013, 296, 531–534. [Google Scholar] [CrossRef]
- Kobayashi, N.I.; Iwata, N.; Saito, T.; Suzuki, H.; Iwata, R.; Tanoi, K.; Nakanishi, T.M. Different magnesium uptake and transport activity along the rice root axis revealed by 28Mg tracer experiments. Soil Sci. Plant Nutr. 2013, 59, 149–155. [Google Scholar] [CrossRef]
- Tanoi, K.; Kobayashi, N.; Saito, T.; Iwata, N.; Kamada, R.; Iwata, R.; Suzuki, H.; Hirose, A.; Ohmae, Y.; Sugita, R.; et al. Effects of magnesium deficiency on magnesium uptake activity of rice root, evaluated using 28Mg as a tracer. Plant Soil 2014, 384, 69–77. [Google Scholar] [CrossRef]
- Sugita, R.; Kobayashi, N.I.; Saito, T.; Hirose, A.; Iwata, R.; Tanoi, K.; Nakanishi, T.M. Quantitative analysis of 28Mg in Arabidopsis using real-time radioisotope imaging system (RRIS). Radioisotopes 2014, 63, 227–237. [Google Scholar] [CrossRef]
- Kanno, S.; Yamawaki, M.; Ishibashi, H.; Kobayashi, N.I.; Hirose, A.; Tanoi, K.; Nussaume, L.; Nakanishi, T.M. Development of real-time radioisotope imaging systems for plant nutrient uptake studies. Philos. Trans. R. Soc. B 2012, 367, 1501–1508. [Google Scholar] [CrossRef] [PubMed]
- Bose, J.; Babourina, O.; Shabala, S.; Rengel, Z. Low-pH and aluminum resistance in Arabidopsis correlates with high cytosolic magnesium content and increased magnesium uptake by plant roots. Plant Cell Physiol. 2013, 54, 1093–1104. [Google Scholar] [CrossRef] [PubMed]
- Schmitz, J.; Tierbach, A.; Lenz, H.; Meschenmoser, K.; Knoop, V. Membrane protein interactions between different Arabidopsis thaliana MRS2-type magnesium transporters are highly permissive. Biochim. Biophys. Acta 2013, 1828, 2032–2040. [Google Scholar] [CrossRef] [PubMed]
- Payandeh, J.; Li, C.; Ramjeesingh, M.; Poduch, E.; Bear, C.E.; Pai, E.F. Probing structure-function relationships and gating mechanisms in the CorA Mg2+ transport system. J. Biol. Chem. 2008, 283, 11721–11733. [Google Scholar] [CrossRef] [PubMed]
- Mäser, P.; Thomine, S.; Schroeder, J.I.; Ward, J.M.; Hirschi, K.; Sze, H.; Talke, I.N.; Amtmann, A.; Maathuis, F.J.; Sanders, D.; et al. Phylogenetic relationships within cation transporter families of Arabidopsis. Plant Physiol. 2001, 126, 1646–1667. [Google Scholar] [CrossRef] [PubMed]
- Szczerba, M.W.; Britto, D.T.; Kronzucker, H.J. K+ transport in plants: Physiology and molecular biology. J. Plant Physiol. 2009, 166, 447–466. [Google Scholar] [CrossRef] [PubMed]
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kobayashi, N.I.; Tanoi, K. Critical Issues in the Study of Magnesium Transport Systems and Magnesium Deficiency Symptoms in Plants. Int. J. Mol. Sci. 2015, 16, 23076-23093. https://doi.org/10.3390/ijms160923076
Kobayashi NI, Tanoi K. Critical Issues in the Study of Magnesium Transport Systems and Magnesium Deficiency Symptoms in Plants. International Journal of Molecular Sciences. 2015; 16(9):23076-23093. https://doi.org/10.3390/ijms160923076
Chicago/Turabian StyleKobayashi, Natsuko I., and Keitaro Tanoi. 2015. "Critical Issues in the Study of Magnesium Transport Systems and Magnesium Deficiency Symptoms in Plants" International Journal of Molecular Sciences 16, no. 9: 23076-23093. https://doi.org/10.3390/ijms160923076
APA StyleKobayashi, N. I., & Tanoi, K. (2015). Critical Issues in the Study of Magnesium Transport Systems and Magnesium Deficiency Symptoms in Plants. International Journal of Molecular Sciences, 16(9), 23076-23093. https://doi.org/10.3390/ijms160923076