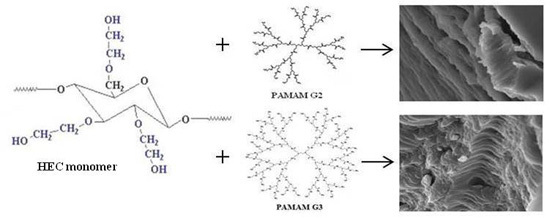
The Effect of Cationic Polyamidoamine Dendrimers on Physicochemical Characteristics of Hydrogels with Erythromycin
Abstract
:1. Introduction
2. Results and Discussion
2.1. Characterization of Erythromycin Hydrogels with PAMAM-NH2 Generation 2 (G2) and Generation 3 (G3)
| Ingredient (g) | Formulation Code | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| E1 | EP1 | EP2 | EP3 | EP4 | EP5 | EP6 | EP7 | EP8 | |
| EM | 2.0 | 2.0 | 2.0 | 2.0 | 2.0 | 2.0 | 2.0 | 2.0 | 2.0 |
| HEC | 2.5 | 2.5 | 2.5 | 2.5 | 2.5 | 2.5 | 2.5 | 2.5 | 2.5 |
| Propylene glycol | 10.0 | 10.0 | 10.0 | 10.0 | 10.0 | 10.0 | 10.0 | 10.0 | 10.0 |
| Bronopol | 0.02 | 0.02 | 0.02 | 0.02 | 0.02 | 0.02 | 0.02 | 0.02 | 0.02 |
| PAMAM-NH2 G2 | - | 0.003 | 0.03 | 0.3 | 0.6 | - | - | - | - |
| PAMAM-NH2 G3 | - | - | - | - | - | 0.003 | 0.03 | 0.3 | 0.6 |
| Purified water (up to) | 100 | 100 | 100 | 100 | 100 | 100 | 100 | 100 | 100 |
| Formulation Code | Drug Content (%) | pH | Particle Size (µm) | Viscosity ** (mPa·s) |
|---|---|---|---|---|
| E1 | 98.00 ± 1.30 | 8.43 ± 0.01 | 29.2 ± 22.41 | 9038.94 ± 26.28 |
| EP1 | 100.95 ± 0.48 | 8.50 ± 0.01 | 28.5 ± 22.95 | 8810.74 ± 84.19 |
| EP2 | 99.75 ± 0.13 | 8.53 ± 0.01 | 24.8 ± 17.75 | 6995.01 ± 14.03 * |
| EP3 | 100.00 ± 0.29 | 8.56 ± 0.01 | 22.4 ± 18.98 | 4583.97 ± 84.20 * |
| EP4 | 99.95 ± 0.20 | 8.58 ± 0.02 | 19.6 ± 14.25 | 3690.99 ± 14.04 * |
| EP5 | 102.10 ± 0.96 | 8.51 ± 0.01 | 28.9 ± 15.78 | 8969.49 ± 56.12 |
| EP6 | 98.15 ± 0.37 | 8.53 ± 0.01 | 25.5 ± 18.68 | 7332.36 ± 24.67 |
| EP7 | 99.20 ± 0.41 | 8.58 ± 0.02 | 23.1 ± 22.27 | 4752.64 ± 58.22 * |
| EP8 | 102.25 ± 0.23 | 8.59 ± 0.02 | 20.8 ± 19.06 | 3939.04 ± 84.20 * |
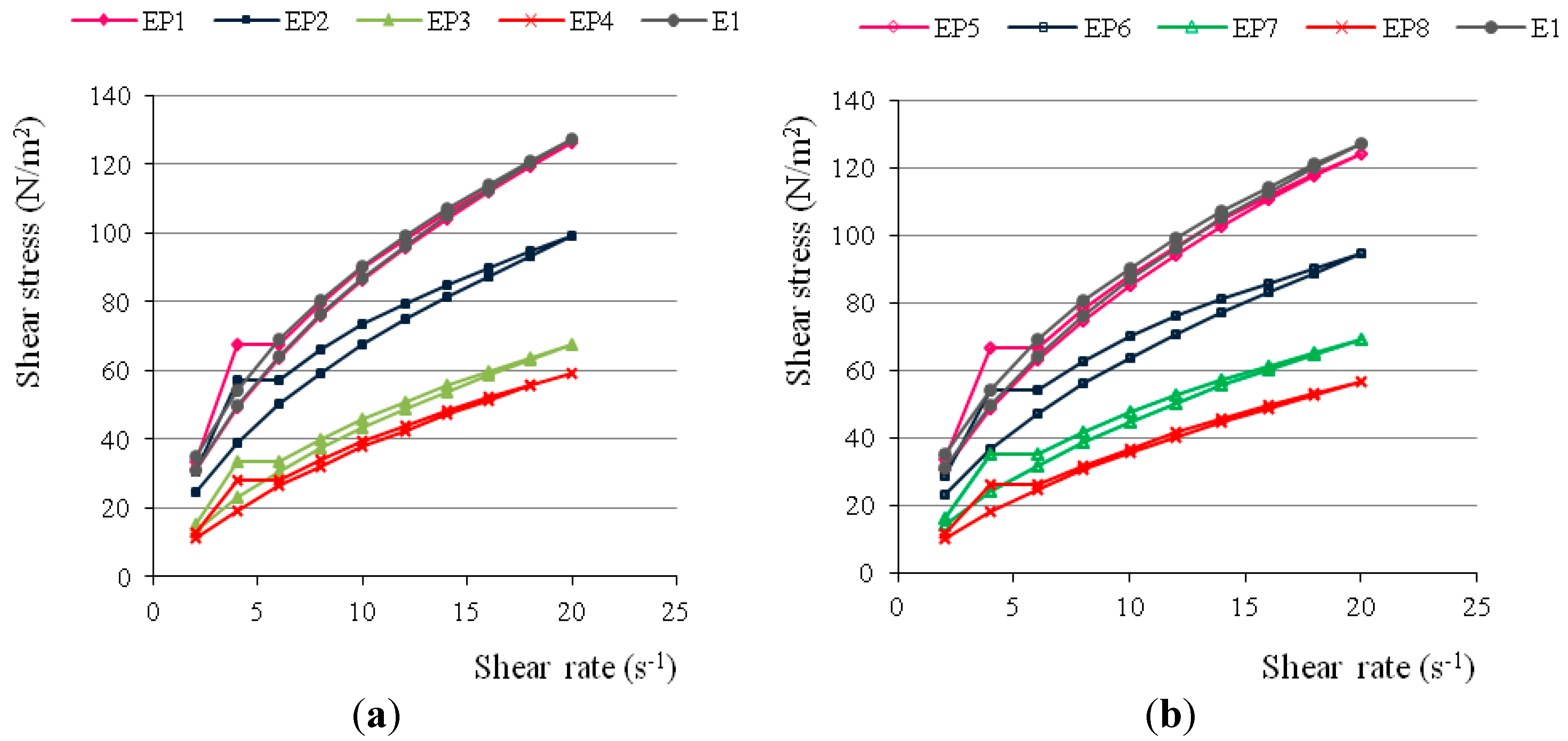
| Formulation Code | Hardness (g) | Cohesiveness (g·s) | Adhesiveness (g·s) |
|---|---|---|---|
| E1 | 56.51 ± 1.26 | 115.91 ± 3.22 | −128.25 ± 3.08 |
| EP1 | 54.97 ± 2.17 | 111.97 ± 3.69 | −132.00 ± 3.57 |
| EP2 | 43.68 ± 2.28 | 89.87 ± 3.04 | −107.72 ± 0.83 * |
| EP3 | 26.30 ± 0.43 | 58.59 ± 1.17 | −70.43 ± 0.09 * |
| EP4 | 22.67 ± 1.03 | 52.02 ± 0.05 | −66.56 ± 2.78 * |
| EP5 | 56.46 ± 1.42 | 114.75 ± 3.61 | −132.24 ± 3.69 |
| EP6 | 46.46 ± 1.32 | 95.67 ± 0.82 | −111.95 ± 1.11 |
| EP7 | 28.49 ± 0.55 | 63.60 ± 0.33 | −73.44 ± 0.87 * |
| EP8 | 26.72 ± 0.11 | 62.38 ± 0.17 | −70.77 ± 0.96 * |
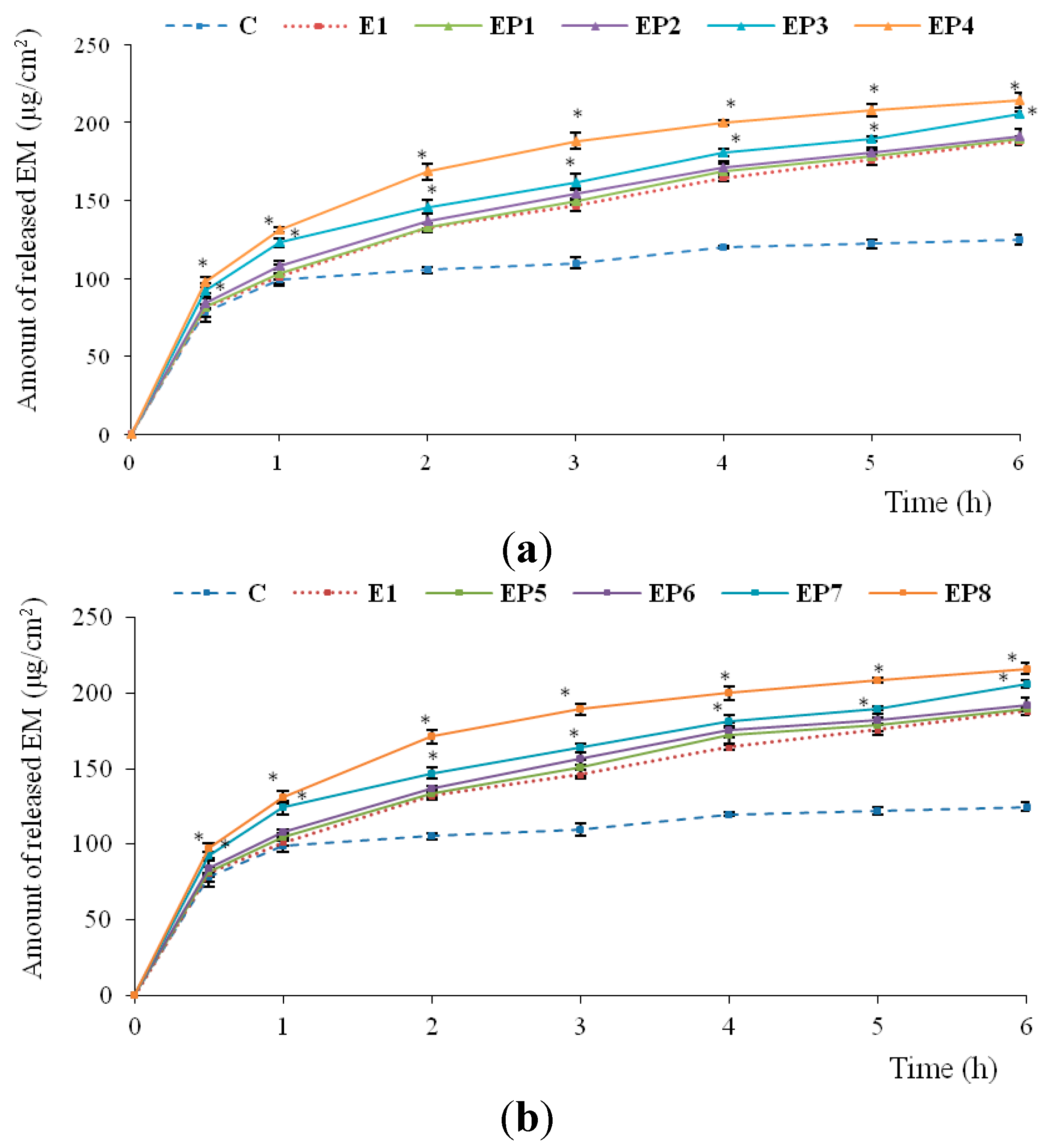
2.2. In Vitro Release of EM
2.3. Antimicrobial Activity of Hydrogels
| Zone of Inhibition (mm) | Name of the Strain | |||
|---|---|---|---|---|
| S. aureus ATCC 29213 | S. aureus (Clinical Strain) | S. aureus (Clinical Strain) | E. faecalis ATCC 29212 | |
| EM | 37.3 ± 2.3 | 36.7 ± 1.2 | 34.5 ± 2.5 | 32.0 ± 0.0 |
| C | 32.0 ± 0.0 | 31.7 ± 1.5 | 31.0 ± 0.0 | 25.3 ± 0.6 |
| H | 5.7 ± 2.3 | 5.5 ± 0.7 | 5.0 ± 2.3 | 5.7 ± 0.6 |
| E1 | 33.0 ± 1.0 | 32.3 ± 0.6 | 35.0 ± 1.0 | 30.0 ± 0.0 |
| EP1 | 36.0 ± 0.0 | 35.3 ± 1.2 | 34.0 ± 0.0 | 31.7 ± 0.6 |
| EP2 | 36.0 ± 0.0 | 39.0 ± 1.41 | 35.5 ± 0.0 | 31.3 ± 1.2 |
| EP3 | 36.0 ± 0.0 | 36.0 ± 0.0 | 33.5 ± 0.0 | 30.7 ± 0.6 |
| EP4 | *- | *- | *- | *- |
| EP5 | 36.0 ± 0.0 | 35.7 ± 0.6 | 34.0 ± 0.0 | 30.3 ± 0.6 |
| EP6 | 37.3 ± 1.2 | 35.3 ± 1.2 | 35.0 ± 0.0 | 30.7 ± 0.6 |
| EP7 | 37.3 ± 0.6 | 36.3 ± 1.5 | 35.0 ± 0.0 | 30.0 ± 0.0 |
| EP8 | *- | *- | *- | *- |
2.4. Stability Studies
3. Experimental Section
3.1. Chemicals and Materials
3.2. Preparation of Hydrogels
3.3. Physicochemical Properties of Hydrogels
3.3.1. HPLC Analysis
3.3.2. Particle Size Analysis
3.3.3. Viscosity Measurement and Determination of Rheological Properties
3.3.4. Texture Analysis
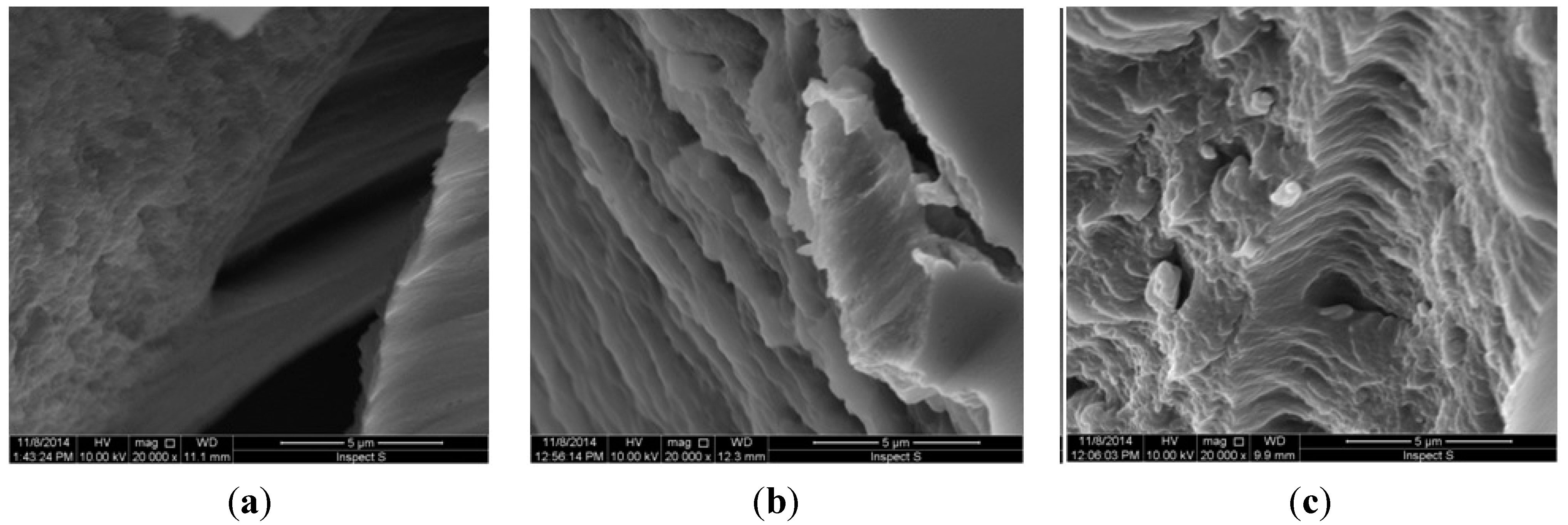
3.3.5. Morphology of the Hydrogels
3.4. In Vitro Release of EM
3.5. Antimicrobial Activity of Hydrogels
3.6. Stability Studies
3.7. Statistical Analysis
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Rehman, K.; Zulfakar, M.H. Recent advances in gel technologies for topical and transdermal drug delivery. Drug Dev. Ind. Pharm. 2014, 40, 433–440. [Google Scholar] [CrossRef] [PubMed]
- Buwalda, S.J.; Boere, K.W.; Dijkstra, P.J.; Feijen, J.; Vermonden, T.; Hennink, W.E. Hydrogels in a historical perspective: From simple networks to smart materials. J. Control. Release 2014, 190, 254–273. [Google Scholar] [CrossRef] [PubMed]
- Peppas, N.A.; Bures, P.; Leobandung, W.; Ichikawa, H. Hydrogels in pharmaceutical formulations. Eur. J. Pharm. Biopharm. 2000, 50, 27–46. [Google Scholar] [CrossRef]
- Svenson, S.; Tomalia, D.A. Dendrimers in biomedical applications-reflections on the field. Adv. Drug Deliv. Rev. 2005, 57, 2106–2129. [Google Scholar] [CrossRef] [PubMed]
- Winnicka, K.; Bielawski, K.; Rusak, M.; Bielawska, A. The effect of generation 2 and 3 poly (amidoamine) dendrimers on viability of human breast cancer cells. J. Health Sci. 2009, 55, 169–177. [Google Scholar] [CrossRef]
- Bharti, J.P.; Prajapati, S.K.; Jaiswal, M.K.; Yadav, R.D. Dendrimer multifunctional nano-device: A review. Int. J. Pharm. Sci. Res. 2011, 2, 1947–1960. [Google Scholar]
- Winnicka, K.; Wroblewska, M.; Sosnowska, K.; Car, H.; Kasacka, I. Evaluation of cationic polyamidoamine dendrimers’ dermal toxicity in the rat skin model. Drug Des. Dev. Ther. 2015, 5, 1367–1377. [Google Scholar] [CrossRef] [PubMed]
- Janiszewska, J.; Sowińska, M.; Rajnisz, A.; Solecka, J.; Łącka, I.; Milewski, S.; Urbańczyk-Lipkowska, Z. Novel dendrimeric lipopeptides with antifungal activity. Bioorg. Med. Chem. Lett. 2012, 22, 1388–1393. [Google Scholar] [CrossRef] [PubMed]
- Wang, B.; Navath, R.S.; Menjoge, A.R.; Balakrishnan, B.; Bellair, R.; Dai, H.; Romero, R.; Kannan, S.; Kannan, R.M. Inhibition of bacterial growth and intramniotic infection in a guinea pig model of chorioamnionitis using PAMAM dendrimers. Int. J. Pharm. 2010, 395, 298–308. [Google Scholar] [CrossRef] [PubMed]
- Noriega-Luna, B.; Godínez, L.A.; Rodríguez, F.J.; Rodríguez, A.; Zaldívar-Lelo de Larrea, G.; Sosa-Ferreyra, C.F.; Mercado-Curiel, R.F.; Manríquez, J.; Bustos, E. Applications of dendrimers in drug delivery agents, diagnosis, therapy, and detection. Sci. World J. 2014, 2014, 1–13. [Google Scholar] [CrossRef]
- Ma, M.; Cheng, Y.; Xu, Z.; Xu, P.; Qu, H.; Fang, Y. Evaluation of polyamidoamine (PAMAM) dendrimers as drug carriers of anti-bacterial drugs using sulfamethoxazole (SMZ) as a model drug. Eur. J. Med. Chem. 2007, 42, 93–98. [Google Scholar] [CrossRef] [PubMed]
- Cheng, Y.; Qu, H.; Ma, M.; Xu, Z.; Xu, P.; Fang, Y.; Xu, T. Polyamidoamine (PAMAM) dendrimers as biocompatible carriers of quinolone antimicrobials: An in vitro study. Eur. J. Med. Chem. 2007, 42, 1032–1038. [Google Scholar] [CrossRef] [PubMed]
- Winnicka, K.; Wroblewska, M.; Wieczorek, P.; Sacha, P.T.; Tryniszewska, E. Hydrogel of ketoconazole and PAMAM dendrimers: Formulation and antifungal activity. Molecules 2012, 17, 4612–4624. [Google Scholar] [CrossRef] [PubMed]
- Strydom, S.J.; Rose, W.E.; Otto, D.P.; Liebenberg, W.; de Villiers, M.M. Poly(amidoamine) dendrimer-mediated synthesis and stabilization of silver sulfonamide nanoparticles with increased antibacterial activity. Nanomedicine 2013, 9, 85–93. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Pornpattananangku, D.; Hu, C.M.; Huang, C.M. Development of nanoparticles for antimicrobial drug delivery. Curr. Med. Chem. 2010, 17, 585–94. [Google Scholar] [CrossRef] [PubMed]
- Huh, A.J.; Kwon, Y.J. “Nanoantibiotics”: A new paradigm for treating infectious diseases using nanomaterials in the antibiotics resistant era. J. Control. Release 2011, 156, 128–145. [Google Scholar] [CrossRef] [PubMed]
- Council of Europe. European Pharmacopoeia, 6th ed.; Council of Europe: Strasbourg, France, 2007; Volume 2, pp. 1801–1803. [Google Scholar]
- Mazzei, T.; Mini, E.; Novelli, A.A. Chemistry and mode of action of macrolides. J. Antimicrob. Chemother. 1993, 31, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Jigar, V.; Vishal, G.; Tejas, G.; Vishal, Ch.; Umesh, U. Formulation and characterization of topical gel of erythromycin entrapped into niosomes. Int. J. PharmTech Res. 2011, 3, 1714–1718. [Google Scholar]
- Jayaraman, S.C.; Ramachandran, C.; Weiner, N. Topical delivery of erythromycin from various formulations: An in vivo hairless mouse study. J. Pharm. Sci. 1996, 85, 1082–1084. [Google Scholar] [CrossRef] [PubMed]
- Lane, M.E. Skin penetration enhancers. Int. J. Pharm. 2013, 447, 12–21. [Google Scholar] [CrossRef] [PubMed]
- Venuganti, V.V.; Perumal, O.P. Poly (amidoamine) dendrimers as skin penetration enhancers: Influence of charge, generation, and concentration. J. Pharm. Sci. 2009, 98, 2345–2356. [Google Scholar] [CrossRef] [PubMed]
- Yiyun, C.; Na, M.; Tongwen, X. Transdermal delivery of nonsteroidal anti-inflammatory drug mediated by polyamidoamine (PAMAM) dendrimers. J. Pharm. Sci. 2007, 96, 595–602. [Google Scholar] [CrossRef] [PubMed]
- Winnicka, K.; Wroblewska, M.; Wieczorek, P.; Sacha, P.T.; Tryniszewska, E.A. The effect of PAMAM dendrimers on the antibacterial activity of antibiotics with different water solubility. Molecules 2013, 18, 8607–8617. [Google Scholar] [CrossRef] [PubMed]
- Brisaert, M.; Heylen, M.; Plaizier-Vercammen, J. Investigation on the chemical stability of erythromycin in solutions using an optimization system. Pharm. World Sci. 1996, 18, 182–186. [Google Scholar] [CrossRef] [PubMed]
- Vandenbossche, G.M.R.; Vanhaecke, E.; de Muynck, C.; Remon, J.P. Stability of topical erythromycin formulations. Int. J. Pharm. 1991, 67, 195–199. [Google Scholar] [CrossRef]
- Carvalho, F.C.; Calixto, G.; Hatakeyama, I.N.; Luz, G.M.; Gremião, M.P.; Chorilli, M. Rheological, mechanical, and bioadhesive behavior of hydrogels to optimize skin delivery systems. Drug Dev. Ind. Pharm. 2013, 39, 1750–1757. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Zhou, R.; Li, L.; Li, B.; Zhang, X.; Su, J. Mechanical, rheological and release behaviors of a poloxamer 407/poloxamer 188/carbopol 940 thermosensitive composite hydrogel. Molecules 2013, 18, 12415–12425. [Google Scholar] [CrossRef] [PubMed]
- Gandra, S.C.; Nguyen, S.; Nazzal, S.; Alayoubi, A.; Jung, R.; Nesamony, J. Thermoresponsive fluconazole gels for topical delivery: Rheological and mechanical properties, in vitro drug release and anti-fungal efficacy. Pharm. Dev. Technol. 2015, 20, 41–49. [Google Scholar] [CrossRef] [PubMed]
- Calejo, M.T.; Kjøniksen, A-L.; Marques, E.F.; Araújo, M.J.; Sande, S.A.; Nyström, B. Interactions between ethyl(hydroxyethyl) cellulose and lysine-based surfactants in aqueous media. Eur. Polym. J. 2012, 48, 1622–1631. [Google Scholar] [CrossRef]
- Lauer, H.; Stark, A.; Hoffmann, H.; Dönges, R. Interactions between anionically modified hydroxyethyl cellulose and cationic surfactants. J. Surfactant Deterg. 1999, 2, 181–191. [Google Scholar]
- Petró, E.; Balogh, A.; Blazsó, G.; Erös, I.; Csóka, I. In vitro and in vivo evaluation of drug release from semisolid dosage forms. Pharmazie 2011, 66, 936–941. [Google Scholar] [CrossRef] [PubMed]
- Ates, S.; Tunçel, T.; Otük, G. In vitro release of erythromycin from hydrogels. Pharmazie 1994, 49, 459–460. [Google Scholar] [PubMed]
- Devarakonda, B.; Li, N.; de Villiers, M.M. Effect of polyamidoamine (PAMAM) dendrimers on the in vitro release of water-insoluble nifedipine from aqueous gels. AAPS PharmSciTech 2005, 6, 504–512. [Google Scholar] [CrossRef] [PubMed]
- Sosnowska, K.; Winnicka, K. PAMAM dendrimers affect the in vitro release of clotrimazole from hydrogels irrespective of its molecular state. Afr. J. Pharm. Pharmacol. 2013, 7, 567–573. [Google Scholar] [CrossRef]
- U.S. Pharmacopeial Convention. United States Pharmacopeia, 32; U.S. Pharmacopeial Convention: Rockville, USA, 2009; Volume 2, pp. 2282–2284. [Google Scholar]
- Kamarei, F.; Movaghari, F.; Ghaffari, A.; Bozchalooi, I.S.; Zamani, A.; Jabbari, A. Development of stability-indicating high performance liquid chromathography method for assay of erythromycin ethylosuccinate in powder for oral suspension dosage form. Arab. J. Chem. 2014, 7, 1079–1085. [Google Scholar] [CrossRef]
- Kanfer, I.; Skinner, M.F.; Walker, R.B. Analysis of macrolide antibiotics. J. Chromatogr. A 1998, 812, 255–286. [Google Scholar] [CrossRef]
- Hurler, J.; Engesland, A.; Poorahmary Kermany, B.; Škalko-Basnet, N. Improved texture analysis for hydrogel characterization: Gel cohesiveness, adhesiveness, and hardness. J. Appl. Polym. Sci. 2012, 125, 180–188. [Google Scholar] [CrossRef]
- Hurler, J.; Škalko-Basnet, N. Potentials of chitosan-based delivery systems in wound therapy: Bioadhesion study. J. Funct. Biomater. 2012, 6, 37–48. [Google Scholar] [CrossRef] [PubMed]
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wróblewska, M.; Winnicka, K. The Effect of Cationic Polyamidoamine Dendrimers on Physicochemical Characteristics of Hydrogels with Erythromycin. Int. J. Mol. Sci. 2015, 16, 20277-20289. https://doi.org/10.3390/ijms160920277
Wróblewska M, Winnicka K. The Effect of Cationic Polyamidoamine Dendrimers on Physicochemical Characteristics of Hydrogels with Erythromycin. International Journal of Molecular Sciences. 2015; 16(9):20277-20289. https://doi.org/10.3390/ijms160920277
Chicago/Turabian StyleWróblewska, Magdalena, and Katarzyna Winnicka. 2015. "The Effect of Cationic Polyamidoamine Dendrimers on Physicochemical Characteristics of Hydrogels with Erythromycin" International Journal of Molecular Sciences 16, no. 9: 20277-20289. https://doi.org/10.3390/ijms160920277
APA StyleWróblewska, M., & Winnicka, K. (2015). The Effect of Cationic Polyamidoamine Dendrimers on Physicochemical Characteristics of Hydrogels with Erythromycin. International Journal of Molecular Sciences, 16(9), 20277-20289. https://doi.org/10.3390/ijms160920277