Does Short-Term Dietary Omega-3 Fatty Acid Supplementation Influence Brain Hippocampus Gene Expression of Zinc Transporter-3?
Abstract
:1. Introduction
2. Results
2.1. Body Weight, Food and Water Intake
2.2. Y-Maze Performance
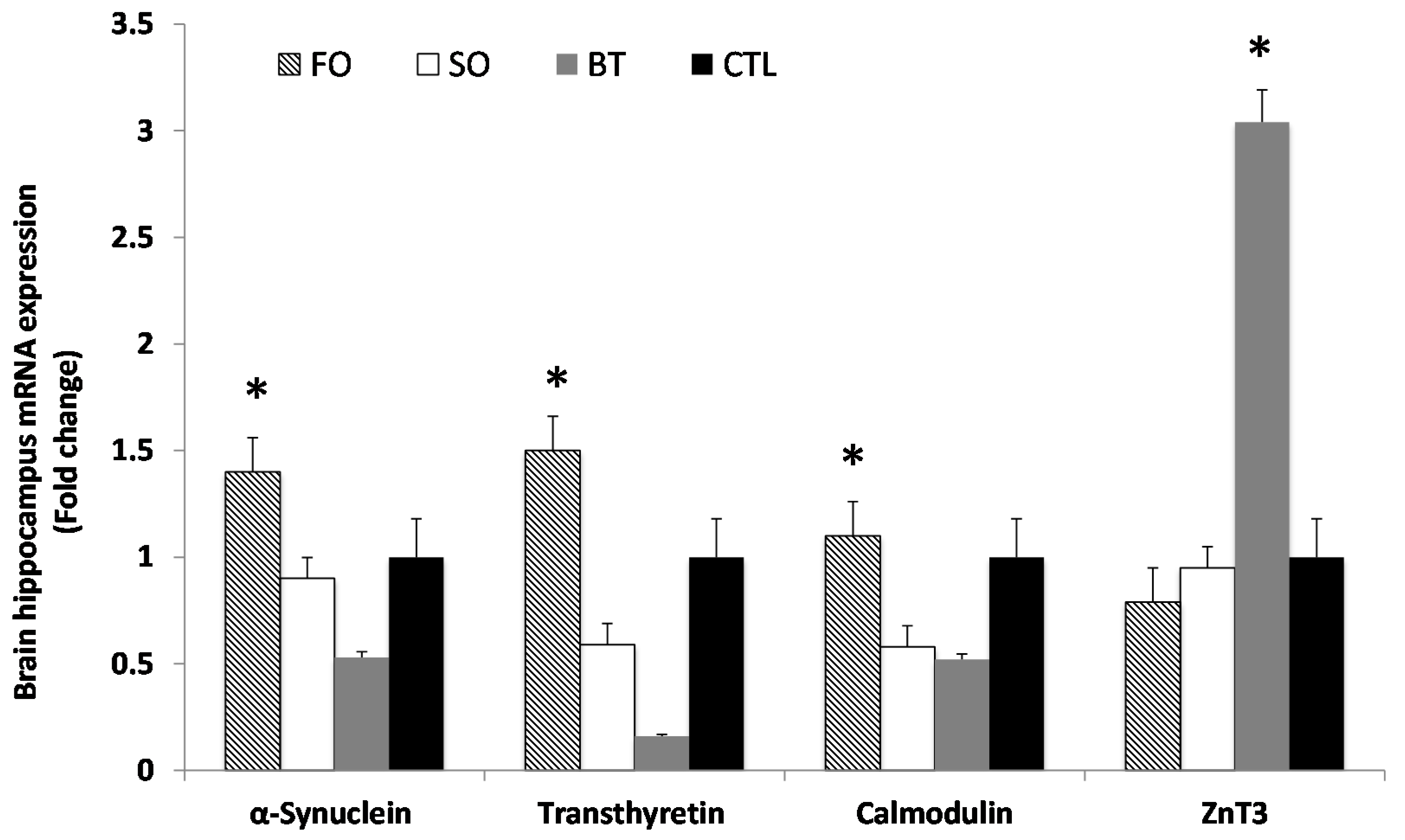
2.3. Brain Hippocampus mRNA Expression
2.4. Brain Fatty Acids
| Treatments | FO | SO | BT | CTRL | SEM |
|---|---|---|---|---|---|
| C16:0 (Palmitic acid) | 32.43 | 32.48 | 34.27 | 32.89 | 0.69 |
| C16:1n-7 (Palmitoleic acid) | 1.01 | 0.77 | 1.21 | 1.11 | 0.15 |
| C18:0 (Stearic acid) | 7.36 | 7.96 | 7.63 | 7.66 | 0.29 |
| C18:1n-9 (Oleic acid) | 29.09 b | 29.84 b | 29.86 b | 33.46 a | 0.75 |
| C18:2n-6 (Linoleic acid) | 5.72 | 5.55 | 5.82 | 6.44 | 0.16 |
| C18:3n-6 (Linolenic acid) | 0.83 | 0.99 | 0.77 | 0.75 | 0.06 |
| C18:3n-3 (α-linolenic acid) | 0.76 | 1.00 | 1.18 | 0.42 | 0.23 |
| C20:1n-9 (Arachidic acid) | 1.99 | 2.03 | 1.29 | 0.87 | 0.19 |
| C20:4n-6 (Arachidonic acid) | 1.03 | 1.03 | 1.29 | 1.36 | 0.12 |
| C20:5n-3 (Eicoapentaenoic acid) | 1.03 | 1.05 | 1.33 | 0.45 | 0.21 |
| C22:4n-6 (Docosatetraenoic acid) | 3.02 | 3.72 | 3.12 | 3.03 | 0.15 |
| C22:5n-3 (Docosapentaenoic acid) | 1.25 | 1.04 | 1.78 | 1.61 | 0.27 |
| C22:6n-3 (Docosahexaenoic acid) | 14.47 a | 12.55 ab | 10.44 b | 9.95 b | 0.73 |
| TOTAL SFA | 39.79 | 40.43 | 41.90 | 40.55 | 0.67 |
| TOTAL MUFA | 35.11 | 36.37 | 35.49 | 38.47 | 0.59 |
| TOTAL n-6 PUFA | 10.60 | 11.28 | 10.99 | 11.57 | 0.23 |
| TOTAL n-3 PUFA | 17.51 a | 15.64 ab | 14.74 ab | 12.44 b | 1.07 |
| n-6:n-3 Ratio | 0.63 b | 0.72 b | 0.84 ab | 0.93 a | 0.06 |
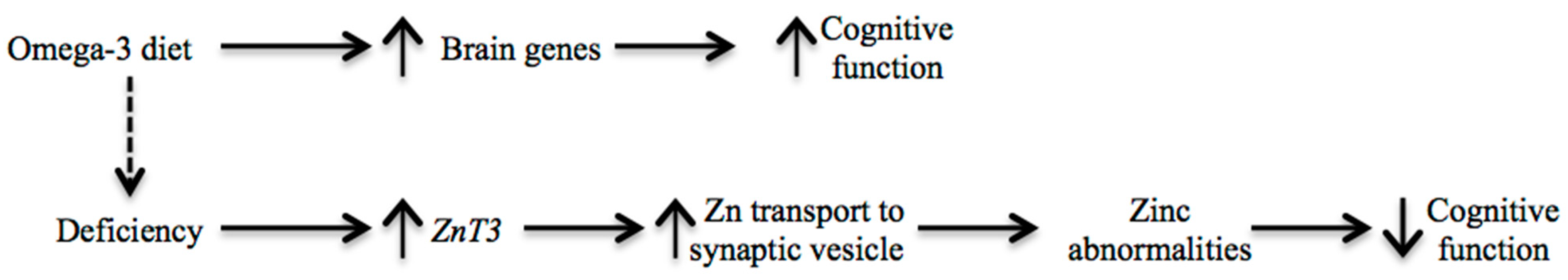
3. Discussion
4. Experimental Section
4.1. Animals and Diet
4.2. Y-Maze Test
4.3. RNA Extraction and Real-Time RT-PCR
4.4. Brain Fatty Acid Analysis
4.5. Statistical Analysis
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Vedin, I.; Cederholm, T.; Freund-Levi, Y.; Basun, H.; Garlind, A.; Irving, G.; Eriksdotter-Jonhagen, M.; Wahlund, L.-O.; Dahlman, I.; Palmblad, J. Effects of DHA-rich n-3 fatty acid supplementation on gene expression in blood mononuclear leukocytes: The OmegAD study. PLoS ONE 2012, 7, e35425. [Google Scholar] [CrossRef]
- Puskas, L.G.; Kitajka, K.; Nyakas, C.; Barcelo-Coblijn, G.; Farkas, T. Short-term administration of omega 3 fatty acids from fish oil results in increased transthyretin transcription in old rat hippocampus. Proc. Natl. Acad. Sci. USA 2003, 100, 1580–1585. [Google Scholar]
- Kitajka, K.; Sinclair, A.J.; Weisinger, R.S.; Weisinger, H.S.; Mathai, M.; Jayasooriya, A.P.; Halver, J.E.; Puskas, L.G. Effects of dietary omega-3 polyunsaturated fatty acids on brain gene expression. Proc. Natl. Acad. Sci. USA 2004, 101, 10931–10936. [Google Scholar]
- Sessler, A.M.; Ntambi, J.M. Polyunsaturated fatty acid regulation of gene expression. J. Nutr. 1998, 128, 923–926. [Google Scholar]
- Barcelo-Coblijn, G.; Hogyes, E.; Kitajka, K.; Puska, L.G.; Zvara, A.; Hackler, L., Jr.; Nyakas, C.; Penke, Z.; Farkas, T. Modification by docosahexaenoic acid of age-induced alterations in gene expression and molecular composition of rat brain phospholipids. Proc. Natl. Acad. Sci. USA 2003, 100, 11321–11326. [Google Scholar]
- Guschina, I.; Millership, S.; O’Donnell, V.; Ninkina, N.; Harwood, J.; Buchman, V. Lipid classes and fatty acid patterns are altered in the brain of c-Synuclein null mutant mice. Lipids 2011, 46, 121–130. [Google Scholar]
- Avraham, Y.; Saidian, M.; Burston, J.J.; Mevorach, R.; Vorobiev, L.; Magen, I.; Kunkes, E.; Borges, B.; Lichtman, A.H.; Berry, E.M. Fish oil promotes survival and protects against cognitive decline in severely undernourished mice by normalizing satiety signals. J. Nutr. Biochem. 2011, 22, 766–776. [Google Scholar]
- Wu, A.; Ying, Z.; Gomez-Pinilla, F. DHA dietary supplementation enhances the effects of exercise on synaptic plasticity and cognition. Neuroscience 2008, 155, 751–759. [Google Scholar]
- Perez-Rosello, T.; Anderson, C.T.; Schopfer, F.J.; Zhao, Y.; Gilad, D.; Salvatore, S.R.; Freeman, B.A.; Hershfinkel, M.; Aizenman, E.; Tzounopoulos, T. Synaptic Zn2+ inhibits neurotransmitter release by promoting endocannabinoid synthesis. J. Neurosci. 2013, 33, 9259–9272. [Google Scholar]
- Sindreu, C.; Storm, D.R. Modulation of neuronal signal transduction and memory formation by synaptic zinc. Front. Behav. Neurosci. 2011, 5, 1–14. [Google Scholar]
- Takeda, A.; Tamano, H.; Tochigi, M.; Oku, N. Zinc homeostasis in the hippocampus of zinc-deficient young adult rats. Neurochem. Int. 2005, 46, 221–225. [Google Scholar]
- Takeda, A.; Tamano, H.; Nagayoshi, A.; Yamada, K.; Oku, N. Increase in hippocampal cell death after treatment with kainate in zinc deficiency. Neurochem. Int. 2005, 47, 539–544. [Google Scholar]
- Adlard, P.A.; Parncutt, J.M.; Finkelstein, D.I.; Bush, A.I. Cognitive loss in zinc transporter-3 knock-out mice: A phenocopy for the synaptic and memory deficits of Alzheimer’s disease? J. Neurosci. 2010, 30, 1631–1636. [Google Scholar]
- Suphioglu, C.; DeMel, D.; Kumar, L.; Sadli, N.; Freestone, D.; Michalczyk, A.; Sinclair, A.; Ackland, M.L. The omega-3 fatty acid, DHA, decreases neuronal cell death in association with altered zinc transport. FEBS Lett. 2010, 584, 612–618. [Google Scholar]
- Hafandi, A.; Begg, D.P.; Premaratna, S.D.; Sinclair, A.J.; Jois, M.; Weisinger, R.S. Dietary repletion with ω3 fatty acid or with COX inhibition reverses cognitive effects in F3 ω3 fatty-acid—Deficient mice. Am. Assoc. Lab. Anim. Sci. 2014, 64, 106–109. [Google Scholar]
- Labrousse, V.F.; Nadjar, A.; Joffre, C.; Costes, L.; Aubert, A.; Gregoire, S.; Bretillon, L.; Laye, S. Short-term long chain omega-3 diet protects from neuroinflammatory processes and memory impairment in aged mice. PLoS ONE 2012, 7, e36861. [Google Scholar] [CrossRef]
- Carrie, I.; Guesnet, P.; Bourre, J.-M.; France’s, H. Diets containing long-chain n-3 polyunsaturated fatty acids affect behaviour differently during development than ageing in mice. Br. J. Nutr. 2000, 83, 439–447. [Google Scholar]
- Hajjar, T.; Meng, G.Y.; Rajion, M.A.; Vidyadaran, S.; Othman, F.; Farjam, A.S.; Li, T.A.; Ebrahimi, M. Omega-3 polyunsaturated fatty acid improves spatial learning and hippocampal peroxisome proliferator activated receptors (PPARα and PPARγ) gene expression in rats. BMC Neurosci. 2012, 13, 109. [Google Scholar] [CrossRef] [PubMed]
- Tofail, F.; Kabir, I.; Hamadani, J.D.; Chowdhury, F.; Yesmin, S.; Mehreen, F.; Huda, S.N. Supplementation of fish-oil and soy-oil during pregnancy and psychomotor development of infants. J. Health Popul. Nutr. 2006, 24, 48–56. [Google Scholar] [PubMed]
- Ueda, Y.; Wang, M.-F.; Irei, A.V.; Sarukura, N.; Sakai, T.; Hsu, T.F. Effect of dietary lipids on longevity and memory in the SAMP8 mice. J. Nutr. Sci. Vitaminol. 2011, 57, 36–41. [Google Scholar] [CrossRef] [PubMed]
- Volpicelli-Daley, L.A.; Luk, K.C.; Patel, T.P.; Tanik, S.A.; Riddle, D.M.; Stieber, A.; Meany, D.F.; Trojanowski, J.Q.; Lee, V.M. Exogenous α-synuclein fibrils induce lewy body pathology leading to synaptic dysfunction and neuron death. Neuron 2011, 72, 57–71. [Google Scholar]
- Murphy, D.D.; Rueter, S.M.; Trojanowski, J.Q.; Lee, V.M. Synucleins are developmentally expressed, and α-synuclein regulates the size of the presynaptic vesicular pool in primary hippocampal neurons. J. Neurosci. 2000, 20, 3214–3220. [Google Scholar]
- Hartman, V.N.; Miller, M.A.; Clayton, D.F.; Liu, W.C.; Kroodsma, D.E.; Brenowitz, E.A. Testosterone regulates α-synuclein mRNA in the avian song system. Neuroreport 2001, 12, 943–946. [Google Scholar]
- Rellos, P.; Pike, A.C.W.; Niesen, F.H.; Salah, E.; Lee, W.H.; von Delft, F.; Knapp, S. Structure of the CaMKIId/calmodulin complex reveals the molecular mechanism of CaMKII kinase activation. PLoS Biol. 2010, 8, e1000426. [Google Scholar] [CrossRef]
- Silva, A.J.; Stevens, C.F.; Tonegawa, S.; Wang, Y. Deficient hippocampal long-term potentiation in α-calcium-calmodulin kinase II mutant mice. Science 1992, 257, 201–206. [Google Scholar]
- Kitajka, K.; Puskas, L.G.; Zvara, A.; Hackler, L., Jr.; Barcelo-Coblijn, G.; Yeo, Y.K.; Farkas, T. The role of n-3 polyunsaturated fatty acids in brain: Modulation of rat brain gene expression by dietary n-3 fatty acids. Proc. Natl. Acad. Sci. USA 2002, 99, 2619–2624. [Google Scholar]
- Sousa, J.C.; Grandela, C.; Fernández-Ruiz, J.; de Miguel, R.; de Sousa, L.; Magalhães, A.I.; Saraiva, M.J.; Sousa, N.; Palha, J.A. Transthyretin is involved in depression-like behavior and exploratory activity. J. Neurochem. 2004, 88, 1052–1058. [Google Scholar]
- Choi, S.H.; Leight, S.N.; Lee, V.M.; Li, T.; Wong, P.C.; Johnson, J.A.; Saraiva, M.J.; Sisodia, S.S. Accelerated Aβ deposition in APPswe/PS1δE9 mice with hemizygous deletions of TTR (transthyretin). J. Neurosci. 2007, 27, 7006–7010. [Google Scholar]
- Sousa, J.C.; Marques, F.; Dias-Ferreira, E.; Cerqueira, J.J.; Sousa, N.; Palha, J.A. Transthyretin influences spatial reference memory. Neurobiol. Learn. Mem. 2007, 88, 381–385. [Google Scholar]
- Jayasooriya, A.P.; Ackland, M.L.; Mathai, M.L.; Sinclair, A.J.; Weisinger, H.S.; Weisinger, R.S.; Halver, J.E.; Kitajka, K.; Puskas, L.G. Perinatal ω-3 polyunsaturated fatty acid supply modifies brain zinc homeostasis during adulthood. Proc. Natl. Acad. Sci. USA 2005, 102, 7133–7138. [Google Scholar]
- Cole, T.B.; Martyanova, A.; Palmiter, R.D. Removing zinc from synaptic vesicles does not impair spatial learning, memory, or sensori motor functions in the mouse. Brain Res. 2001, 891, 253–265. [Google Scholar]
- Hellmich, H.L.; Eidson, K.A.; Capra, B.A.; Garcia, J.M.; Boone, D.R.; Hawkins, B.E.; Uchida, T.; DeWitt, D.S.; Prough, D.S. Injured fluoro-jade positive hippocampal neurons contain high levels of zinc after traumatic brain injury. Brain Res. 2007, 1127, 119–126. [Google Scholar]
- Mao, L.; Chen, J.; Peng, Q.; Zhou, A.; Wang, Z. Effects of different sources and levels of zinc on H2O2-induced apoptosis in IEC-6 cells. Biol. Trace Elem. Res. 2013, 155, 132–141. [Google Scholar]
- Franklin, R.B.; Costello, L.C. The important role of the apoptotic effects of zinc in the development of cancers. J. Cell. Biochem. 2009, 106, 750–757. [Google Scholar]
- Onaolapo, O.J.; Onaolapo, A.Y.; Mosaku, T.J.; Akanji, O.O.; Abiodun, O.R. Elevated plus maze and Y-maze behavioral effects of subchronic, oral low dose monosodium glutamate in Swiss albino mice. J. Pharm. Biol. Sci. 2012, 3, 21–27. [Google Scholar]
- Folch, J.; Lees, M.; Sloane-Stanley, G.H. A simple method for the isolation and purification of total lipids from animal tissues. J. Biol. Chem. 1957, 226, 497–509. [Google Scholar] [PubMed]
- Rajion, M.A.; McLean, J.G.; Cahill, R.N. Essential fatty acids in the fetal and newborn lamb. J. Biol. Sci. 1985, 38, 33–40. [Google Scholar]
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sopian, N.F.A.; Ajat, M.; Shafie, N.I.; Noor, M.H.M.; Ebrahimi, M.; Rajion, M.A.; Meng, G.Y.; Ahmad, H. Does Short-Term Dietary Omega-3 Fatty Acid Supplementation Influence Brain Hippocampus Gene Expression of Zinc Transporter-3? Int. J. Mol. Sci. 2015, 16, 15800-15810. https://doi.org/10.3390/ijms160715800
Sopian NFA, Ajat M, Shafie NI, Noor MHM, Ebrahimi M, Rajion MA, Meng GY, Ahmad H. Does Short-Term Dietary Omega-3 Fatty Acid Supplementation Influence Brain Hippocampus Gene Expression of Zinc Transporter-3? International Journal of Molecular Sciences. 2015; 16(7):15800-15810. https://doi.org/10.3390/ijms160715800
Chicago/Turabian StyleSopian, Nur Farhana Ahmad, Mokrish Ajat, Nurul' Izzati Shafie, Mohd Hezmee Mohd Noor, Mehdi Ebrahimi, Mohamed Ali Rajion, Goh Yong Meng, and Hafandi Ahmad. 2015. "Does Short-Term Dietary Omega-3 Fatty Acid Supplementation Influence Brain Hippocampus Gene Expression of Zinc Transporter-3?" International Journal of Molecular Sciences 16, no. 7: 15800-15810. https://doi.org/10.3390/ijms160715800
APA StyleSopian, N. F. A., Ajat, M., Shafie, N. I., Noor, M. H. M., Ebrahimi, M., Rajion, M. A., Meng, G. Y., & Ahmad, H. (2015). Does Short-Term Dietary Omega-3 Fatty Acid Supplementation Influence Brain Hippocampus Gene Expression of Zinc Transporter-3? International Journal of Molecular Sciences, 16(7), 15800-15810. https://doi.org/10.3390/ijms160715800







