Retinal Cell Death Caused by Sodium Iodate Involves Multiple Caspase-Dependent and Caspase-Independent Cell-Death Pathways
Abstract
:1. Introduction
2. Results
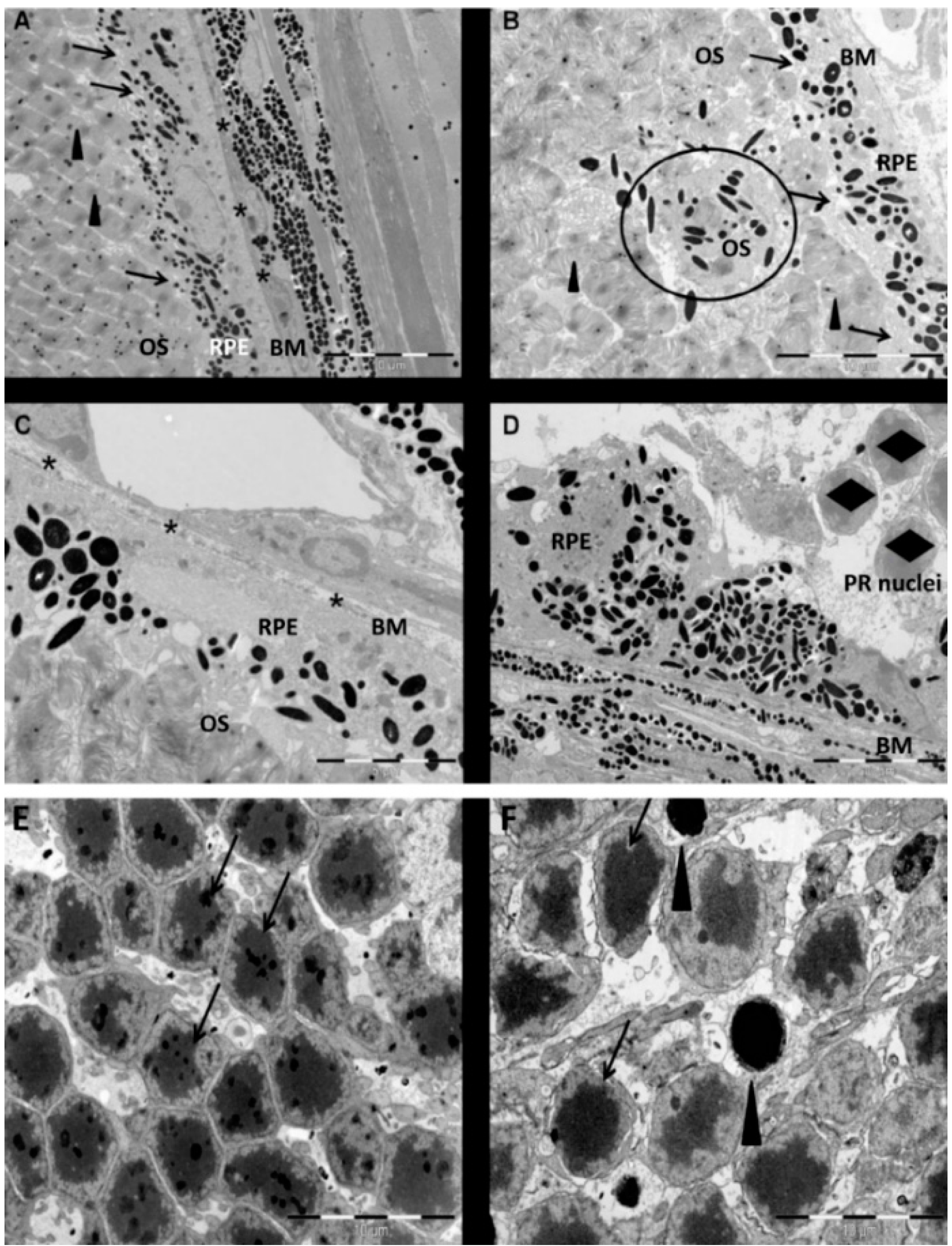
2.1. Ultrastructural Alterations in the Retina Following NaIO3 Administration
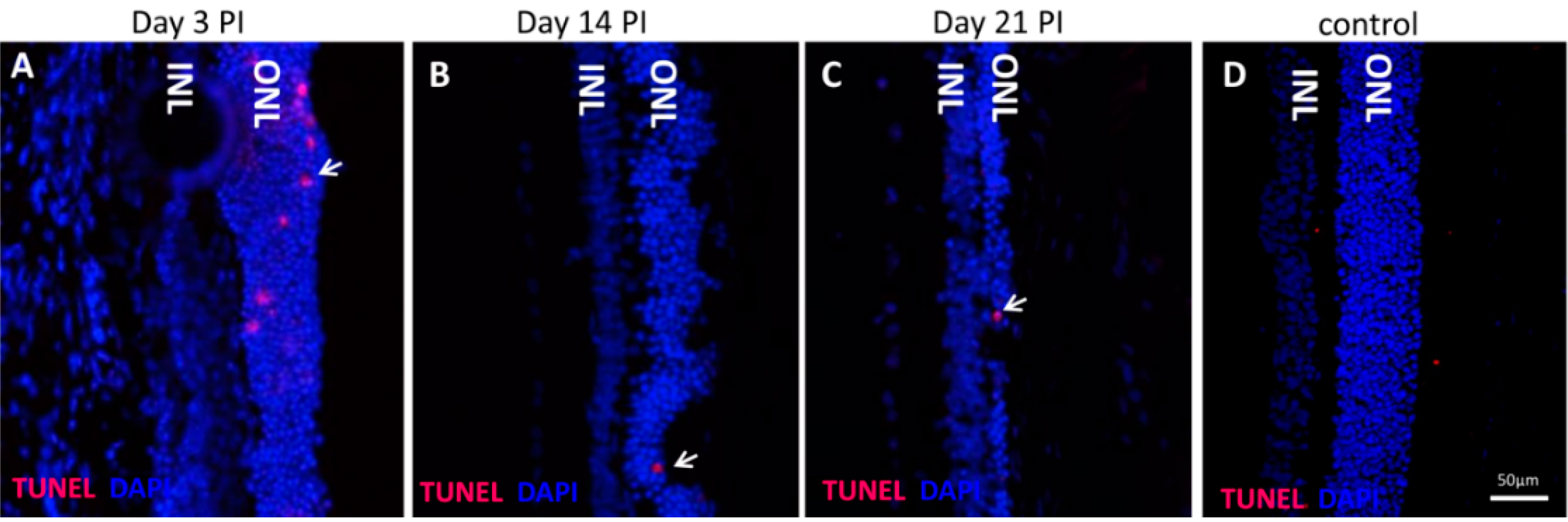
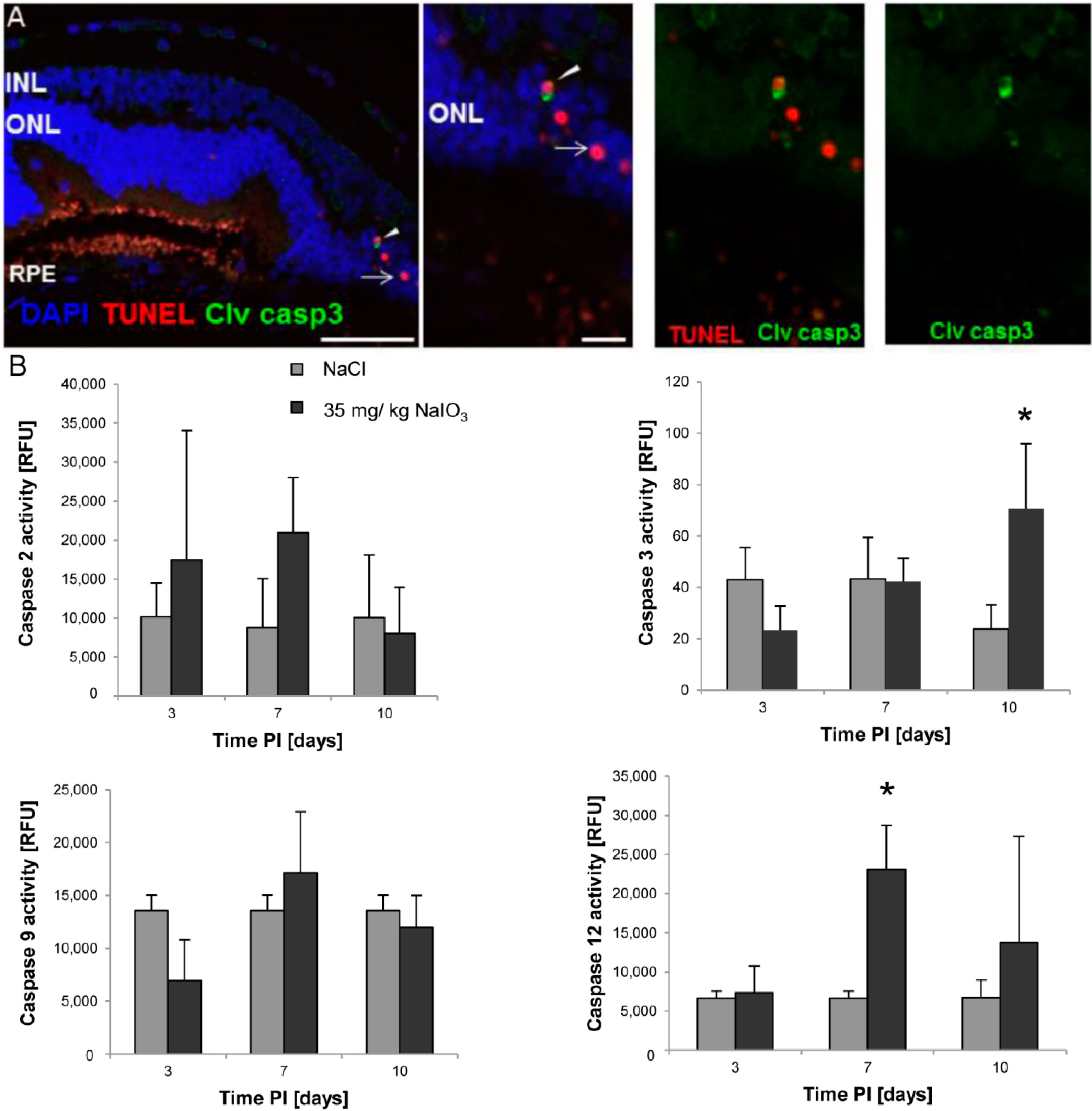
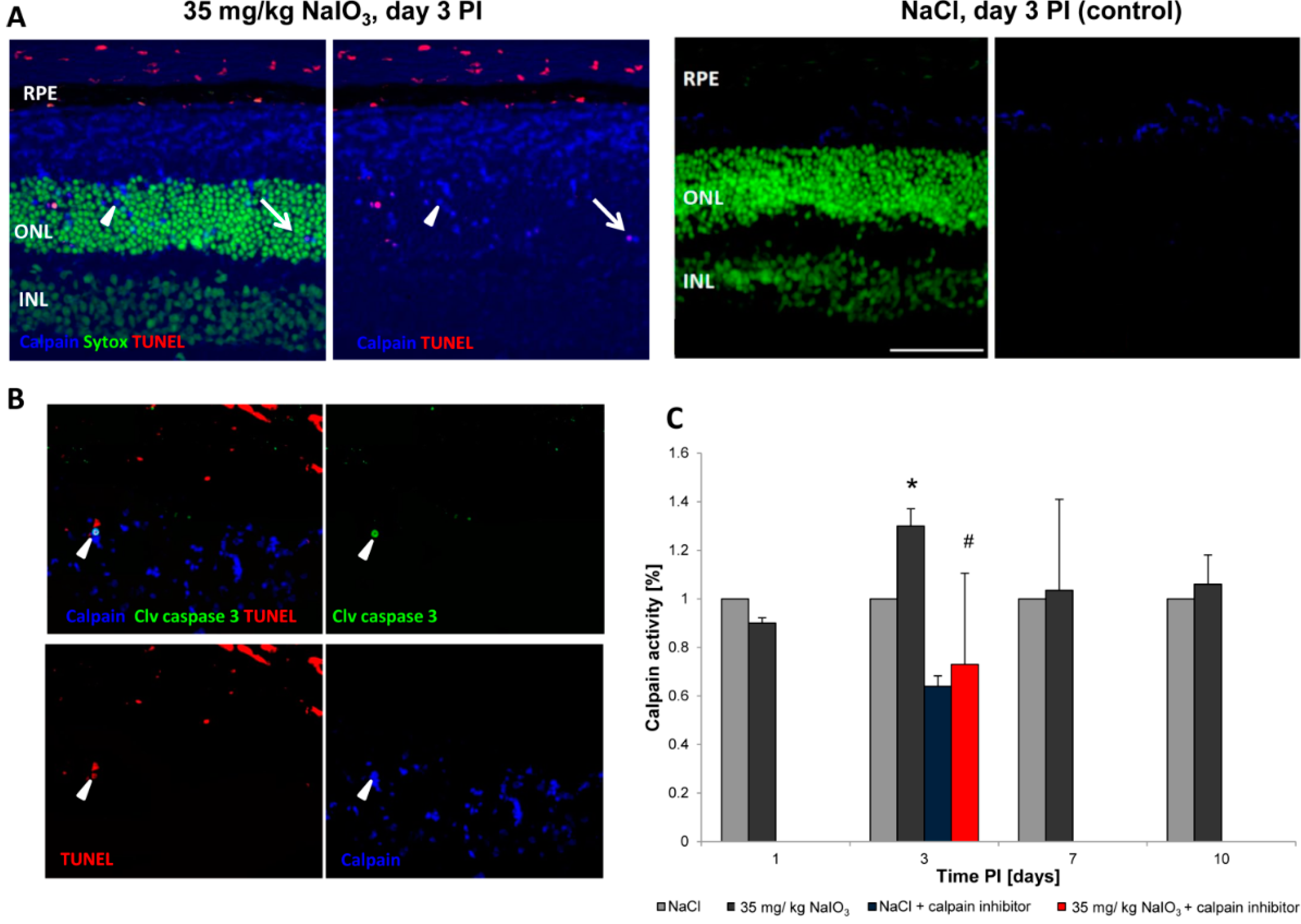
2.2. Calpain and Caspases Are Involved in NaIO3-Induced Photoreceptor Cell Death in Vivo
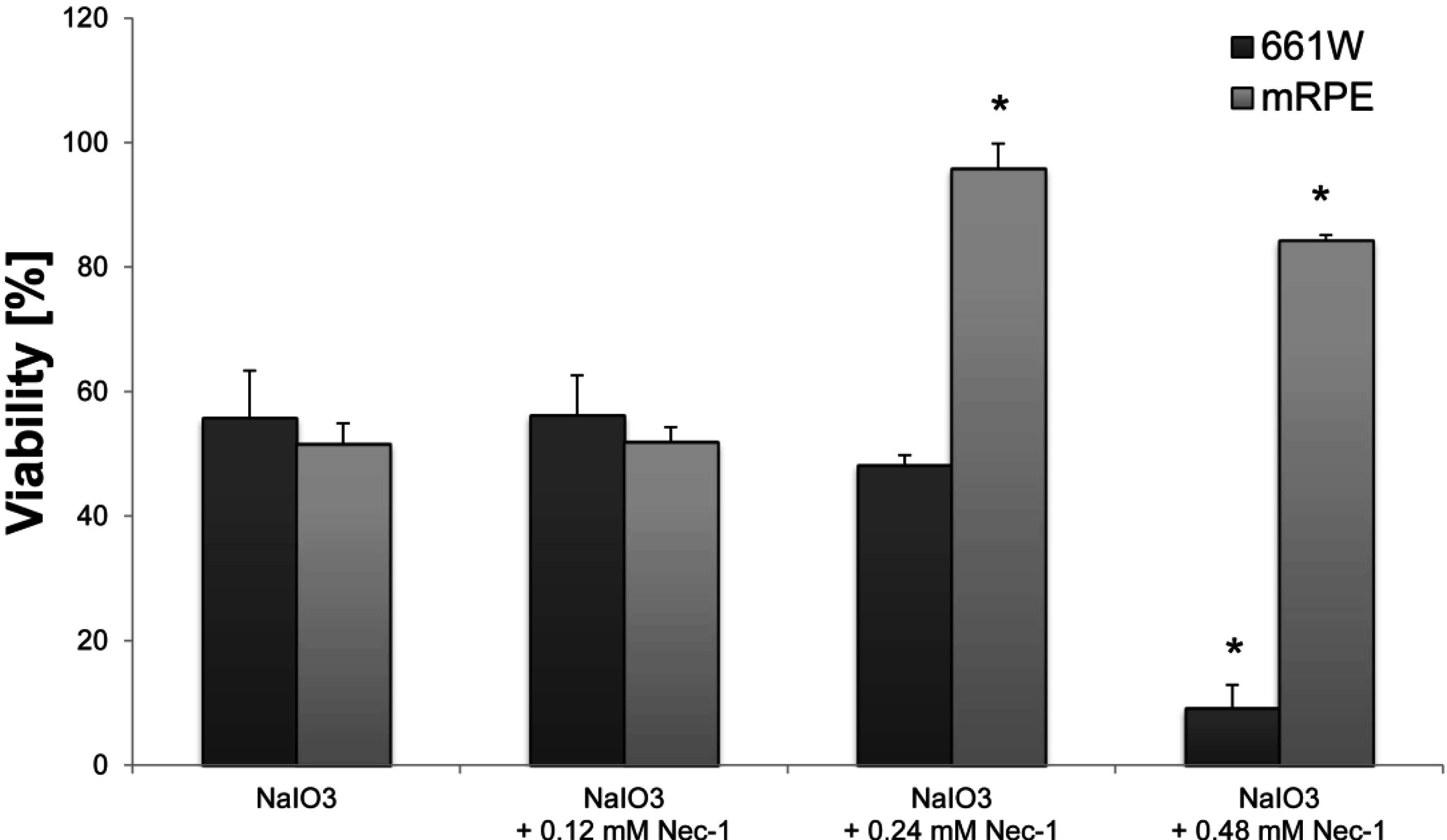
2.3. NaIO3 Induces Necrosis in RPE Cells and Apoptotic Cell Death in 661W Cells in Vitro
 cells,
cells,  + 1 μM staurosporine,
+ 1 μM staurosporine,  + 100% sonication,
+ 100% sonication,  + 6 mM NaIO3,
+ 6 mM NaIO3,  + 12 mM NaIO3,
+ 12 mM NaIO3,  + 48 mM NaIO3); * p < 0.05.
+ 48 mM NaIO3); * p < 0.05.
 cells,
cells,  + 1 μM staurosporine,
+ 1 μM staurosporine,  + 100% sonication,
+ 100% sonication,  + 6 mM NaIO3,
+ 6 mM NaIO3,  + 12 mM NaIO3,
+ 12 mM NaIO3,  + 48 mM NaIO3); * p < 0.05.
+ 48 mM NaIO3); * p < 0.05.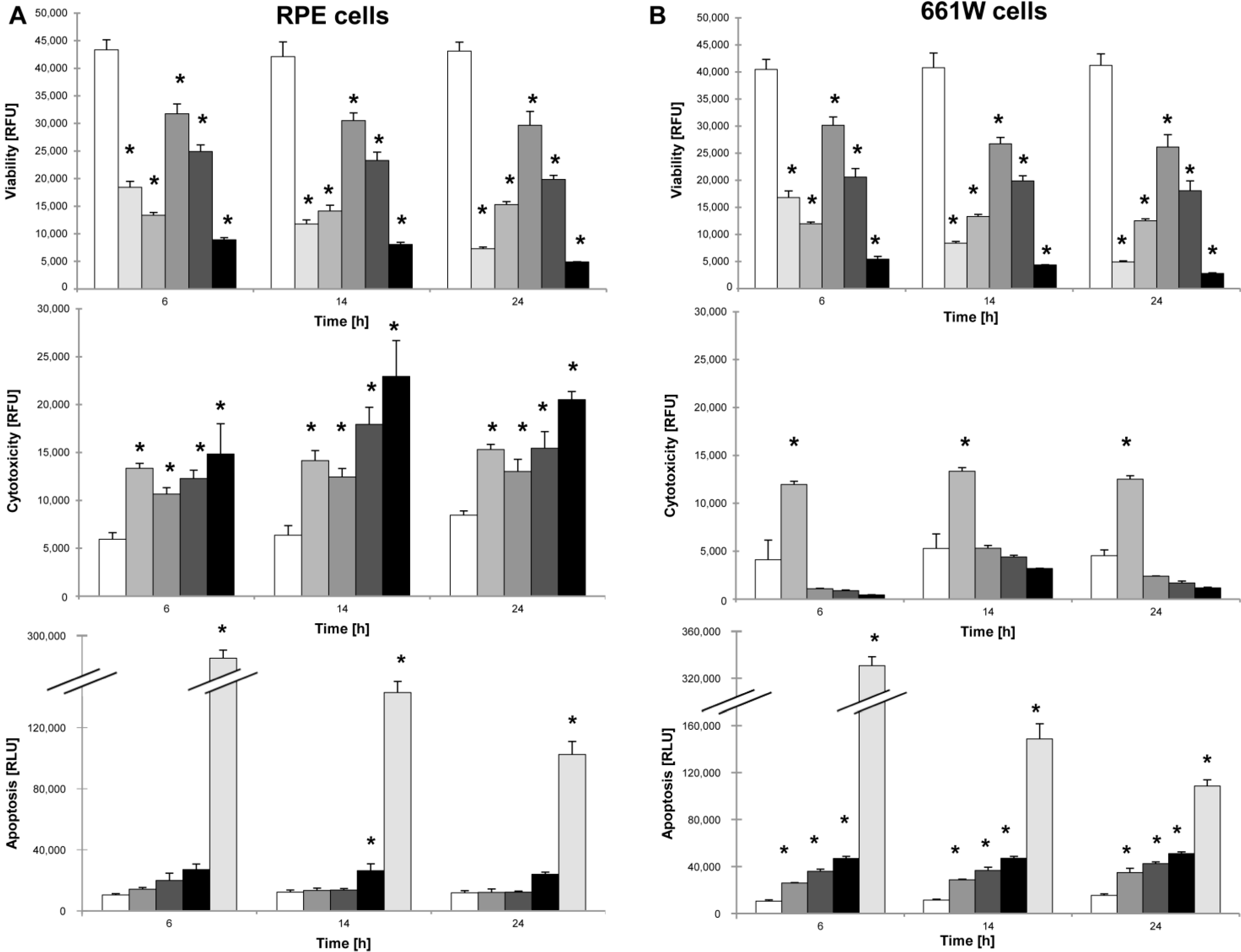
3. Discussion
4. Experimental Section
4.1. Animal Treatment
4.2. Cell Culture
4.3. Electron Microscopy
4.4. Immunohistochemistry
4.5. Calpain and Caspase Activity Assays
4.6. ApoTox Glo Triplex Assay
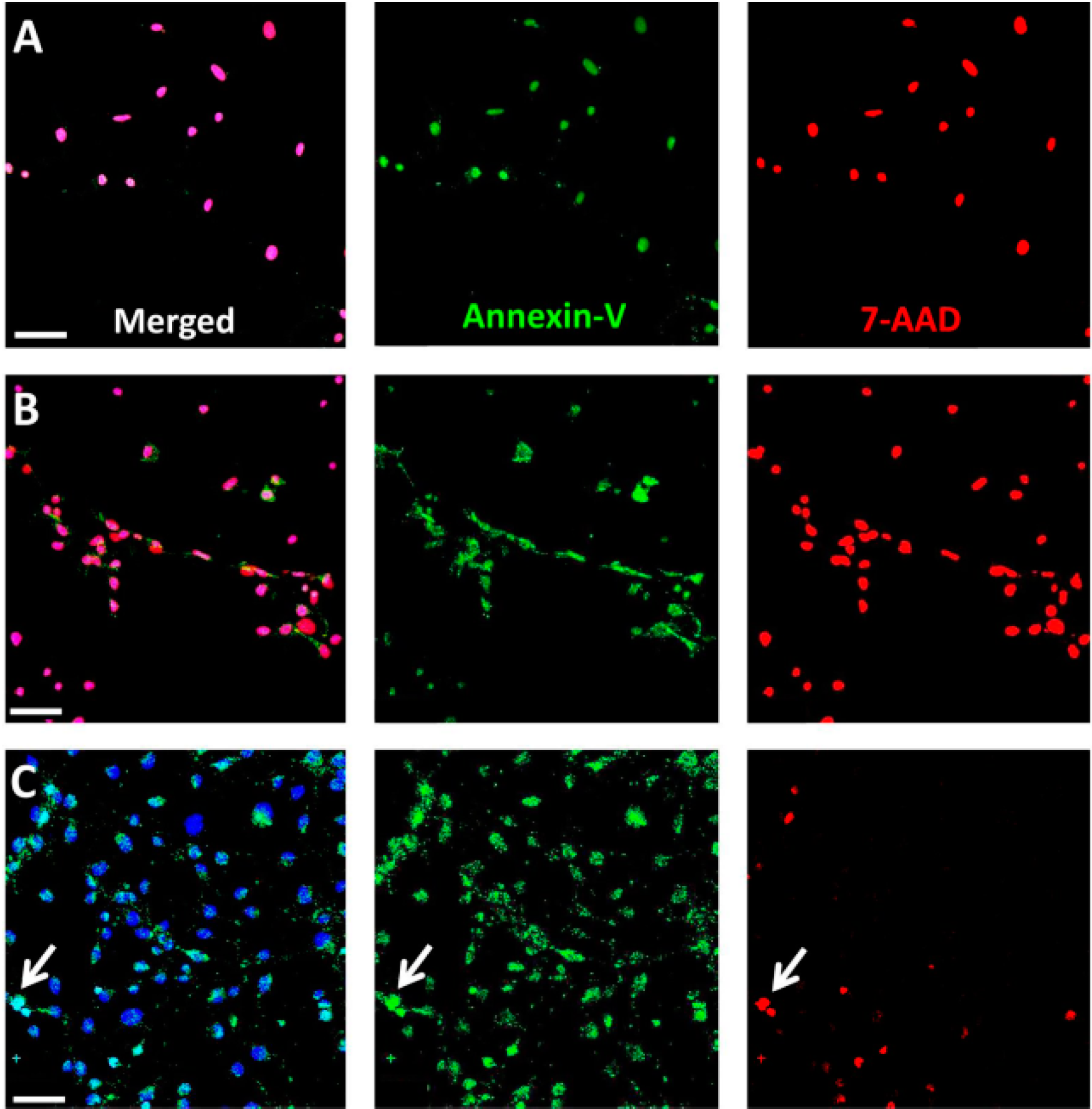
4.7. Annexin-V and 7-AAD Staining
4.8. Necrostatin-1 Treatment
4.9. Statistical Analysis
5. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Webster, S.H.; Rice, M.E.; Highman, B.; von Oettingen, W.F. The toxicology of potassium and sodium iodates: Acute toxicity in mice. J. Pharmacol. Exp. Ther. 1957, 120, 171–178. [Google Scholar] [PubMed]
- Mizota, A.; Adachi-Usami, E. Functional recovery of retina after sodium iodate injection in mice. Vis. Res. 1997, 37, 1859–1865. [Google Scholar] [CrossRef]
- Higuchi, M.; Tomioka, M.; Takano, J.; Shirotani, K.; Iwata, N.; Masumoto, H.; Maki, M.; Itohara, S.; Saido, T.C. Distinct mechanistic roles of calpain and caspase activation in neurodegeneration as revealed in mice overexpressing their specific inhibitors. J. Biol. Chem. 2005, 280, 15229–15237. [Google Scholar] [CrossRef] [PubMed]
- Machalinska, A.; Kawa, M.P.; Pius-Sadowska, E.; Roginska, D.; Klos, P.; Baumert, B.; Wiszniewska, B.; Machalinski, B. Endogenous regeneration of damaged retinal pigment epithelium following low dose sodium iodate administration: An insight into the role of glial cells in retinal repair. Exp. Eye Res. 2013, 112, 68–78. [Google Scholar] [CrossRef] [PubMed]
- Gong, L.; Wu, Q.; Song, B.; Lu, B.; Zhang, Y. Differentiation of rat mesenchymal stem cells transplanted into the subretinal space of sodium iodate-injected rats. Clin. Exp. Ophthalmol. 2008, 36, 666–671. [Google Scholar] [CrossRef] [PubMed]
- Korte, G.E.; Reppucci, V.; Henkind, P. RPE destruction causes choriocapillary atrophy. Investig. Ophthalmol. Vis. Sci. 1984, 25, 1135–1145. [Google Scholar]
- Strauss, O. The retinal pigment epithelium in visual function. Physiol. Rev. 2005, 85, 845–881. [Google Scholar] [CrossRef] [PubMed]
- Bazan, N.G. Neurotrophins induce neuroprotective signaling in the retinal pigment epithelial cell by activating the synthesis of the anti-inflammatory and anti-apoptotic neuroprotectin D1. Adv. Exp. Med. Biol. 2008, 613, 39–44. [Google Scholar] [PubMed]
- Franco, L.M.; Zulliger, R.; Wolf-Schnurrbusch, U.E.; Katagiri, Y.; Kaplan, H.J.; Wolf, S.; Enzmann, V. Decreased visual function after patchy loss of retinal pigment epithelium induced by low-dose sodium iodate. Investig. Ophthalmol. Vis. Sci. 2009, 50, 4004–4010. [Google Scholar] [CrossRef] [PubMed]
- Sen, H.A.; Berkowitz, B.A.; Ando, N.; de Juan, E., Jr. In vivo imaging of breakdown of the inner and outer blood-retinal barriers. Investig. Ophthalmol. Vis. Sci. 1992, 33, 3507–3512. [Google Scholar]
- Baich, A.; Ziegler, M. The effect of sodium iodate and melanin on the formation of glyoxylate. Pigment Cell Res. 1992, 5, 394–395. [Google Scholar] [CrossRef] [PubMed]
- Stern, W.H.; Ernest, J.T.; Steinberg, R.H.; Miller, S.S. Interrelationships between the retinal pigment epithelium and the neurosensory retina. Aust. J. Ophthalmol. 1980, 8, 281–288. [Google Scholar] [CrossRef] [PubMed]
- Ashburn, F.S., Jr.; Pilkerton, A.R.; Rao, N.A.; Marak, G.E. The effects of iodate and iodoacetate on the retinal adhesion. Investig. Ophthalmol. Vis. Sci. 1980, 19, 1427–1432. [Google Scholar]
- Yoon, Y.H.; Marmor, M.F. Retinal pigment epithelium adhesion to bruch’s membrane is weakened by hemicholinium-3 and sodium iodate. Ophthalmic Res. 1993, 25, 386–392. [Google Scholar] [CrossRef] [PubMed]
- Tao, Z.; Dai, J.; He, J.; Li, C.; Li, Y.; Yin, Z.Q. The influence of NaIO3-induced retinal degeneration on intra-retinal layer and the changes of expression profile/morphology of DA-ACs and mRGCs. Mol. Neurobiol. 2013, 47, 241–260. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Iacovelli, J.; Spencer, C.; Saint-Geniez, M. Direct effect of sodium iodate on neurosensory retina. Investig. Ophthalmol. Vis. Sci. 2014, 55, 1941–1953. [Google Scholar] [CrossRef] [PubMed]
- Qin, S.; Lu, Y.; Rodrigues, G.A. Resveratrol protects rpe cells from sodium iodate by modulating pparalpha and ppardelta. Exp. Eye Res. 2014, 118, 100–108. [Google Scholar] [CrossRef] [PubMed]
- Zhou, P.; Ye, H.F.; Jiang, Y.X.; Yang, J.; Zhu, X.J.; Sun, X.H.; Luo, Y.; Dou, G.R.; Wang, Y.S.; Lu, Y. αA crystallin may protect against geographic atrophy-meta-analysis of cataract vs. cataract surgery for geographic atrophy and experimental studies. PLoS ONE 2012, 7, e43173. [Google Scholar] [CrossRef] [PubMed]
- Debnath, J.; Baehrecke, E.H.; Kroemer, G. Does autophagy contribute to cell death? Autophagy 2005, 1, 66–74. [Google Scholar] [CrossRef] [PubMed]
- Sperandio, S.; de Belle, I.; Bredesen, D.E. An alternative, nonapoptotic form of programmed cell death. Proc. Natl. Acad. Sci. USA 2000, 97, 14376–14381. [Google Scholar] [CrossRef] [PubMed]
- Syntichaki, P.; Xu, K.; Driscoll, M.; Tavernarakis, N. Specific aspartyl and calpain proteases are required for neurodegeneration in C. elegans. Nature 2002, 419, 939–944. [Google Scholar] [CrossRef] [PubMed]
- Yamashima, T.; Kohda, Y.; Tsuchiya, K.; Ueno, T.; Yamashita, J.; Yoshioka, T.; Kominami, E. Inhibition of ischaemic hippocampal neuronal death in primates with cathepsin B inhibitor CA-074: A novel strategy for neuroprotection based on “calpain-cathepsin hypothesis”. Eur. J. Neurosci. 1998, 10, 1723–1733. [Google Scholar] [CrossRef] [PubMed]
- Paquet-Durand, F.; Azadi, S.; Hauck, S.M.; Ueffing, M.; van Veen, T.; Ekstrom, P. Calpain is activated in degenerating photoreceptors in the rd1 mouse. J. Neurochem. 2006, 96, 802–814. [Google Scholar] [CrossRef] [PubMed]
- Kaur, J.; Mencl, S.; Sahaboglu, A.; Farinelli, P.; van Veen, T.; Zrenner, E.; Ekstrom, P.; Paquet-Durand, F.; Arango-Gonzalez, B. Calpain and PARP activation during photoreceptor cell death in P23H and S334ter rhodopsin mutant rats. PLoS ONE 2011, 6, e22181. [Google Scholar] [CrossRef] [PubMed]
- Trichonas, G.; Murakami, Y.; Thanos, A.; Morizane, Y.; Kayama, M.; Debouck, C.M.; Hisatomi, T.; Miller, J.W.; Vavvas, D.G. Receptor interacting protein kinases mediate retinal detachment-induced photoreceptor necrosis and compensate for inhibition of apoptosis. Proc. Natl. Acad. Sci. USA 2010, 107, 21695–21700. [Google Scholar] [CrossRef] [PubMed]
- Carido, M.; Zhu, Y.; Postel, K.; Benkner, B.; Cimalla, P.; Karl, M.O.; Kurth, T.; Paquet-Durand, F.; Koch, E.; Münch, T.; et al. Characterization of a mouse model with complete RPE loss and its use for RPE cell transplantation. Investig. Ophthalmol. Vis. Sci. 2014, 55, 5431–5444. [Google Scholar] [CrossRef] [PubMed]
- Nakagawa, T.; Zhu, H.; Morishima, N.; Li, E.; Xu, J.; Yankner, B.A.; Yuan, J. Caspase-12 mediates endoplasmic-reticulum-specific apoptosis and cytotoxicity by amyloid-β. Nature 2000, 403, 98–103. [Google Scholar] [CrossRef] [PubMed]
- Mead, B.; Berry, M.; Logan, A.; Scott, R.A.; Leadbeater, W.; Scheven, B.A. Stem cell treatment of degenerative eye disease. Stem Cell Res. 2015, 14, 243–257. [Google Scholar] [CrossRef] [PubMed]
- Sanvicens, N.; Gomez-Vicente, V.; Masip, I.; Messeguer, A.; Cotter, T.G. Oxidative stress-induced apoptosis in retinal photoreceptor cells is mediated by calpains and caspases and blocked by the oxygen radical scavenger CR-6. J. Biol. Chem. 2004, 279, 39268–39278. [Google Scholar] [CrossRef] [PubMed]
- Ringvold, A.; Olsen, E.G.; Flage, T. Transient breakdown of the retinal pigment epithelium diffusion barrier after sodium iodate: A fluorescin angiographic study in the rabbit. Exp. Eye Res. 1981, 33, 361–369. [Google Scholar] [CrossRef]
- Hariri, S.; Tam, M.C.; Lee, D.; Hileeto, D.; Moayed, A.A.; Bizheva, K. Noninvasive imaging of the early effect of sodium iodate toxicity in a rat model of outer retina degeneration with spectral domain optical coherence tomography. J. Biomed. Opt. 2013, 18, 26017. [Google Scholar] [CrossRef] [PubMed]
- Kiuchi, K.; Yoshizawa, K.; Shikata, N.; Moriguchi, K.; Tsubura, A. Morphologic characteristics of retinal degeneration induced by sodium iodate in mice. Curr. Eye Res. 2002, 25, 373–379. [Google Scholar] [CrossRef] [PubMed]
- Ablonczy, Z.; Dahrouj, M.; Tang, P.H.; Liu, Y.; Sambamurti, K.; Marmorstein, A.D.; Crosson, C.E. Human retinal pigment epithelium cells as functional models for the RPE in vivo. Investig. Ophthalmol. Vis. Sci. 2011, 52, 8614–8620. [Google Scholar] [CrossRef] [PubMed]
- Degterev, A.; Huang, Z.; Boyce, M.; Li, Y.; Jagtap, P.; Mizushima, N.; Cuny, G.D.; Mitchison, T.J.; Moskowitz, M.A.; Yuan, J. Chemical inhibitor of nonapoptotic cell death with therapeutic potential for ischemic brain injury. Nat. Chem. Biol. 2005, 1, 112–119. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.; Chua, C.C.; Kong, J.; Kostrzewa, R.M.; Kumaraguru, U.; Hamdy, R.C.; Chua, B.H. Necrostatin-1 protects against glutamate-induced glutathione depletion and caspase-independent cell death in HT-22 cells. J. Neurochem. 2007, 103, 2004–2014. [Google Scholar] [CrossRef] [PubMed]
- Juel, H.B.; Faber, C.; Svendsen, S.G.; Vallejo, A.N.; Nissen, M.H. Inflammatory cytokines protect retinal pigment epithelial cells from oxidative stress-induced death. PLoS ONE 2013, 8, e64619. [Google Scholar] [CrossRef] [PubMed]
- Tan, E.; Ding, X.P.; Saadi, A.; Agarwal, N.; Naash, M.I.; Al-Ubaidi, M.R. Expression of cone-photoreceptor-specific antigens in a cell line derived from retinal tumors in transgenic mice. Investig. Ophthalmol. Vis. Sci. 2004, 45, 768. [Google Scholar] [CrossRef]
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Balmer, J.; Zulliger, R.; Roberti, S.; Enzmann, V. Retinal Cell Death Caused by Sodium Iodate Involves Multiple Caspase-Dependent and Caspase-Independent Cell-Death Pathways. Int. J. Mol. Sci. 2015, 16, 15086-15103. https://doi.org/10.3390/ijms160715086
Balmer J, Zulliger R, Roberti S, Enzmann V. Retinal Cell Death Caused by Sodium Iodate Involves Multiple Caspase-Dependent and Caspase-Independent Cell-Death Pathways. International Journal of Molecular Sciences. 2015; 16(7):15086-15103. https://doi.org/10.3390/ijms160715086
Chicago/Turabian StyleBalmer, Jasmin, Rahel Zulliger, Stefano Roberti, and Volker Enzmann. 2015. "Retinal Cell Death Caused by Sodium Iodate Involves Multiple Caspase-Dependent and Caspase-Independent Cell-Death Pathways" International Journal of Molecular Sciences 16, no. 7: 15086-15103. https://doi.org/10.3390/ijms160715086
APA StyleBalmer, J., Zulliger, R., Roberti, S., & Enzmann, V. (2015). Retinal Cell Death Caused by Sodium Iodate Involves Multiple Caspase-Dependent and Caspase-Independent Cell-Death Pathways. International Journal of Molecular Sciences, 16(7), 15086-15103. https://doi.org/10.3390/ijms160715086





