Statin, Calcium Channel Blocker and Beta Blocker Therapy May Decrease the Incidence of Tuberculosis Infection in Elderly Taiwanese Patients with Type 2 Diabetes
Abstract
:1. Introduction
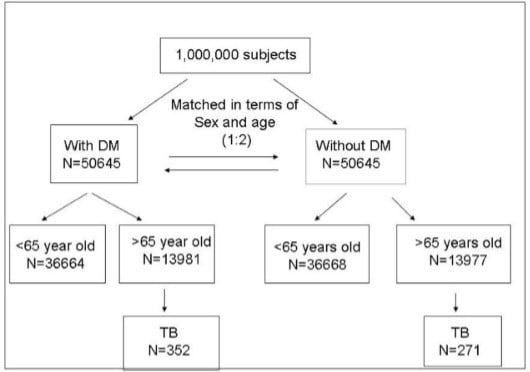
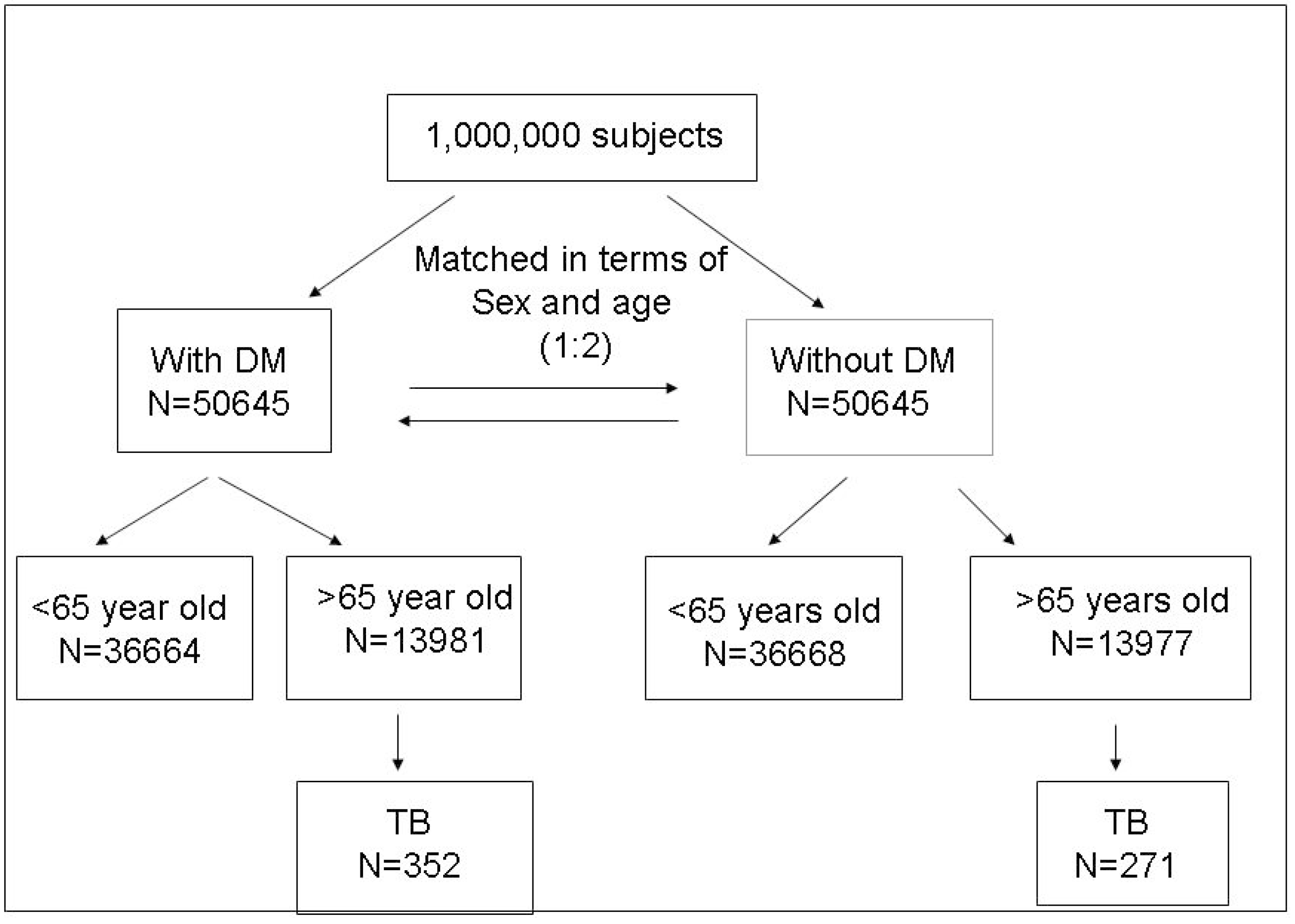
2. Results
| Variables | Total | DM | Without DM | p Value |
|---|---|---|---|---|
| N | N (%) | N (%) | ||
| Sex | ||||
| Female | 15,083 | 7543 (54.0%) | 7540 (53.9%) | |
| Male | 12,875 | 6438 (46.0%) | 6437 (46.1%) | 0.9919 |
| Income category | ||||
| unemployed | 10,374 | 5220 (37.3%) | 5154 (36.9%) | |
| <20,000 NTD | 16,592 | 8168 (58.4%) | 8424 (60.3%) | |
| >20,000 NTD | 992 | 593 (4.2%) | 399 (2.9%) | <0.0001 |
| Residence | ||||
| Metropolitan | 6054 | 3028 (21.7%) | 3026 (21.6%) | |
| Northern cities | 1893 | 975 (7.0%) | 918 (6.6%) | |
| Southern cities | 1067 | 518 (3.7%) | 549 (3.9%) | |
| Northern counties | 12,401 | 6180 (44.2%) | 6221 (44.5%) | |
| Southern counties | 6421 | 3232 (23.1%) | 3189 (22.8%) | 0.1270 |
| Co morbidities | ||||
| Gout | 928 | 597 (4.3%) | 331 (2.4%) | <0.0001 |
| Hypertension | 6285 | 3893 (27.8%) | 2392 (17.1%) | <0.0001 |
| Hyperlipidemia | 1858 | 1162 (8.3%) | 696 (5.0%) | <0.0001 |
| Asthma | 1190 | 736 (5.3%) | 454 (3.2%) | <0.0001 |
| COPD | 2629 | 1507 (10.8%) | 1122 (8.0%) | <0.0001 |
| AIDS | 1 | 1 (0.01%) | 0 (0.00%) | 0.3174 |
| Connective tissue disease | 399 | 217 (1.6%) | 182 (1.3%) | 0.0781 |
| End stage renal disease | 329 | 204 (1.5%) | 125 (0.9%) | <0.0001 |
| Heart failure | 931 | 647 (4.6%) | 284 (2.0%) | <0.0001 |
| Other cardiovascular disease | 2093 | 1309 (9.4%) | 784 (5.6%) | <0.0001 |
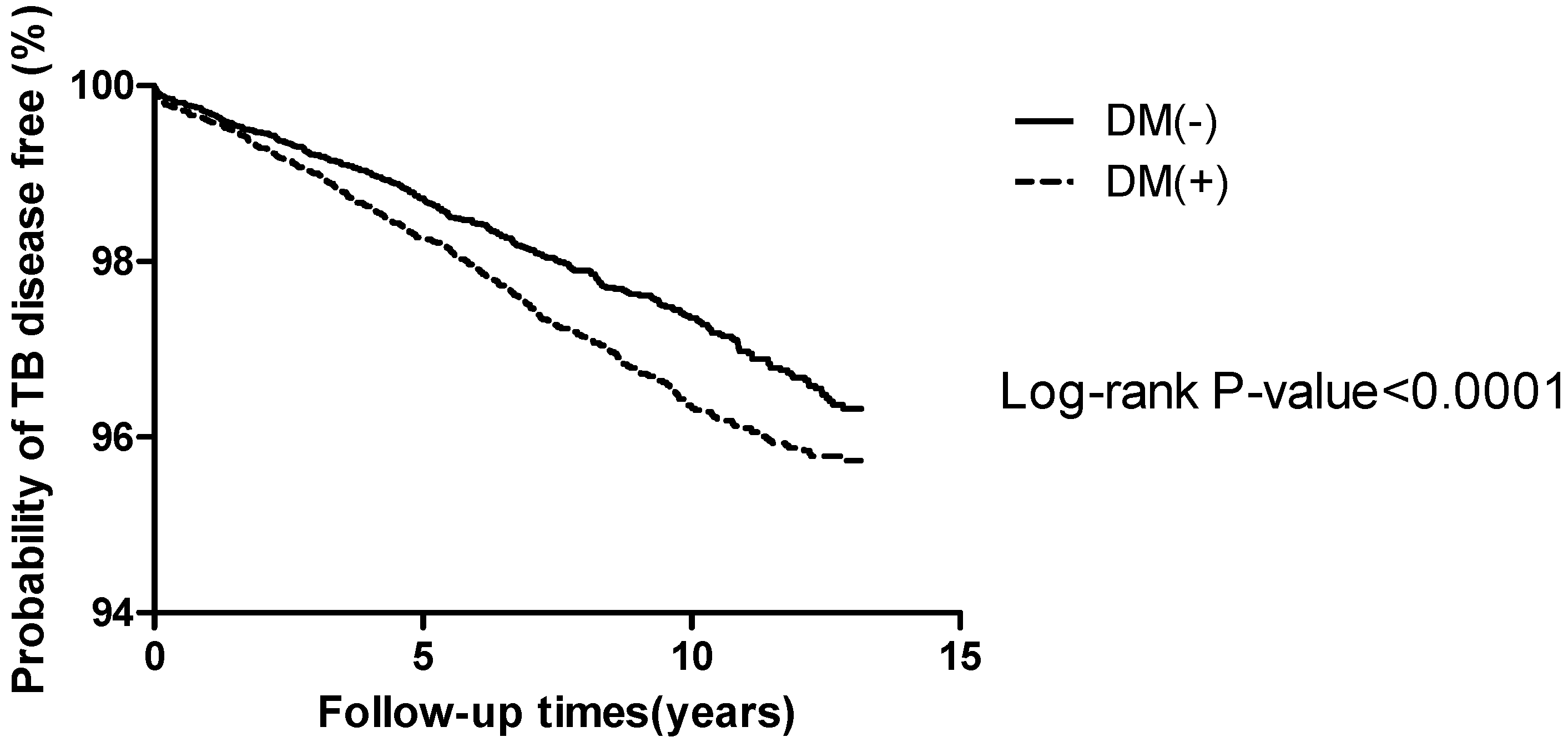
| Tuberculosis | 623 | 352 (2.6%) | 271 (2.0%) | 0.0006 |
| Medications | ||||
| Sulfonylureas | 10,212 | 10,212 (73.0%) | - | <0.0001 |
| Metformin | 8508 | 8508 (60.8%) | - | <0.0001 |
| Acarbose | 2502 | 2502 (17.9%) | - | <0.0001 |
| Thiazolidinediones | 1305 | 1305 (9.3%) | - | <0.0001 |
| Meglitinides | 2508 | 2508 (17.9%) | - | <0.0001 |
| Insulin | 1531 | 1531 (10.9%) | - | <0.0001 |
| ACEI | 8083 | 5472 (39.1%) | 2611 (18.7%) | <0.0001 |
| ARB | 10,578 | 6969 (49.8%) | 3609 (25.3%) | <0.0001 |
| Beta blockers | 11,916 | 6991 (50.0%) | 4925 (35.2%) | <0.0001 |
| Calcium channel lockers | 17,240 | 10,078 (72.1%) | 7162 (51.2%) | <0.0001 |
| Diuretics | 11,007 | 6857 (49.0%) | 4150 (29.7%) | <0.0001 |
| Hydralazine + nitrate | 76 | 49 (0.4%) | 27 (0.2%) | 0.0115 |
| All nitrates | 36 | 23 (0.2%) | 13 (0.1%) | 0.0955 |
| Isosorbide | 3586 | 2326 (16.6%) | 1260 (9.0%) | <0.0001 |
| Other anti-hypertensives | 8977 | 5379 (38.5%) | 3598 (25.7%) | <0.0001 |
| Statins | 6441 | 4715 (33.7%) | 1726 (12.3%) | <0.0001 |
| Other anti-hyperlipidemia agents | 2796 | 2222 (15.9%) | 574 (4.1%) | <0.0001 |
| Variable | Univariate | Multivariate | ||||
|---|---|---|---|---|---|---|
| RR | 95% CI | p Value | ARR | 95% CI | p Value | |
| Gout | 0.92 | 0.49–1.73 | 0.8192 | 0.93 | 0.48–1.77 | 0.8192 |
| Hypertension | 0.90 | 0.68–1.18 | 0.4281 | 0.90 | 0.66–1.22 | 0.4837 |
| Hyperlipidemia | 0.47 | 0.25–0.88 | 0.0179 | 0.57 | 0.30–1.09 | 0.0867 |
| Age (mean ± SD) | 1.04 | 1.03–1.06 | <0.0001 | 1.04 | 1.02–1.06 | <0.0001 |
| Sex (Male) | 2.02 | 1.64–2.49 | <0.0001 | 1.84 | 1.46–2.31 | <0.0001 |
| Income category monthly income >NT $20,000 | 1.40 | 0.89–2.20 | 0.1429 | 1.33 | 0.84–2.09 | 0.2206 |
| Northern cities | 1.05 | 0.67–1.64 | 0.8336 | 1.01 | 0.64–1.57 | 0.9828 |
| Southern cities | 0.96 | 0.54–1.74 | 0.9027 | 0.89 | 0.50–1.61 | 0.7038 |
| Northern counties | 0.94 | 0.71–1.24 | 0.6676 | 0.94 | 0.71–1.24 | 0.6480 |
| Southern counties | 1.46 | 1.09–1.94 | 0.011 | 1.39 | 1.03–1.86 | 0.0288 |
| Asthma | 2.07 | 1.39–3.09 | 0.0004 | 1.83 | 1.17–2.86 | 0.0080 |
| COPD | 1.63 | 1.18–2.26 | 0.0031 | 1.34 | 0.92–1.95 | 0.1260 |
| AIDS | - | - | - | - | - | - |
| Connective tissue disease | 0.52 | 0.13–2.08 | 0.3533 | 0.53 | 0.13–2.15 | 0.3732 |
| End stage renal disease | 0.62 | 0.16–2.50 | 0.5042 | 0.63 | 0.15–2.50 | 0.5150 |
| Heart failure | 0.98 | 0.54–1.78 | 0.9441 | 0.72 | 0.38–1.35 | 0.3035 |
| Other cardiovascular disease | 1.31 | 0.91–1.91 | 0.1538 | 1.33 | 0.89–1.99 | 0.1652 |
| Sulfonylureas | 1.17 | 0.90–1.53 | 0.2460 | 1.30 | 0.99–1.78 | 0.0624 |
| Metformin | 0.93 | 0.76–1.16 | 0.5303 | 0.93 | 0.73–1.17 | 0.5209 |
| Acarbose | 1.31 | 1.04–1.66 | 0.0215 | 1.29 | 1.00–1.66 | 0.0493 |
| Thiazolidinediones | 0.91 | 0.65–1.27 | 0.5740 | 0.88 | 0.62–1.25 | 0.4842 |
| Meglitinides | 1.50 | 1.19–1.88 | 0.0005 | 1.45 | 1.14–1.85 | 0.0026 |
| Insulin | 1.50 | 1.05–1.93 | 0.0246 | 1.42 | 1.04–1.96 | 0.0286 |
| ACEI | 1.08 | 0.88–1.33 | 0.4463 | 1.14 | 0.92–1.43 | 0.2380 |
| ARB | 0.80 | 0.66–0.99 | 0.0362 | 0.85 | 0.68–1.07 | 0.1748 |
| Beta-blockers | 0.70 | 0.57–0.86 | 0.0008 | 0.72 | 0.58–0.91 | 0.0048 |
| Calcium channel blockers | 0.76 | 0.60–0.96 | 0.0199 | 0.76 | 0.58–0.98 | 0.0374 |
| Diuretics | 1.35 | 1.09–1.66 | 0.0053 | 1.41 | 1.13–1.77 | 0.0028 |
| Hydralazine plus nitrate | 1.09 | 0.27–4.37 | 0.9013 | 1.23 | 0.31–4.97 | 0.7703 |
| All nitrates | - | - | - | - | - | - |
| Isosorbide | 1.24 | 0.98–1.59 | 0.0791 | 1.32 | 1.02–1.71 | 0.0323 |
| Other anti-hypertensives | 1.42 | 1.16–1.74 | 0.0008 | 1.09 | 0.87–1.37 | 0.4617 |
| Statins | 0.66 | 0.53–0.83 | 0.0004 | 0.76 | 0.60–0.97 | 0.0291 |
| Other anti-hyperlipidemia agents | 0.77 | 0.58–1.03 | 0.0775 | 0.93 | 0.69–1.25 | 0.6123 |
3. Discussion
4. Materials and Methods
4.1. Setting
4.2. Study end Points
4.3. Possible Associated Risk Factors of Diabetes and Tuberculosis Infection
4.4. Statistical Methods
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- IDF Diabetes Atlas. Available online: http://www.eatlas.idf.org (accessed on 2013).
- Lönnroth, K.; Castro, K.G.; Chakaya, J.M.; Chauhan, L.S.; Floyd, K.; Glaziou, P.; Raviglione, M.C. Tuberculosis control and elimination 2010–50: Cure, care, and social development. Lancet 2010, 375, 1814–1829. [Google Scholar] [PubMed]
- Global Tuberculosis Report 2013; World Health Organization: Geneva, Switzerland, 2013.
- Holliday, R. Understanding ageing. Philos. Trans. Roy. Soc. Lond. B 1997, 352, 1793–1797. [Google Scholar] [CrossRef]
- Rymkiewicz, P.D.; Heng, Y.X.; Vasudev, A.; Larbi, A. The immune system in the aging human. Immunol. Res. 2012, 53, 235–250. [Google Scholar] [CrossRef] [PubMed]
- Dooley, K.E.; Tang, T.; Golub, J.E.; Dorman, S.E.; Cronin, W. Impact of diabetes mellitus on treatment outcomes of patients with active tuberculosis. Am. J. Trop. Med. Hyg. 2009, 80, 634–639. [Google Scholar] [PubMed]
- Dye, C.; Trunz, B.B.; Lönnroth, K.; Roglic, G.; Williams, B.G. Nutrition, diabetes and tuberculosis in the epidemiological transition. PLoS ONE 2011, 6, e21161. [Google Scholar] [CrossRef] [PubMed]
- Baker, M.A.; Harries, A.D.; Jeon, C.Y.; Hart, J.E.; Kapur, A.; Lönnroth, K.; Ottmani, S.E.; Goonesekera, S.D.; Murray, M.B. Systematic review: The impact of diabetes on tuberculosis treatment outcomes. BMC Med. 2011, 9, 81. [Google Scholar] [CrossRef] [PubMed]
- Giovannucci, E.; Harlan, D.M.; Archer, M.C.; Bergenstal, R.M.; Gapstur, S.M.; Habel, L.A.; Pollak, M.; Regensteiner, J.G.; Yee, D. Diabetes and cancer: A consensus report. Diabetes Care 2010, 33, 1674–1685. [Google Scholar] [CrossRef] [PubMed]
- West, K.M. Epidemiology of Diabetes and Its Vascular Lesions; Elsevier: New York, NY, USA, 1978; p. 351. [Google Scholar]
- Tattersall, R.B.; Gale, E.A.M. Infections. In Diabetes, Clinical Management; Tattersall, R.B., Gale, E.A.M., Eds.; Churchill Livingstone: Edinburgh, UK, 1990; pp. 358–364. [Google Scholar]
- Wilson, R.M. Infection and diabetes mellitus. In Textbook of Diabetes; Pickup, J.C., Williams, G., Eds.; Blackwell Scientific Publication: Oxford, UK, 1991; pp. 813–819. [Google Scholar]
- Ruslami, R.; Aarnoutse, R.E.; Alisjahbana, B.; van Der Ven, A.J.A.M.; van Crevel, R. Implications of the global increase of diabetes for tuberculosis controland patient care. Trop. Med. Int. Health 2010, 15, 1289–1299. [Google Scholar] [CrossRef] [PubMed]
- Stevenson, C.R.; Critchley, J.A.; Forouhi, N.G.; Roglic, G.; Williams, B.G.; Dye, C.; Unwin, N.C. Diabetes and the risk of tuberculosis: A neglected threat to publichealth? Chronic Illn. 2007, 3, 228–245. [Google Scholar] [CrossRef] [PubMed]
- Jeon, C.; Murray, M. Diabetes mellitus increases the risk of activetuberculosis: A systematic review of 13 observational studies. PLoS Med. 2008, 5, e152. [Google Scholar] [CrossRef] [PubMed]
- Alisjahbana, B.; Sahiratmadja, E.; Nelwan, E.J.; Purva, A.M.; Ahmad, Y.; Ottenhoff, T.H.; Nelwan, R.H.; Parwati, I.; van der Meer, J.W.; van Crevel, R. The effect of type 2 diabetes mellitus on the presentation andtreatment response of pulmonary tuberculosis. Clin. Infect. Dis. 2007, 45, 428–435. [Google Scholar] [CrossRef] [PubMed]
- Bautista, L.E.; Vera, L.M.; Arenas, I.A.; Gamarra, G. Independent associationbetween inflammatory markers (C-reactive protein, interleukin-6, and TNFalpha) and essential hypertension. J. Hum. Hypertens. 2005, 19, 149–154. [Google Scholar] [CrossRef] [PubMed]
- Chae, C.U.; Lee, R.T.; Rifai, N.; Ridker, P.M. Blood pressure and inflammationin apparently healthy men. Hypertension 2001, 38, 399–403. [Google Scholar] [CrossRef] [PubMed]
- Laviades, C.; Varo, N.; Diez, J. Transforming growth factor beta in hypertensives with cardiorenal damage. Hypertension 2000, 36, 517–522. [Google Scholar] [CrossRef] [PubMed]
- Dorffel, Y.; Latsch, C.; Stuhlmuller, B.; Schreiber, S.; Scholze, S.; Burmester, G.R.; Scholze, J. Preactivated peripheral blood monocytes in patients with essential hypertension. Hypertension 1999, 34, 113–117. [Google Scholar] [CrossRef] [PubMed]
- Frossard, P.M.; Gupta, A.; Pravica, V.; Perrey, C.; Hutchinson, I.V.; Lukic, M.L. Astudy of five human cytokine genes in human essential hypertension. Mol. Immunol. 2002, 38, 969–976. [Google Scholar] [CrossRef] [PubMed]
- Muller, D.N.; Shagdarsuren, E.; Park, J.K.; Dechend, R.; Mervaala, E.; Hampich, F.; Fiebeler, A.; Ju, X.; Finckenberg, P.; Theuer, J.; et al. Immunosuppressive treatment protects against angiotensin II-induced renal damage. Am. J. Pathol. 2002, 161, 1679–1693. [Google Scholar] [CrossRef] [PubMed]
- Muller, D.N.; Dechend, R.; Mervaala, E.M.; Park, J.K.; Schmidt, F.; Fiebeler, A.; Theuer, J.; Breu, V.; Ganten, D.; Haller, H.; et al. NF-kappaB inhibition ameliorates angiotensin II-induced inflammatory damage in rats. Hypertension 2000, 35, 193–201. [Google Scholar] [CrossRef] [PubMed]
- Shagdarsuren, E.; Wellner, M.; Braesen, J.H.; Park, J.K.; Fiebeler, A.; Henke, N.; Dechend, R.; Gratze, P.; Luft, F.C.; Muller, D.N. Complement activation inangiotensin II-induced organ damage. Circ. Res. 2005, 97, 716–724. [Google Scholar] [CrossRef] [PubMed]
- Crowley, S.D.; Frey, C.W.; Gould, S.K.; Griffiths, R.; Ruiz, P.; Burchette, J.L.; Howell, D.N.; Makhanova, N.; Yan, M.; Kim, H.S.; et al. Stimulation of lymphocyte responses by angiotensin II promotes kidneyinjury in hypertension. Am. J. Physiol. Ren. Physiol. 2008, 295, F515–F524. [Google Scholar] [CrossRef]
- Franco, M.; Martinez, F.; Quiroz, Y.; Galicia, O.; Bautista, R.; Johnson, R.J.; Rodriguez-Iturbe, B. Renal angiotensin II concentration and interstitial infiltration of immune cells are correlated with blood pressure levels insalt-sensitive hypertension. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2007, 293, R251–R256. [Google Scholar] [CrossRef] [PubMed]
- Guzik, T.J.; Hoch, N.E.; Brown, K.A.; McCann, L.A.; Rahman, A.; Dikalov, S.; Goronzy, J.; Weyand, C.; Harrison, D.G. Role of the T cell in the genesis ofangiotensin II induced hypertension and vascular dysfunction. J. Exp. Med. 2007, 204, 2449–2460. [Google Scholar] [CrossRef] [PubMed]
- Madhur, M.S.; Lob, H.E.; McCann, L.A.; Iwakura, Y.; Blinder, Y.; Guzik, T.J.; Harrison, D.G. Interleukin 17 promotes angiotensin II-induced hypertension and vascular dysfunction. Hypertension 2010, 55, 500–507. [Google Scholar] [CrossRef] [PubMed]
- Gupta, S.; Salam, N.; Srivastava, V.; Singla, R.; Behera, D.; Khayyam, K.U.; Korde, R.; Malhotra, P.; Saxena, R.; Natarajan, K. Voltage gated calcium channels negatively regulate protective immunity to Mycobacterium tuberculosis. PLoS ONE 2009, 4, e5305. [Google Scholar] [CrossRef] [PubMed]
- Gupta, S.; Tyagi, S.; Almeida, D.V.; Maiga, M.C.; Ammerman, N.C.; Bishai, W.R. Acceleration of tuberculosis treatment by adjunctive therapy with verapamil as an efflux inhibitor. Am. J. Respir. Crit. Care Med. 2013, 188, 600e7. [Google Scholar]
- Arcangelo, V.P.; Peterson, A.M. Pharmacotherapeutics For Advanced Practice: A Practical Approach; Lippincott Williams Wilkins: Philadelphia, PA, USA, 2006; p. 205. [Google Scholar]
- Kohm, A.P.; Sanders, V.M. Norepinephrine: A messenger from the brain to the immune system. Immunol. Today 2000, 21, 539–542. [Google Scholar] [CrossRef] [PubMed]
- Shilov, J.I.; Gein, S.V.; Chereshnev, V.A. Influence of Blockade of beta-Adreno receptors and Acute Stress on Antibody Formation, Delayed Type of Hypersensitivity. Phagocytic Cell Act. Local Immune Response 2001, 6, 301–308. [Google Scholar]
- Kohm, A.P.; Sanders, V.M. Norepinephrine and beta 2-adrenergic receptor stimulation regulate CD4+ T and B lymphocyte function in vitro and in vivo. Pharmacol. Rev. 2001, 53, 487–525. [Google Scholar] [PubMed]
- Argmann, C.A.; Edwards, J.Y.; Sawyez, C.G.; O’Neil, C.H.; Hegele, R.A.; Pickering, J.G.; Huff, M.W. Regulation of macrophage cholesterol efflux through hydroxyl methylglutaryl-CoA reductase inhibition: A role for RhoA in ABCA1-mediated cholesterol efflux. J. Biol. Chem. 2005, 280, 22212–22221. [Google Scholar] [CrossRef] [PubMed]
- Qiu, G.; Hill, J.S. Atorvastatin inhibits ABCA1 expression and cholesterol efflux in THP-1 macrophages by an LXR-dependent pathway. J. Cardiovasc. Pharmacol. 2008, 51, 388–395. [Google Scholar] [CrossRef] [PubMed]
- Loike, J.D.; Shabtai, D.Y.; Neuhut, R.; Malitzky, S.; Lu, E.; Husemann, J.; Goldberg, I.J.; Silverstein, S.C. Statin inhibition of Fc receptor-mediated phagocytosis by macrophages is modulated by cell activation and cholesterol. Arterioscler. Thromb. Vasc. Biol. 2004, 24, 2051–2056. [Google Scholar] [CrossRef] [PubMed]
- Nnoaham, K.E.; Clarke, A. Low serum vitamin D levels and tuberculosis: Asystematic review and meta-analysis. Int. J. Epidemiol. 2008, 37, 113–119. [Google Scholar] [CrossRef] [PubMed]
- Chocano-Bedoya, P.; Ronnenberg, A.G. Vitamin D and tuberculosis. Nutr. Rev. 2009, 67, 289–293. [Google Scholar] [CrossRef] [PubMed]
- Banerjee, D.; Bhattacharyya, R.; Kaul, D.; Sharma, P. Diabetes and tuberculosis: Analysis of a paradox. Adv. Clin. Chem. 2011, 53, 139–153. [Google Scholar] [PubMed]
- Wolden-Kirk, H.; Overbergh, L.; Christesen, H.T.; Brusgaard, K.; Mathieu, C. Vitamin D and diabetes: Its importance for beta cell and immune function. Mol. Cell. Endocrinol. 2011, 347, 106–120. [Google Scholar] [CrossRef] [PubMed]
- Yilmaz, H.; Kaya, M.; Sahin, M.; Delibasi, T. Is vitamin D status a predictorglycaemic regulation and cardiac complication in type 2 diabetes mellituspatients? Diabetes Metab. Syndr. 2012, 6, 28–31. [Google Scholar] [CrossRef] [PubMed]
- Pauwels, R.A.; Buist, A.S.; Calverley, P.M.; Jenkins, C.R.; Hurd, S.S. GOLD Scientific Committee. Global strategy for thediagnosis, management, and prevention of chronic obstructive pulmonarydisease. NHLBI/WHO Global Initiative for Chronic ObstructiveLung Disease (GOLD) Workshop summary. Am. J. Respir. Crit. Care Med. 2001, 163, 1256–1276. [Google Scholar] [CrossRef] [PubMed]
- Murray, J.F.; Mason, R.J.; Broaddus, V.C.; Nadel, J.A.; Martin, T.R.; King, T.E., Jr.; Schraufnagel, D.E. Murray and Nadel’s Textbook of Respiratory Medicine, 5th ed.; Elsevier: Philadelphia, PA, USA, 2010. [Google Scholar]
- Orme, I.M.; Andersen, P.; Boom, W.H. T cell response to Mycobacterium tuberculosis. J. Infect. Dis. 1993, 167, 1481–1497. [Google Scholar] [CrossRef] [PubMed]
- Segal, B.H.; Sneller, M.C. Infectious complications of immunosuppressive therapy in patients with rheumatic diseases. Rheum. Dis. Clin. N. Am. 1997, 23, 219–237. [Google Scholar] [CrossRef]
- Fauci, A.S.; Dale, D.C.; Balow, J.E. Glucocortico steroid therapy: Mechanisms of action and clinical considerations. Ann. Intern. Med. 1976, 84, 304–315. [Google Scholar] [CrossRef] [PubMed]
- Rosenfeld, M.E.; Campbell, L.A. Pathogens and atherosclerosis: Update onthe potential contribution of multiple infectious organisms to the pathogenesis of atherosclerosis. Thromb. Haemost. 2011, 106, 858–867. [Google Scholar] [CrossRef] [PubMed]
- Gibbons, R.J.; Abrams, J.; Chatterjee, K.; Daley, J.; Deedwania, P.C.; Douglas, J.S.; Ferguson, T.B.; Fihn, S.D.; Fraker, T.D.; Gardin, J.M. ACC/AHA 2002 Guideline Update for the Management of Patients With Chronic Stable Angina—Summary Article: A Report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Committee on the Management of Patients With Chronic Stable Angina). J. Am. Coll. Cardiol. 2003, 41, 159–168. [Google Scholar] [CrossRef]
- Hofbauer, R.; Frass, M.; Pasching, E.; Gmeiner, B.; Kaye, A.D.; Kapiotis, S. Furosemide and spironolactone reduce transmigration of leukocytes through endothelial cell monolayers. J. Toxicol. Environ. Health A 2002, 65, 685–693. [Google Scholar] [CrossRef] [PubMed]
- Miura, R.; Nakamura, K.; Miura, D.; Miura, A.; Hisamatsu, K.; Kajiya, M.; Nagase, S.; Morita, H.; Fukushima, K.; Ohe, T.; et al. Anti-inflammatory effect of spironolactone on human peripheral blood mononuclear cells. J. Pharmacol. Sci. 2006, 101, 256–259. [Google Scholar] [CrossRef] [PubMed]
- Crowe, J.H.; Crowe, L.M.; Chapman, D. Preservation of membranes in anhydrobiotic organisms: The role of trehalose. Science 1984, 223, 701–703. [Google Scholar] [CrossRef] [PubMed]
- Daffe, M.; Draper, P. The envelope layers of mycobacteria with reference to their pathogenicity. Adv. Microb. Physiol. 1998, 39, 131–203. [Google Scholar] [PubMed]
- Pan, Y.T.; Carroll, J.D.; Asano, N.; Pastuszak, I.; Edavana, V.K.; Elbein, A.D. Trehalose synthase converts glycogen to trehalose. FEBS J. 2008, 275, 3408–3420. [Google Scholar] [CrossRef] [PubMed]
- Caner, S.; Nguyen, N.; Aguda, A.; Zhang, R.; Pan, Y.T.; Withers, S.G.; Brayer, G.D. The structure of the Mycobacterium smegmatis trehalose synthase reveals an unusual active site configuration and acarbose-binding mode. Soc. Glycobiol. 2013, 23, 1075–1083. [Google Scholar]
- Niazi, A.K.; Kalra, S. Diabetes and tuberculosis: A review of the role of optimal glycemic control. J. Diabetes Metab. Disord. 2012, 11, 28. [Google Scholar] [CrossRef] [PubMed]
- Rodbard, H.W.; Jelleinger, P.S.; Davidson, J.A.; Einhorn, D.; Garber, A.J.; Grunberger, G.; Handelsman, Y.; Horton, E.S.; Lebovitz, H.; Levy, P.; et al. Statement by an AACE/ACEConsensus Panel on type 2 diabetes mellitus An algorithm for glycemiccontrol. Endocr. Pr. 2009, 15, 540–559. [Google Scholar] [CrossRef]
- Dobler, C.C.; Flack, J.R.; Marks, G.B. Risk of tuberculosis among people with diabetes mellitus: An Australian nationwide cohort study. BMJ Open 2012, 2, e000666. [Google Scholar] [CrossRef] [PubMed]
- Bureau of National Health Insurance. Available online: http://www.nhi.gov.tw/ 533information/bulletin_file/421_0890036465-19.doc (accessed on 1 April 2009).
- Lo, H.Y.; Yang, S.L.; Chou, P.; Chuang, J.H.; Chiang, C.Y. Completeness and timeliness of tuberculosis notification in Taiwan. BMC Public Health 2011, 11, 915. [Google Scholar] [CrossRef] [PubMed]
- Taiwan Centers for Disease Control. Treatment guidelines for tuberculosis, 2nd Edition ed2006. Available online: http://www.cdc.gov.tw/infectionreportinfo.aspx?treeid=075874dc882a5bfd&nowtreeid=8dba723ff186fac0&tid=BAB48CF8772C3B05 (accessed on 5 March 2013).
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, M.-Y.; Lin, K.-D.; Hsu, W.-H.; Chang, H.-L.; Yang, Y.-H.; Hsiao, P.-J.; Shin, S.-J. Statin, Calcium Channel Blocker and Beta Blocker Therapy May Decrease the Incidence of Tuberculosis Infection in Elderly Taiwanese Patients with Type 2 Diabetes. Int. J. Mol. Sci. 2015, 16, 11369-11384. https://doi.org/10.3390/ijms160511369
Lee M-Y, Lin K-D, Hsu W-H, Chang H-L, Yang Y-H, Hsiao P-J, Shin S-J. Statin, Calcium Channel Blocker and Beta Blocker Therapy May Decrease the Incidence of Tuberculosis Infection in Elderly Taiwanese Patients with Type 2 Diabetes. International Journal of Molecular Sciences. 2015; 16(5):11369-11384. https://doi.org/10.3390/ijms160511369
Chicago/Turabian StyleLee, Mei-Yueh, Kun-Der Lin, Wei-Hao Hsu, Hsiu-Ling Chang, Yi-Hsin Yang, Pi-Jung Hsiao, and Shyi-Jang Shin. 2015. "Statin, Calcium Channel Blocker and Beta Blocker Therapy May Decrease the Incidence of Tuberculosis Infection in Elderly Taiwanese Patients with Type 2 Diabetes" International Journal of Molecular Sciences 16, no. 5: 11369-11384. https://doi.org/10.3390/ijms160511369
APA StyleLee, M.-Y., Lin, K.-D., Hsu, W.-H., Chang, H.-L., Yang, Y.-H., Hsiao, P.-J., & Shin, S.-J. (2015). Statin, Calcium Channel Blocker and Beta Blocker Therapy May Decrease the Incidence of Tuberculosis Infection in Elderly Taiwanese Patients with Type 2 Diabetes. International Journal of Molecular Sciences, 16(5), 11369-11384. https://doi.org/10.3390/ijms160511369