Inhibitory Effect of Statins on Inflammation-Related Pathways in Human Abdominal Aortic Aneurysm Tissue
Abstract
:1. Introduction
2. Results and Discussion
2.1. Effect of Simvastatin on JNK and NF-κB Activation in Human AAA Walls
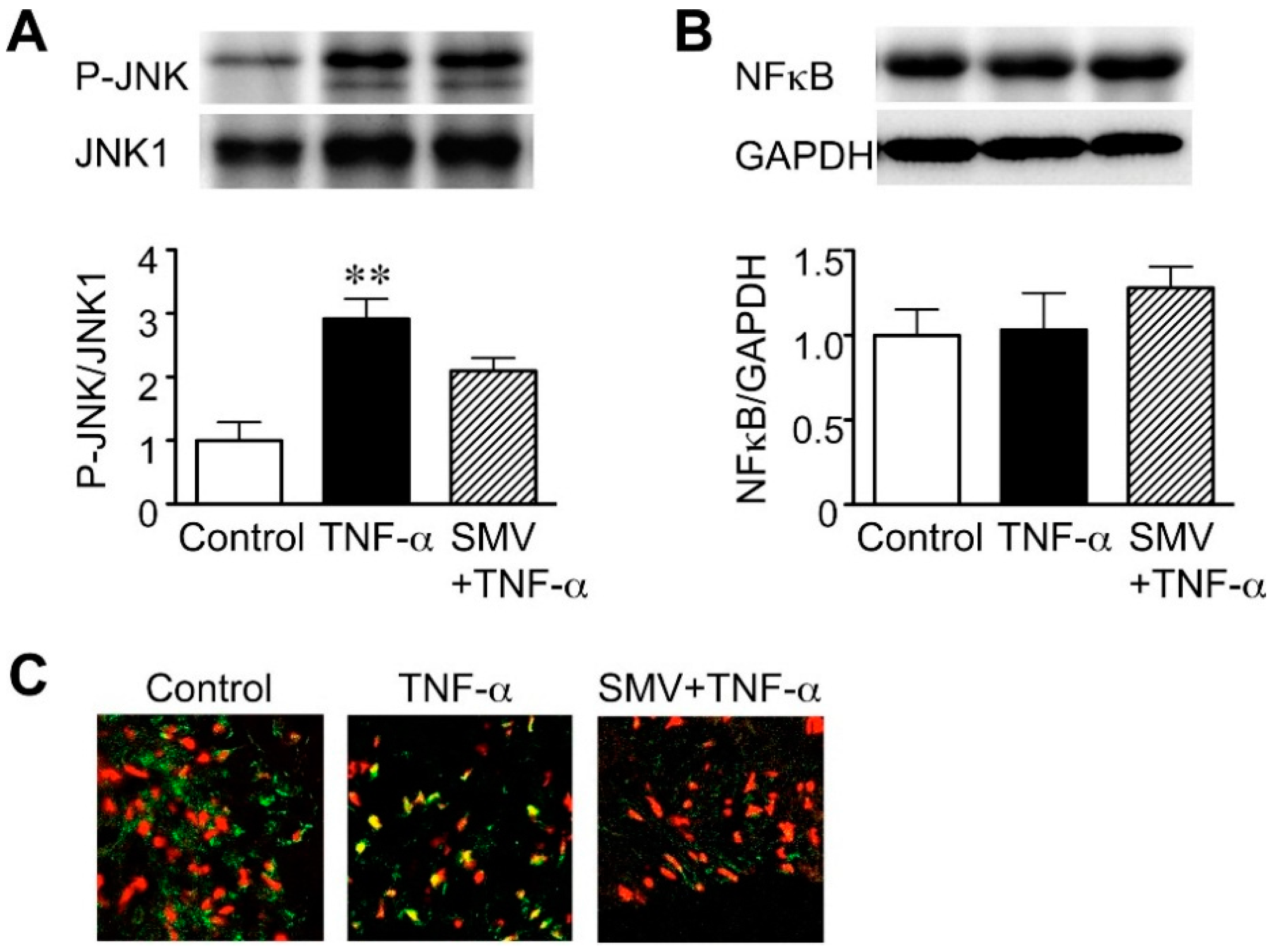
2.2. Effect of Statins in Downstream Pathways after NF-κB
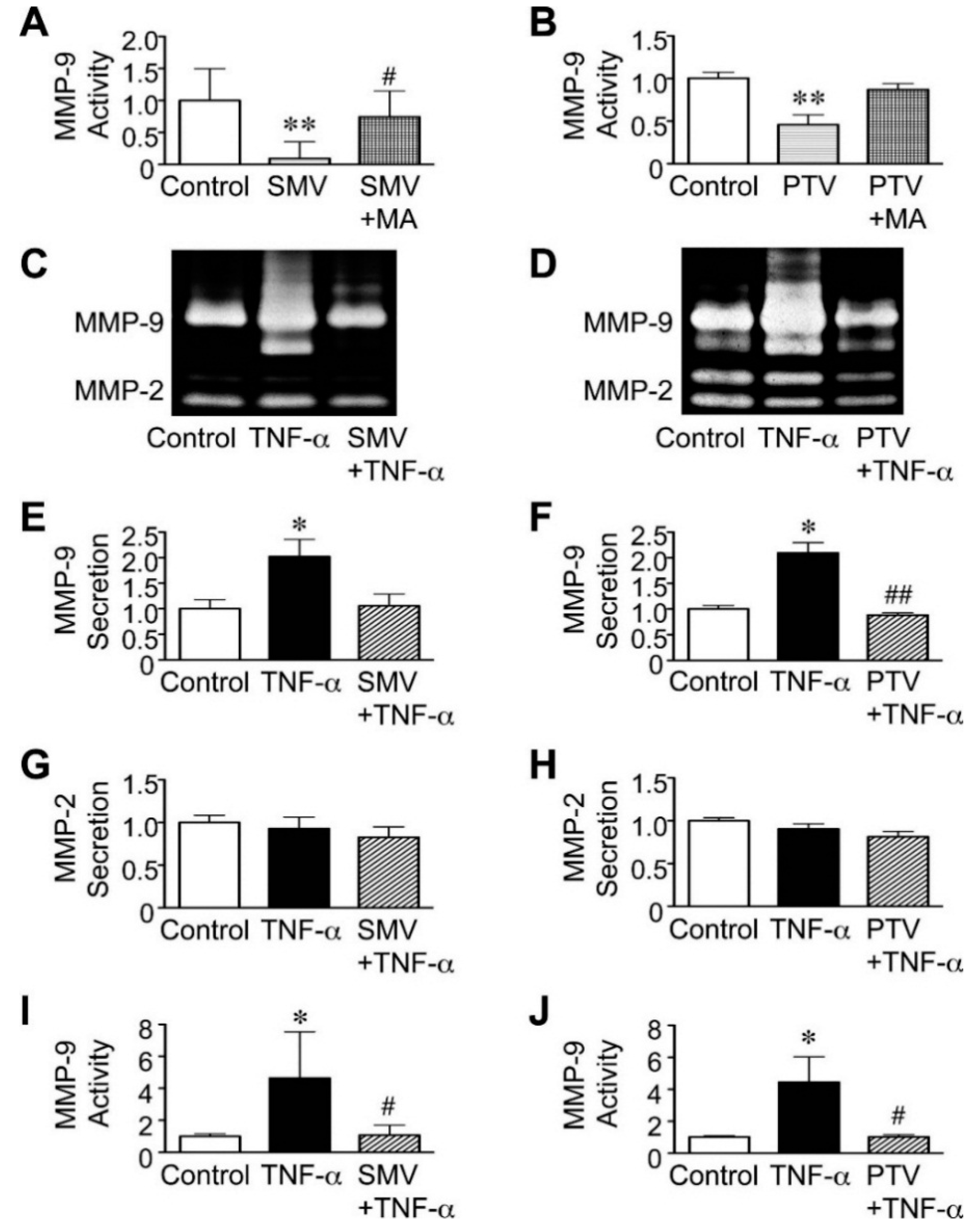
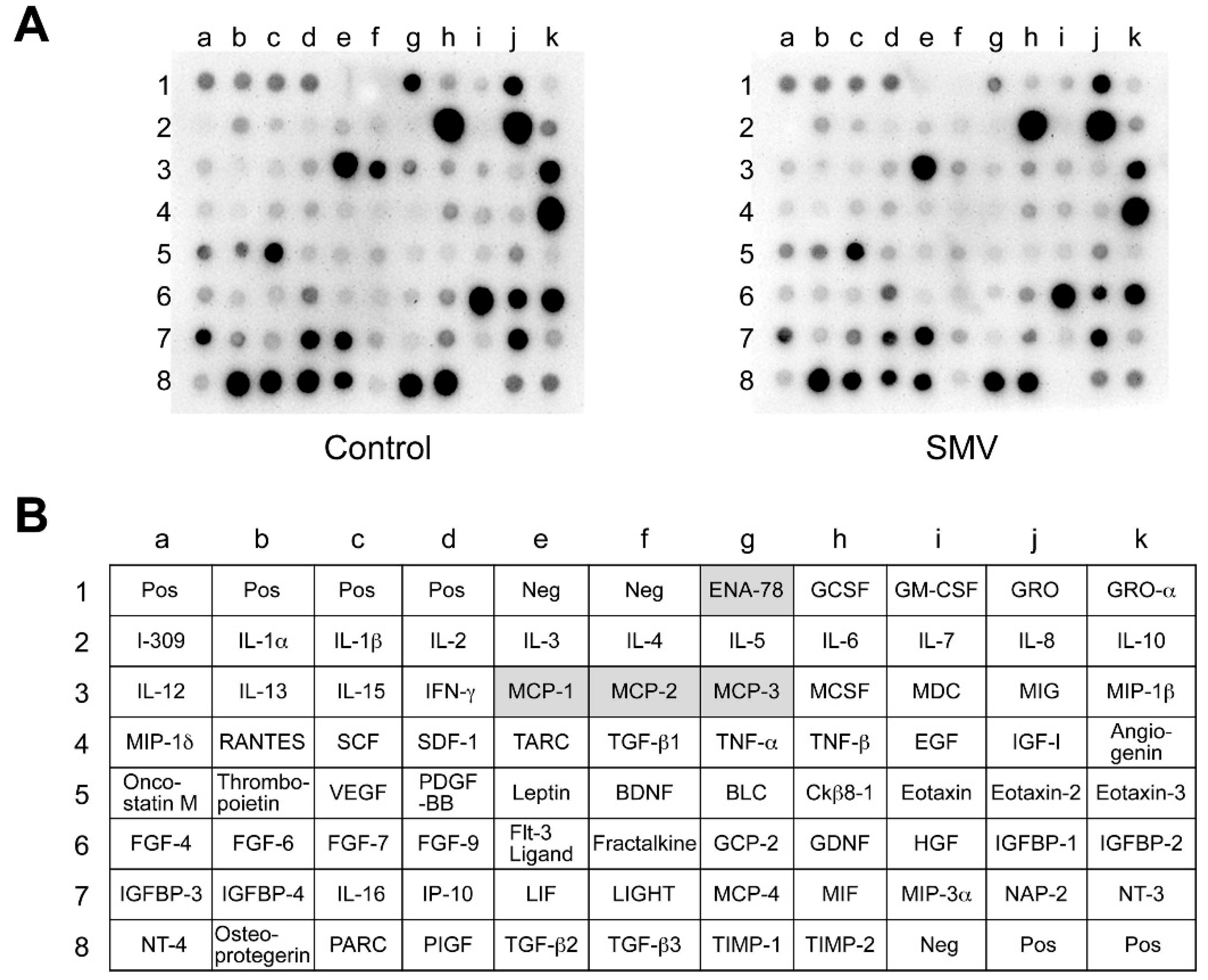
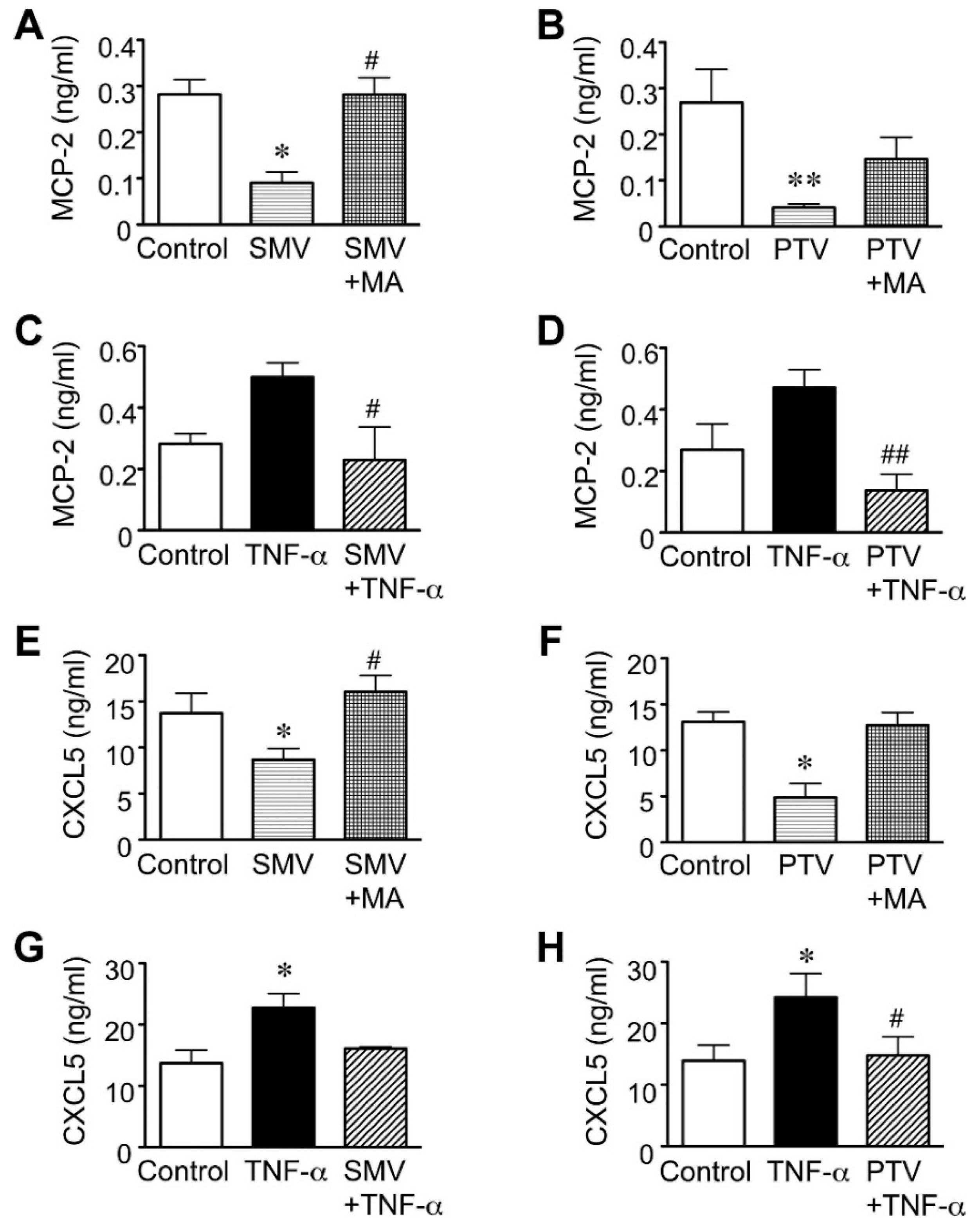
2.3. Importance of the Rac1/NF-κB Pathway Inhibition in the Action of Statins
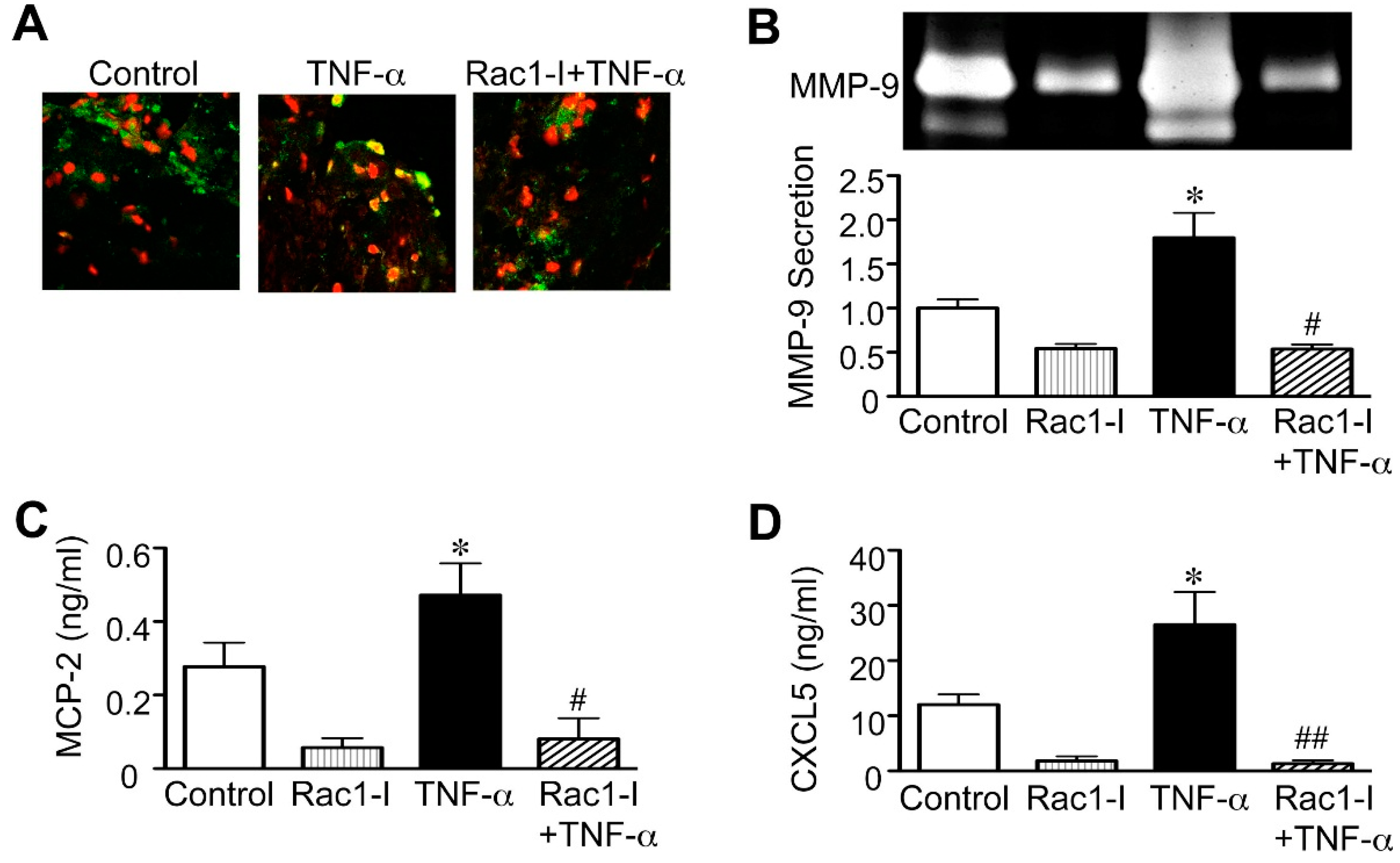
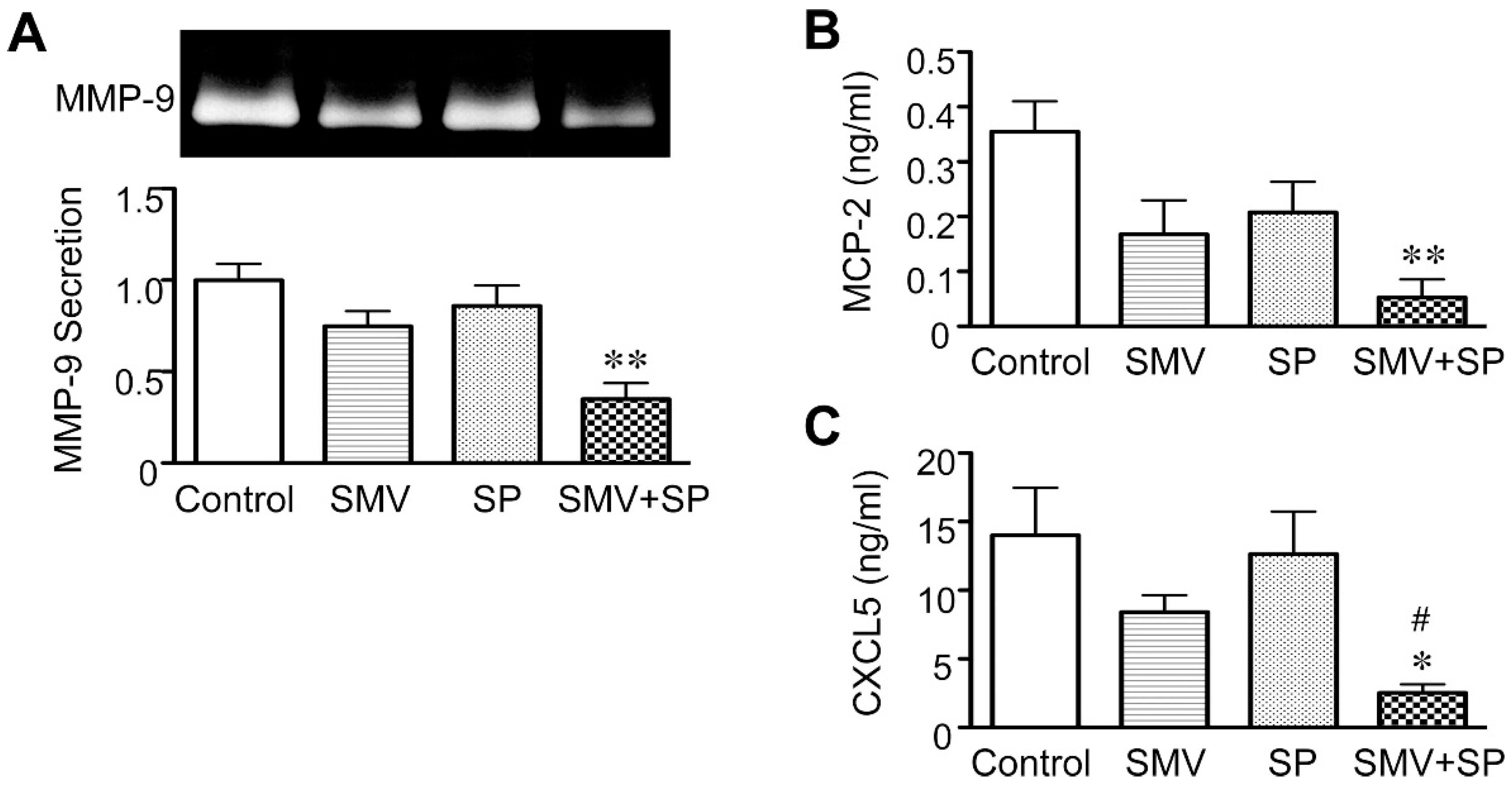
2.4. Clinical Implications and Future Directions
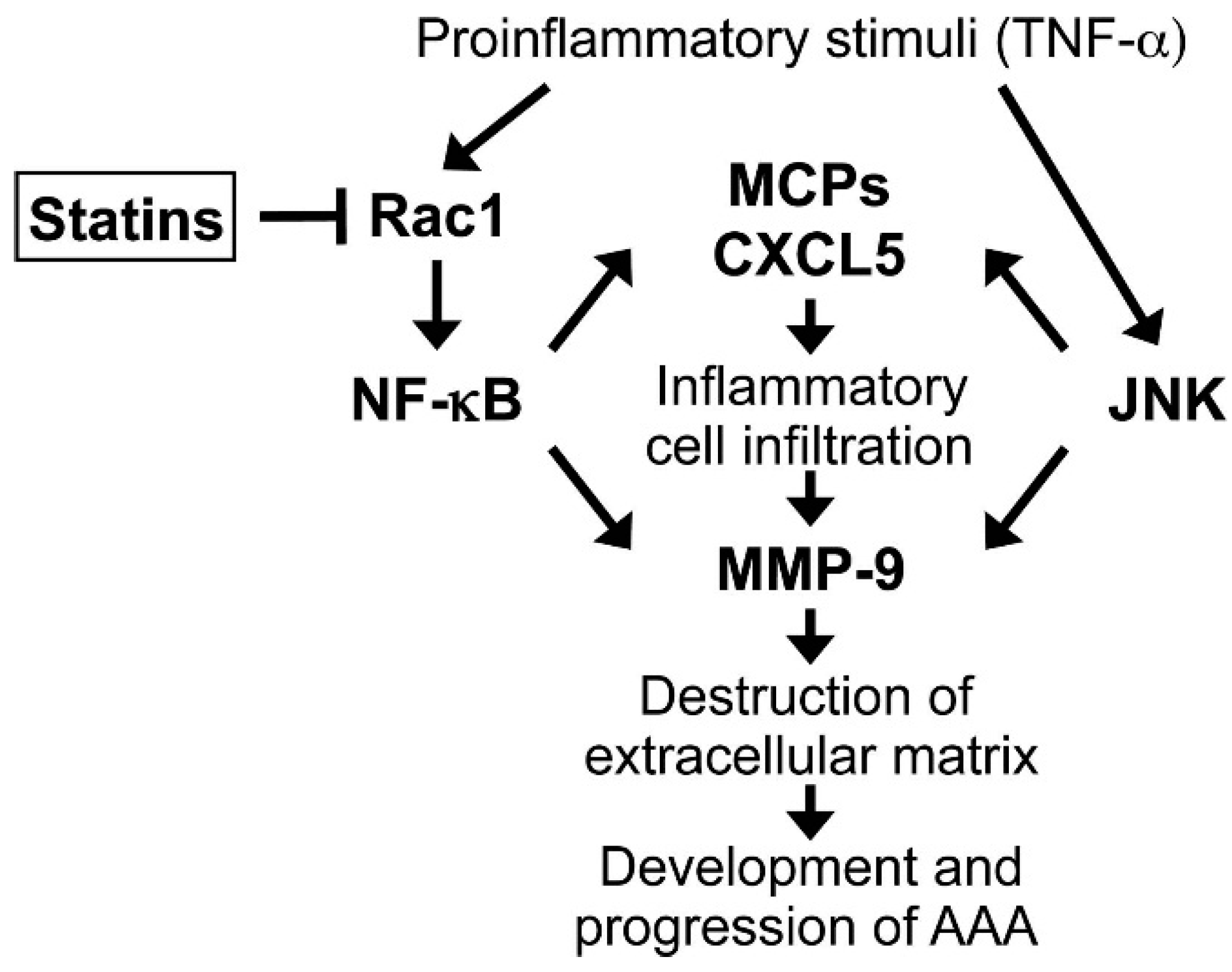
3. Experimental Section
3.1. Organ Culture of Human AAA Walls
3.2. Protein Analyses of Tissue Homogenates and Conditioned Media
3.3. Statistical Analysis
4. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Sakalihasan, N.; Limet, R.; Defawe, O.D. Abdominal aortic aneurysm. Lancet 2005, 365, 1577–1589. [Google Scholar] [CrossRef] [PubMed]
- Moll, F.L.; Powell, J.T.; Fraedrich, G.; Verzini, F.; Haulon, S.; Waltham, M.; van Herwaarden, J.A.; Holt, P.J.E.; van Keulen, J.W.; Rantner, B.; et al. Management of abdominal aortic aneurysms clinical practice guidelines of the european society for vascular surgery. Eur. J. Vasc. Endovasc. Surg. 2011, 41, S1–S58. [Google Scholar] [CrossRef] [PubMed]
- Golledge, J.; Muller, J.; Daugherty, A.; Norman, P. Abdominal aortic aneurysm: Pathogenesis and implications for management. Arterioscler. Thromb. Vasc. Biol. 2006, 26, 2605–2613. [Google Scholar] [CrossRef] [PubMed]
- Aoki, H.; Yoshimura, K.; Matsuzaki, M. Turning back the clock: Regression of abdominal aortic aneurysms via pharmacotherapy. J. Mol. Med. (Berl.) 2007, 85, 1077–1088. [Google Scholar] [CrossRef]
- Yoshimura, K.; Aoki, H. Recent advances in pharmacotherapy development for abdominal aortic aneurysm. Int. J. Vasc. Med. 2012, 2002. [Google Scholar] [CrossRef]
- Golledge, J. Targeting chemokines in aortic aneurysm: Could this be key to a novel therapy for a common problem? Arterioscler. Thromb. Vasc. Biol. 2013, 33, 670–672. [Google Scholar] [CrossRef] [PubMed]
- Yoshimura, K.; Aoki, H.; Ikeda, Y.; Fujii, K.; Akiyama, N.; Furutani, A.; Hoshii, Y.; Tanaka, N.; Ricci, R.; Ishihara, T.; et al. Regression of abdominal aortic aneurysm by inhibition of c-Jun N-terminal kinase. Nat. Med. 2005, 11, 1330–1338. [Google Scholar] [CrossRef] [PubMed]
- Miyake, T.; Aoki, M.; Masaki, H.; Kawasaki, T.; Oishi, M.; Kataoka, K.; Ogihara, T.; Kaneda, Y.; Morishita, R. Regression of abdominal aortic aneurysms by simultaneous inhibition of nuclear factor κB and Ets in a rabbit model. Circ. Res. 2007, 101, 1175–1184. [Google Scholar] [CrossRef] [PubMed]
- Yoshimura, K.; Aoki, H.; Ikeda, Y.; Furutani, A.; Hamano, K.; Matsuzaki, M. Regression of abdominal aortic aneurysm by inhibition of c-Jun N-terminal kinase in mice. Ann. N. Y. Acad. Sci. 2006, 1085, 74–81. [Google Scholar] [CrossRef] [PubMed]
- Golledge, J.; Powell, J.T. Medical management of abdominal aortic aneurysm. Eur. J. Vasc. Endovasc. Surg. 2007, 34, 267–273. [Google Scholar] [CrossRef] [PubMed]
- Golledge, J.; Norman, P.E. Current status of medical management for abdominal aortic aneurysm. Atherosclerosis 2011, 217, 57–63. [Google Scholar] [CrossRef] [PubMed]
- Kurosawa, K.; Matsumura, J.S.; Yamanouchi, D. Current status of medical treatment for abdominal aortic aneurysm. Circ. J. 2013, 77, 2860–2866. [Google Scholar] [CrossRef] [PubMed]
- Jain, M.K.; Ridker, P.M. Anti-inflammatory effects of statins: Clinical evidence and basic mechanisms. Nat. Rev. Drug Discov. 2005, 4, 977–987. [Google Scholar] [CrossRef] [PubMed]
- Takemoto, M.; Liao, J.K. Pleiotropic effects of 3-hydroxy-3-methylglutaryl coenzyme a reductase inhibitors. Arterioscler. Thromb. Vasc. Biol. 2001, 21, 1712–1719. [Google Scholar] [CrossRef] [PubMed]
- Schonbeck, U.; Libby, P. Inflammation, immunity, and HMG-CoA reductase inhibitors: Statins as antiinflammatory agents? Circulation 2004, 109, II18–II126. [Google Scholar] [CrossRef] [PubMed]
- Nomura, S.; Yoshimura, K.; Akiyama, N.; Mikamo, A.; Furutani, A.; Aoki, H.; Matsuzaki, M.; Hamano, K. HMG-CoA reductase inhibitors reduce matrix metalloproteinase-9 activity in human varicose veins. Eur. Surg. Res. 2005, 37, 370–378. [Google Scholar] [CrossRef] [PubMed]
- Nagashima, H.; Aoka, Y.; Sakomura, Y.; Sakuta, A.; Aomi, S.; Ishizuka, N.; Hagiwara, N.; Kawana, M.; Kasanuki, H. A 3-hydroxy-3-methylglutaryl coenzyme A reductase inhibitor, cerivastatin, suppresses production of matrix metalloproteinase-9 in human abdominal aortic aneurysm wall. J. Vasc. Surg. 2002, 36, 158–163. [Google Scholar] [CrossRef] [PubMed]
- Wilson, W.R.; Evans, J.; Bell, P.R.; Thompson, M.M. HMG-CoA reductase inhibitors (statins) decrease MMP-3 and MMP-9 concentrations in abdominal aortic aneurysms. Eur. J. Vasc. Endovasc. Surg. 2005, 30, 259–262. [Google Scholar] [CrossRef] [PubMed]
- Kudo, J.; Yoshimura, K.; Hamano, K. Simvastatin reduces secretion of monocyte chemoattractant proteins and matrix metalloproteinase-9 in human abdominal aortic aneurysms. Bull. Yamaguchi Med. Sch. 2007, 54, 47–56. [Google Scholar]
- Kalyanasundaram, A.; Elmore, J.R.; Manazer, J.R.; Golden, A.; Franklin, D.P.; Galt, S.W.; Zakhary, E.M.; Carey, D.J. Simvastatin suppresses experimental aortic aneurysm expansion. J. Vasc. Surg. 2006, 43, 117–124. [Google Scholar] [CrossRef] [PubMed]
- Steinmetz, E.F.; Buckley, C.; Shames, M.L.; Ennis, T.L.; Vanvickle-Chavez, S.J.; Mao, D.; Goeddel, L.A.; Hawkins, C.J.; Thompson, R.W. Treatment with simvastatin suppresses the development of experimental abdominal aortic aneurysms in normal and hypercholesterolemic mice. Ann. Surg. 2005, 241, 92–101. [Google Scholar] [PubMed]
- Shiraya, S.; Miyake, T.; Aoki, M.; Yoshikazu, F.; Ohgi, S.; Nishimura, M.; Ogihara, T.; Morishita, R. Inhibition of development of experimental aortic abdominal aneurysm in rat model by atorvastatin through inhibition of macrophage migration. Atherosclerosis 2009, 202, 34–40. [Google Scholar] [CrossRef] [PubMed]
- Golledge, J.; Cullen, B.; Moran, C.; Rush, C. Efficacy of simvastatin in reducing aortic dilatation in mouse models of abdominal aortic aneurysm. Cardiovasc. Drugs Ther. 2010, 24, 373–378. [Google Scholar] [CrossRef] [PubMed]
- Karrowni, W.; Dughman, S.; Hajj, G.P.; Miller, F.J., Jr. Statin therapy reduces growth of abdominal aortic aneurysms. J. Investig. Med. 2011, 59, 1239–1243. [Google Scholar] [PubMed]
- Schlosser, F.J.; Tangelder, M.J.; Verhagen, H.J.; van der Heijden, G.J.; Muhs, B.E.; van der Graaf, Y.; Moll, F.L. Growth predictors and prognosis of small abdominal aortic aneurysms. J. Vasc. Surg. 2008, 47, 1127–1133. [Google Scholar] [CrossRef] [PubMed]
- Schouten, O.; van Laanen, J.H.; Boersma, E.; Vidakovic, R.; Feringa, H.H.; Dunkelgrun, M.; Bax, J.J.; Koning, J.; van Urk, H.; Poldermans, D. Statins are associated with a reduced infrarenal abdominal aortic aneurysm growth. Eur. J. Vasc. Endovasc. Surg. 2006, 32, 21–26. [Google Scholar] [CrossRef] [PubMed]
- Sukhija, R.; Aronow, W.S.; Sandhu, R.; Kakar, P.; Babu, S. Mortality and size of abdominal aortic aneurysm at long-term follow-up of patients not treated surgically and treated with and without statins. Am. J. Cardiol. 2006, 97, 279–280. [Google Scholar] [CrossRef] [PubMed]
- Sweeting, M.J.; Thompson, S.G.; Brown, L.C.; Greenhalgh, R.M.; Powell, J.T. Use of angiotensin converting enzyme inhibitors is associated with increased growth rate of abdominal aortic aneurysms. J. Vasc. Surg. 2010, 52, 1–4. [Google Scholar] [CrossRef] [PubMed]
- Thompson, A.; Cooper, J.A.; Fabricius, M.; Humphries, S.E.; Ashton, H.A.; Hafez, H. An analysis of drug modulation of abdominal aortic aneurysm growth through 25 years of surveillance. J. Vasc. Surg. 2010, 52, 55–61. [Google Scholar] [CrossRef] [PubMed]
- Ferguson, C.D.; Clancy, P.; Bourke, B.; Walker, P.J.; Dear, A.; Buckenham, T.; Norman, P.; Golledge, J. Association of statin prescription with small abdominal aortic aneurysm progression. Am. Heart J. 2010, 159, 307–313. [Google Scholar] [CrossRef] [PubMed]
- Twine, C.P.; Williams, I.M. Systematic review and meta-analysis of the effects of statin therapy on abdominal aortic aneurysms. Br. J. Surg. 2011, 98, 346–353. [Google Scholar] [CrossRef] [PubMed]
- Wemmelund, H.; Hogh, A.; Hundborg, H.H.; Thomsen, R.W.; Johnsen, S.P.; Lindholt, J.S. Statin use and rupture of abdominal aortic aneurysm. Br. J. Surg. 2014, 101, 966–975. [Google Scholar] [CrossRef] [PubMed]
- Hamano, K.; Li, T.S.; Takahashi, M.; Kobayashi, T.; Shirasawa, B.; Ito, H.; Zempo, N. Enhanced tumor necrosis factor-α expression in small sized abdominal aortic aneurysms. World J. Surg. 2003, 27, 476–480. [Google Scholar] [CrossRef] [PubMed]
- Juvonen, J.; Surcel, H.M.; Satta, J.; Teppo, A.M.; Bloigu, A.; Syrjala, H.; Airaksinen, J.; Leinonen, M.; Saikku, P.; Juvonen, T. Elevated circulating levels of inflammatory cytokines in patients with abdominal aortic aneurysm. Arterioscler. Thromb. Vasc. Biol. 1997, 17, 2843–2847. [Google Scholar] [CrossRef] [PubMed]
- Xiong, W.; MacTaggart, J.; Knispel, R.; Worth, J.; Persidsky, Y.; Baxter, B.T. Blocking TNF-alpha attenuates aneurysm formation in a murine model. J. Immunol. 2009, 183, 2741–2746. [Google Scholar] [CrossRef] [PubMed]
- Pyo, R.; Lee, J.K.; Shipley, J.M.; Curci, J.A.; Mao, D.; Ziporin, S.J.; Ennis, T.L.; Shapiro, S.D.; Senior, R.M.; Thompson, R.W. Targeted gene disruption of matrix metalloproteinase-9 (gelatinase B) suppresses development of experimental abdominal aortic aneurysms. J. Clin. Investig. 2000, 105, 1641–1649. [Google Scholar] [CrossRef] [PubMed]
- Ortego, M.; Bustos, C.; Hernandez-Presa, M.A.; Tunon, J.; Diaz, C.; Hernandez, G.; Egido, J. Atorvastatin reduces NF-κB activation and chemokine expression in vascular smooth muscle cells and mononuclear cells. Atherosclerosis 1999, 147, 253–261. [Google Scholar] [CrossRef] [PubMed]
- Middleton, R.K.; Lloyd, G.M.; Bown, M.J.; Cooper, N.J.; London, N.J.; Sayers, R.D. The pro-inflammatory and chemotactic cytokine microenvironment of the abdominal aortic aneurysm wall: A protein array study. J. Vasc. Surg. 2007, 45, 574–580. [Google Scholar] [CrossRef] [PubMed]
- Golledge, J.; Clancy, P.; Moran, C.; Biros, E.; Rush, C.; Walker, P.; Norman, P. The novel association of the chemokine CCL22 with abdominal aortic aneurysm. Am. J. Pathol. 2010, 176, 2098–2106. [Google Scholar] [CrossRef] [PubMed]
- Rush, C.; Nyara, M.; Moxon, J.V.; Trollope, A.; Cullen, B.; Golledge, J. Whole genome expression analysis within the angiotensin II-apolipoprotein E deficient mouse model of abdominal aortic aneurysm. BMC Genomics 2009, 10. [Google Scholar] [CrossRef] [PubMed]
- Rashid, M.; Tawara, S.; Fukumoto, Y.; Seto, M.; Yano, K.; Shimokawa, H. Importance of Rac1 signaling pathway inhibition in the pleiotropic effects of HMG-CoA reductase inhibitors. Circ. J. 2009, 73, 361–370. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.Y.; Ptasznik, A.; Pan, Z.K. RhoA and Rac1 signals in fMLP-induced NF-κB activation in human blood monocytes. Biochem. Biophys. Res. Commun. 2004, 319, 629–635. [Google Scholar] [CrossRef] [PubMed]
- Zampetaki, A.; Zhang, Z.; Hu, Y.; Xu, Q. Biomechanical stress induces IL-6 expression in smooth muscle cells via Ras/Rac1-p38 MAPK-NF-κB signaling pathways. Am. J. Physiol. Heart Circ. Physiol. 2005, 288, H2946–H2954. [Google Scholar] [CrossRef] [PubMed]
- Yoshimura, K.; Aoki, H.; Ikeda, Y.; Furutani, A.; Hamano, K.; Matsuzaki, M. Identification of c-Jun N-terminal kinase as a therapeutic target for abdominal aortic aneurysm. Ann. N. Y. Acad. Sci. 2006, 1085, 403–406. [Google Scholar] [CrossRef] [PubMed]
- Bellosta, S.; Paoletti, R.; Corsini, A. Safety of statins: Focus on clinical pharmacokinetics and drug interactions. Circulation 2004, 109, III50–III57. [Google Scholar] [CrossRef] [PubMed]
- Bjorkhem-Bergman, L.; Lindh, J.D.; Bergman, P. What is a relevant statin concentration in cell experiments claiming pleiotropic effects? Br. J. Clin. Pharmacol. 2011, 72, 164–165. [Google Scholar] [CrossRef] [PubMed]
- Kajimoto, K.; Miyauchi, K.; Kasai, T.; Shimada, K.; Kojima, Y.; Shimada, A.; Niinami, H.; Amano, A.; Daida, H. Short-term 20-mg atorvastatin therapy reduces key inflammatory factors including c-Jun N-terminal kinase and dendritic cells and matrix metalloproteinase expression in human abdominal aortic aneurysmal wall. Atherosclerosis 2009, 206, 505–511. [Google Scholar] [CrossRef] [PubMed]
- Piechota-Polanczyk, A.; Goraca, A.; Demyanets, S.; Mittlboeck, M.; Domenig, C.; Neumayer, C.; Wojta, J.; Nanobachvili, J.; Huk, I.; Klinger, M. Simvastatin decreases free radicals formation in the human abdominal aortic aneurysm wall via NF-κB. Eur. J. Vasc. Endovasc. Surg. 2012, 44, 133–137. [Google Scholar] [CrossRef] [PubMed]
- Dawson, J.A.; Choke, E.; Loftus, I.M.; Cockerill, G.W.; Thompson, M.M. A randomised placebo-controlled double-blind trial to evaluate lipid-lowering pharmacotherapy on proteolysis and inflammation in abdominal aortic aneurysms. Eur. J. Vasc. Endovasc. Surg. 2011, 41, 28–35. [Google Scholar] [CrossRef] [PubMed]
- Hurks, R.; Hoefer, I.E.; Vink, A.; Pasterkamp, G.; Schoneveld, A.; Kerver, M.; de Vries, J.P.; Tangelder, M.J.; Moll, F.L. Different effects of commonly prescribed statins on abdominal aortic aneurysm wall biology. Eur. J. Vasc. Endovasc. Surg. 2010, 39, 569–576. [Google Scholar] [CrossRef] [PubMed]
- Van der Meij, E.; Koning, G.G.; Vriens, P.W.; Peeters, M.F.; Meijer, C.A.; Kortekaas, K.E.; Dalman, R.L.; van Bockel, J.H.; Hanemaaijer, R.; Kooistra, T.; et al. A clinical evaluation of statin pleiotropy: Statins selectively and dose-dependently reduce vascular inflammation. PLoS ONE 2013, 8, e53882. [Google Scholar] [CrossRef] [PubMed]
- Takagi, H.; Matsui, M.; Umemoto, T. A meta-analysis of clinical studies of statins for prevention of abdominal aortic aneurysm expansion. J. Vasc. Surg. 2010, 52, 1675–1681. [Google Scholar] [CrossRef] [PubMed]
- Coimbra, M.; Banciu, M.; Fens, M.H.; de Smet, L.; Cabaj, M.; Metselaar, J.M.; Storm, G.; Schiffelers, R.M. Liposomal pravastatin inhibits tumor growth by targeting cancer-related inflammation. J. Control. Release 2010, 148, 303–310. [Google Scholar] [CrossRef] [PubMed]
- Winter, P.M.; Caruthers, S.D.; Zhang, H.; Williams, T.A.; Wickline, S.A.; Lanza, G.M. Antiangiogenic synergism of integrin-targeted fumagillin nanoparticles and atorvastatin in atherosclerosis. JACC Cardiovasc. Imaging 2008, 1, 624–634. [Google Scholar] [CrossRef] [PubMed]
- Katsuki, S.; Matoba, T.; Nakashiro, S.; Sato, K.; Koga, J.; Nakano, K.; Nakano, Y.; Egusa, S.; Sunagawa, K.; Egashira, K. Nanoparticle-mediated delivery of pitavastatin inhibits atherosclerotic plaque destabilization/rupture in mice by regulating the recruitment of inflammatory monocytes. Circulation 2014, 129, 896–906. [Google Scholar] [CrossRef] [PubMed]
- Hackmann, A.E.; Rubin, B.G.; Sanchez, L.A.; Geraghty, P.A.; Thompson, R.W.; Curci, J.A. A randomized, placebo-controlled trial of doxycycline after endoluminal aneurysm repair. J. Vasc. Surg. 2008, 48, 519–526. [Google Scholar] [CrossRef] [PubMed]
- Nagasawa, A.; Yoshimura, K.; Suzuki, R.; Mikamo, A.; Yamashita, O.; Ikeda, Y.; Tsuchida, M.; Hamano, K. Important role of the angiotensin II pathway in producing matrix metalloproteinase-9 in human thoracic aortic aneurysms. J. Surg. Res. 2013, 183, 472–477. [Google Scholar] [CrossRef] [PubMed]
- Yamashita, O.; Yoshimura, K.; Nagasawa, A.; Ueda, K.; Morikage, N.; Ikeda, Y.; Hamano, K. Periostin links mechanical strain to inflammation in abdominal aortic aneurysm. PLoS ONE 2013, 8, e79753. [Google Scholar] [CrossRef] [PubMed]
- Onoda, M.; Yoshimura, K.; Aoki, H.; Ikeda, Y.; Morikage, N.; Furutani, A.; Matsuzaki, M.; Hamano, K. Lysyl oxidase resolves inflammation by reducing monocyte chemoattractant protein-1 in abdominal aortic aneurysm. Atherosclerosis 2010, 208, 366–369. [Google Scholar] [CrossRef] [PubMed]
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yoshimura, K.; Nagasawa, A.; Kudo, J.; Onoda, M.; Morikage, N.; Furutani, A.; Aoki, H.; Hamano, K. Inhibitory Effect of Statins on Inflammation-Related Pathways in Human Abdominal Aortic Aneurysm Tissue. Int. J. Mol. Sci. 2015, 16, 11213-11228. https://doi.org/10.3390/ijms160511213
Yoshimura K, Nagasawa A, Kudo J, Onoda M, Morikage N, Furutani A, Aoki H, Hamano K. Inhibitory Effect of Statins on Inflammation-Related Pathways in Human Abdominal Aortic Aneurysm Tissue. International Journal of Molecular Sciences. 2015; 16(5):11213-11228. https://doi.org/10.3390/ijms160511213
Chicago/Turabian StyleYoshimura, Koichi, Ayako Nagasawa, Junichi Kudo, Masahiko Onoda, Noriyasu Morikage, Akira Furutani, Hiroki Aoki, and Kimikazu Hamano. 2015. "Inhibitory Effect of Statins on Inflammation-Related Pathways in Human Abdominal Aortic Aneurysm Tissue" International Journal of Molecular Sciences 16, no. 5: 11213-11228. https://doi.org/10.3390/ijms160511213
APA StyleYoshimura, K., Nagasawa, A., Kudo, J., Onoda, M., Morikage, N., Furutani, A., Aoki, H., & Hamano, K. (2015). Inhibitory Effect of Statins on Inflammation-Related Pathways in Human Abdominal Aortic Aneurysm Tissue. International Journal of Molecular Sciences, 16(5), 11213-11228. https://doi.org/10.3390/ijms160511213




