Revisiting the Formation and Tunable Dissociation of a [2]Pseudorotaxane Formed by Slippage Approach
Abstract
:1. Introduction
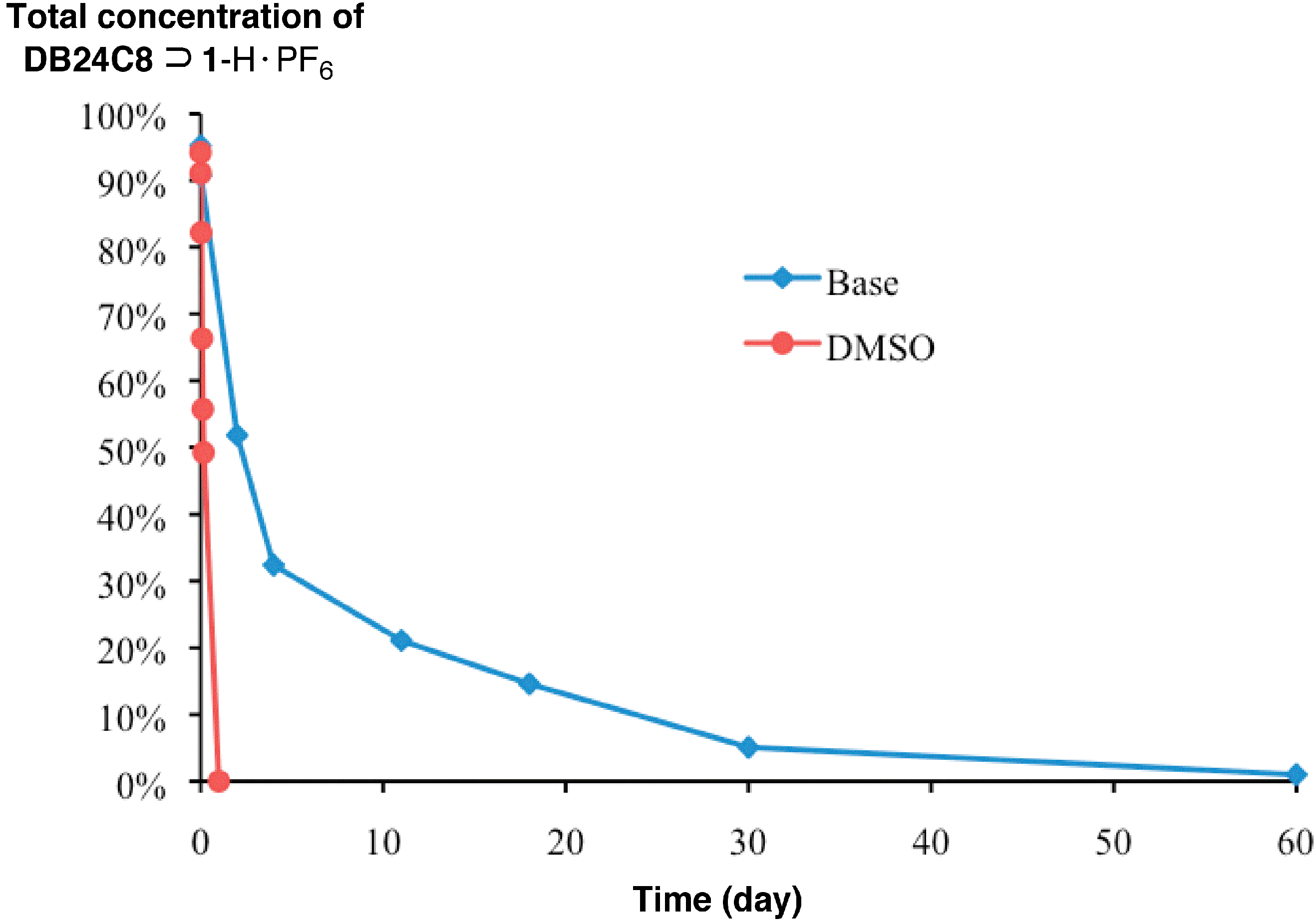
2. Results and Discussion
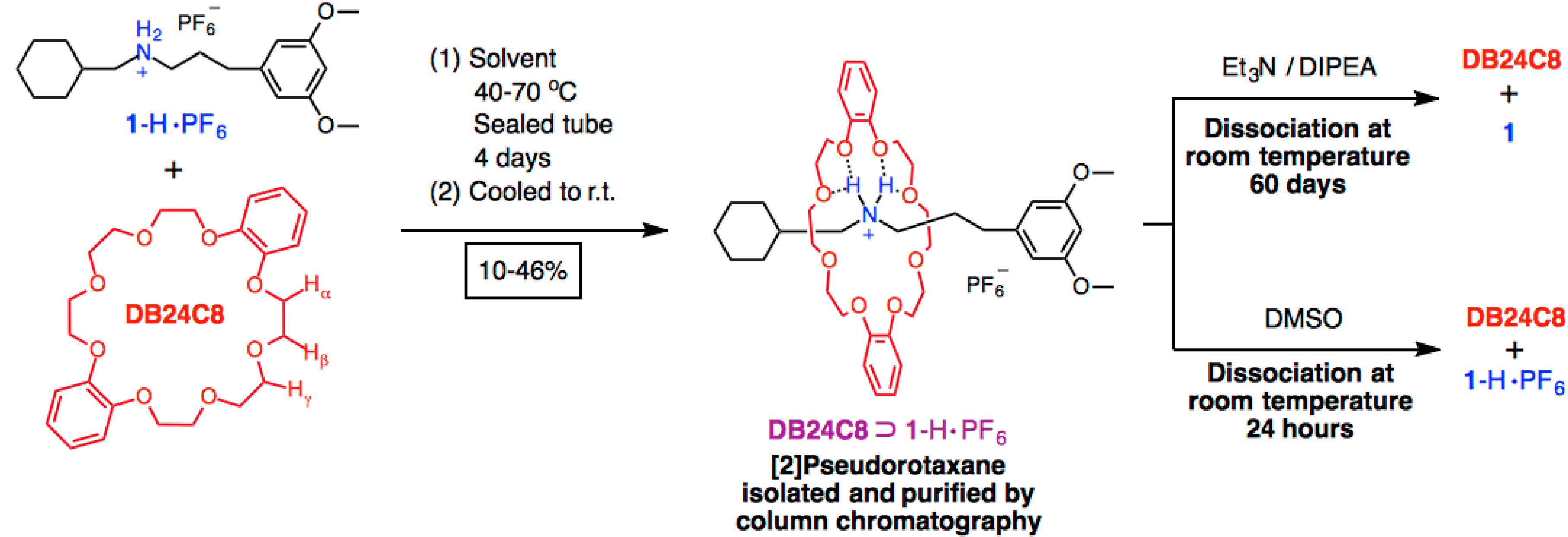
| Solvent (Reaction Temperature in Sealed Tube) | DB24C8⊃1-H·PF6 |
|---|---|
| MeCN (70 °C) | 46% |
| PhMe (70 °C) | 30% |
| CH2Cl2 (40 °C) | 10% |
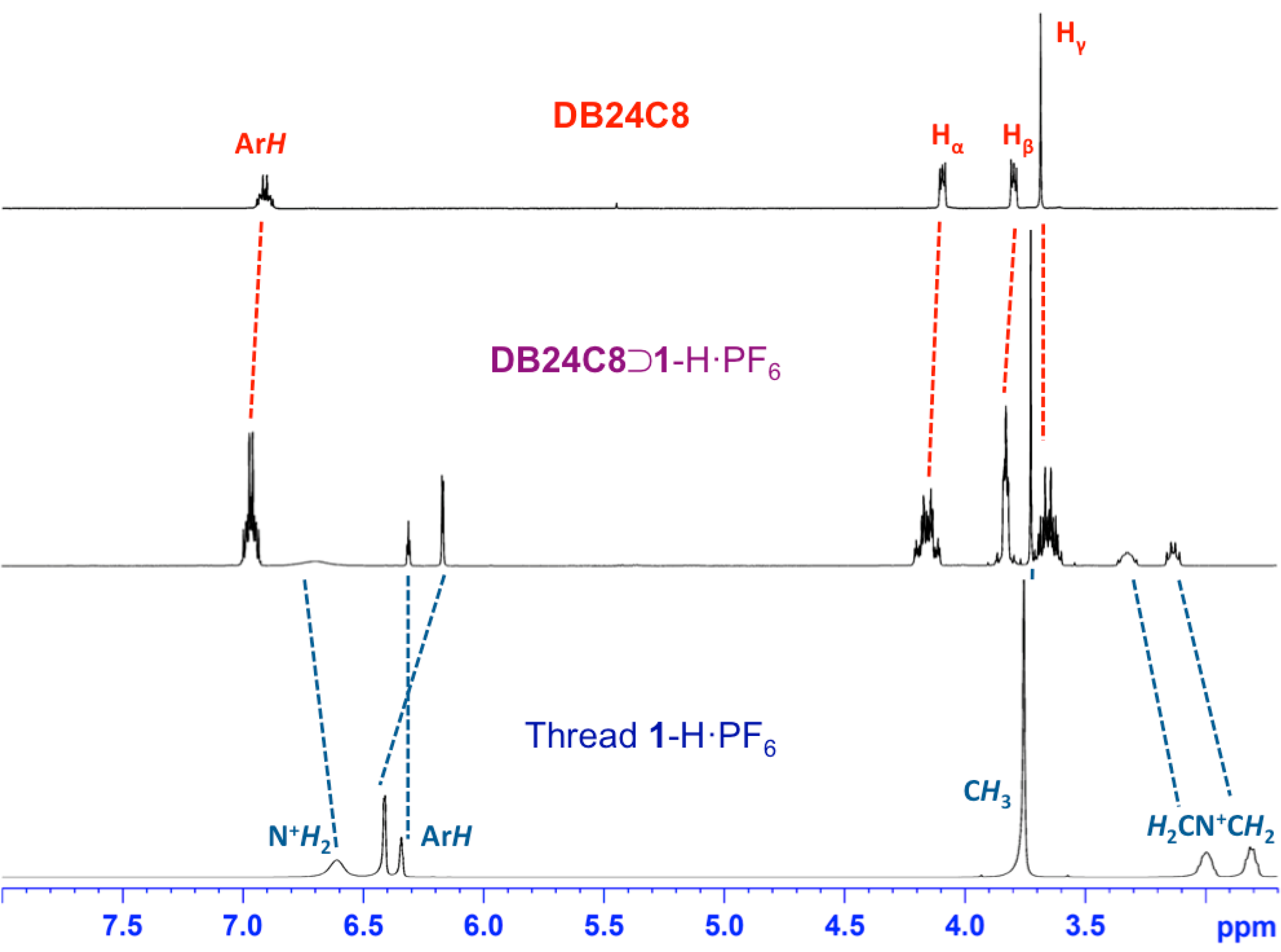
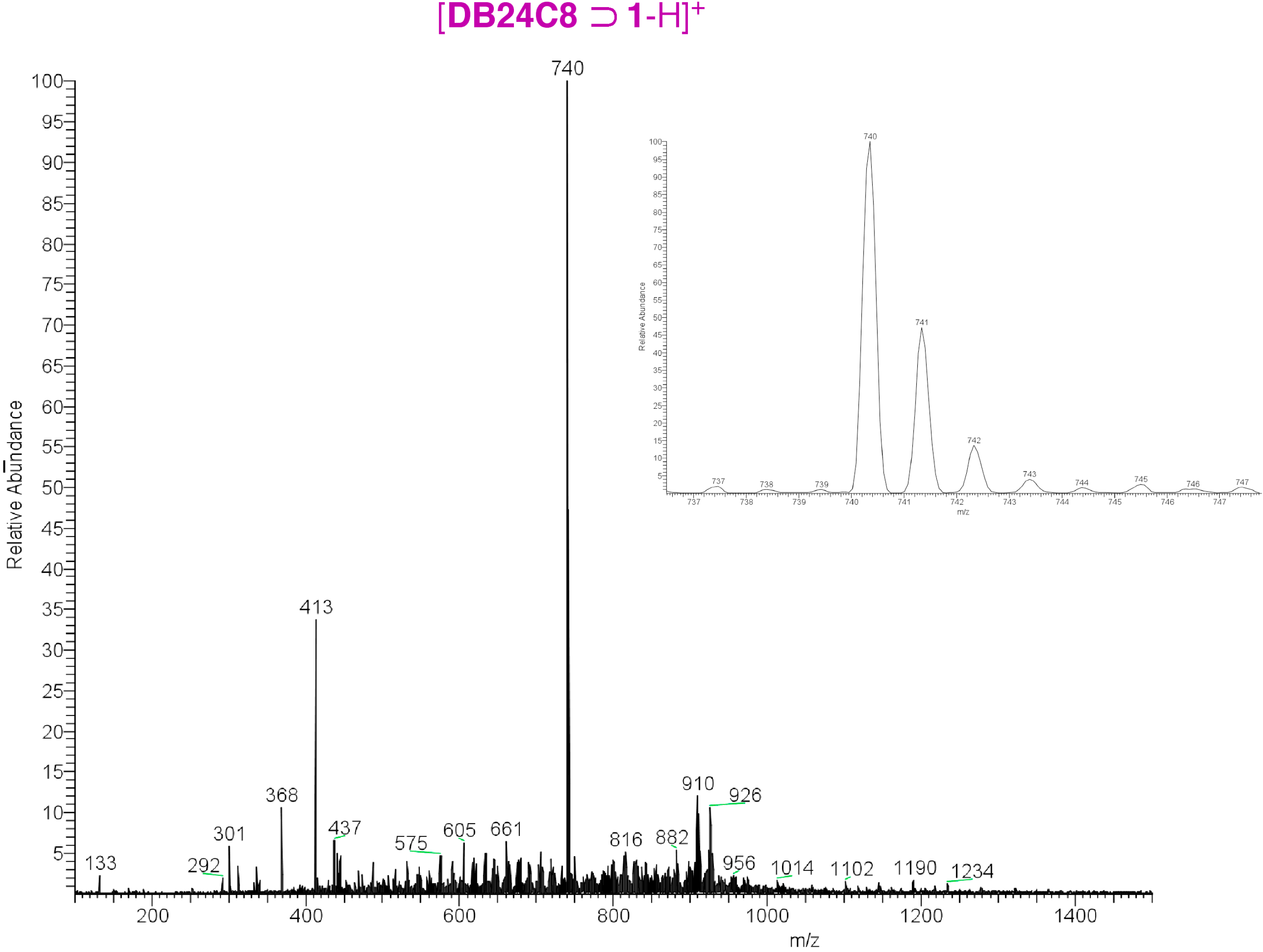
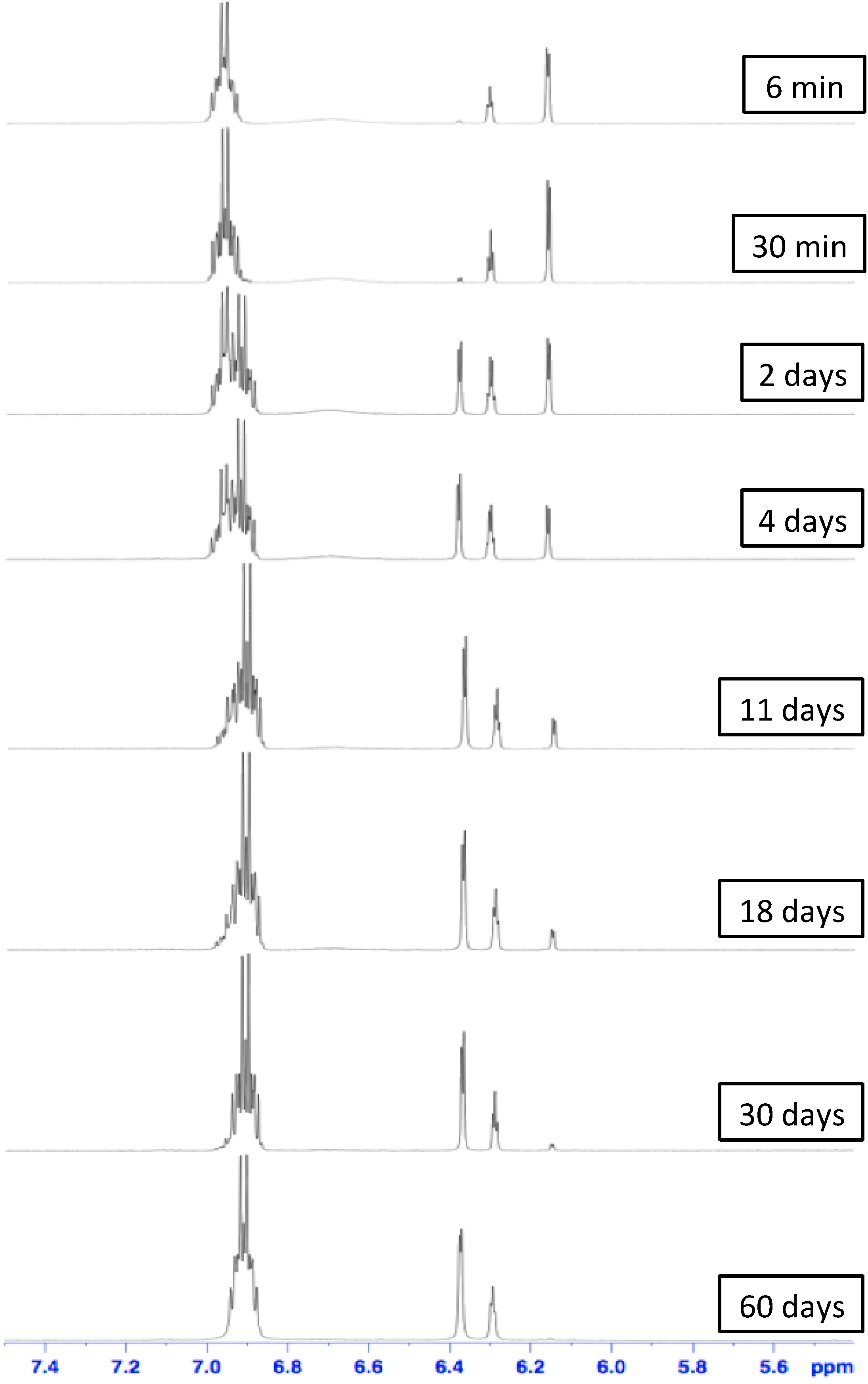
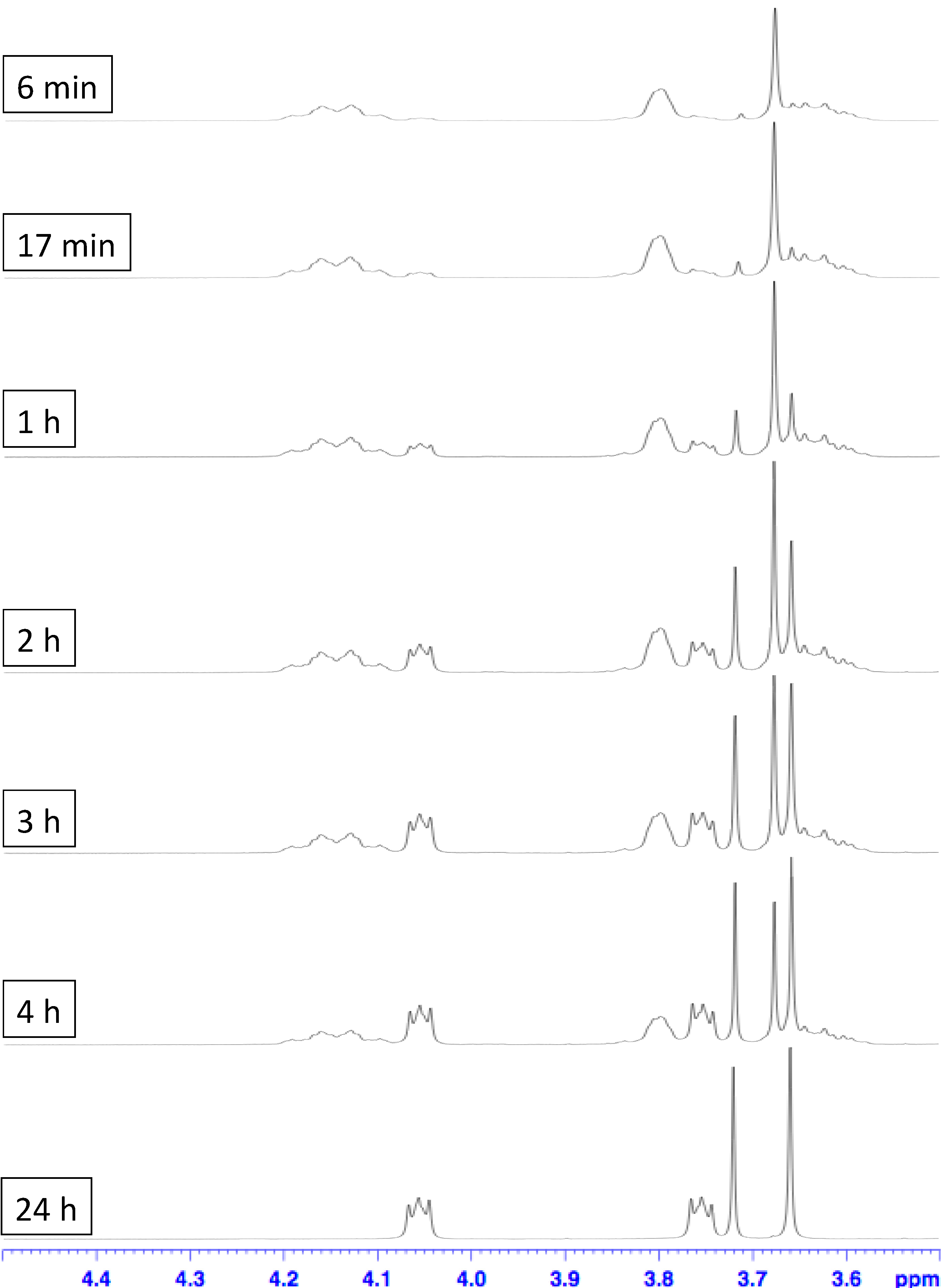
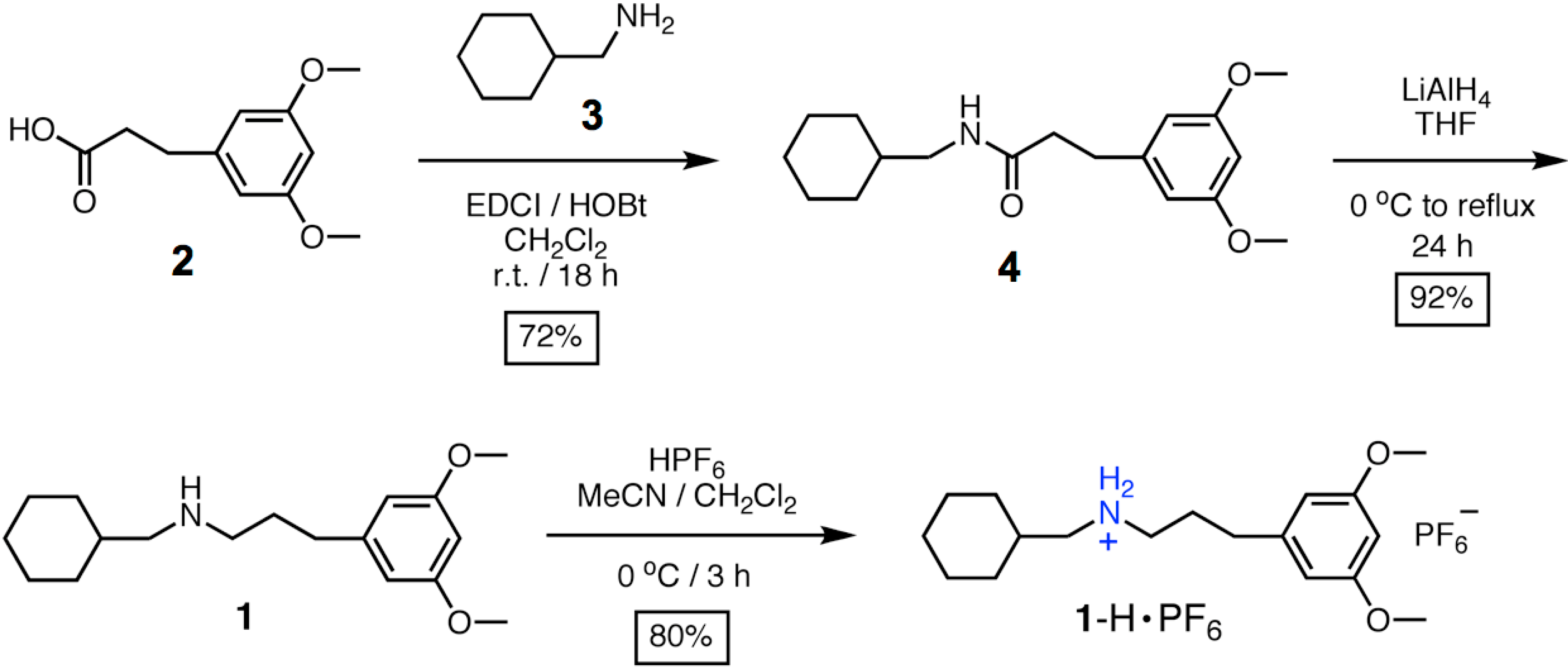
3. Experimental Section
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Ashton, P.R.; Baxter, I.; Fyfe, M.C.T.; Raymo, F.M.; Spencer, N.; Stoddart, J.F.; White, A.J.P.; Williams, D.J. Rotaxane or Pseudorotaxane? That Is The Question! J. Am. Chem. Soc. 1998, 120, 2297–2307. [Google Scholar]
- Affeld, A.; Hübner, G.M.; Seel, C.; Schalley, C.A. Rotaxane or Pseudorotaxane? Effects of Small Structural Variation on the Deslipping Kinetics of Rotaxanes with Stopper Groups of Intermediate Size. Eur. J. Org. Chem 2001, 2001, 2877–2890. [Google Scholar]
- Aricó, F.; Badjic, J.D.; Cantrill, S.J.; Flood, A.H.; Leung, K.C.-F.; Liu, Y.; Stoddart, J.F. Templated Synthesis of Interlocked Molecules. Top. Curr. Chem. 2005, 249, 203–259. [Google Scholar]
- Hirose, K.; Nakamura, Y.; Tobe, Y. Remarkable Effects of Chirality on Deslipping Reactions of Diasteromeric Rotaxanes and Relevant Mechanism Involving Pre-Equilibrium. Org. Lett. 2009, 11, 145–147. [Google Scholar] [CrossRef] [PubMed]
- Leung, K.C.-F.; Chak, C.-P.; Lo, C.-M.; Wong, W.-Y.; Xuan, S.; Cheng, C.H.K. pH-Controllable Supramolecular Systems. Chem. Asian J. 2009, 4, 364–381. [Google Scholar] [CrossRef] [PubMed]
- Wong, W.-Y.; Lee, S.-F.; Chan, H.-S.; Mak, T.C.M.; Wong, C.-H.; Huang, L.-S.; Stoddart, J.F.; Leung, K.C.-F. Recognition between V- and Dumbbell-shaped Molecules. RSC Adv. 2013, 3, 26382–26390. [Google Scholar] [CrossRef]
- Nijhuis, C.A.; Ravoo, B.J.; Huskens, J.; Reinhoudt, D.N. Redox-active Supramolecular Systems. Coord. Chem. Rev. 2007, 251, 1761–1780. [Google Scholar] [CrossRef]
- Liu, Y.; Flood, A. H.; Stoddart, J.F. Thermally and Electrochemically Controllable Self-Complexing Molecular Switches. J. Am. Chem. Soc. 2004, 126, 9150–9151. [Google Scholar] [CrossRef] [PubMed]
- Fernando, I.R.; Bairu, S.G.; Guda, R.; Mezei, G. Single-Color Pseudorotaxane-based Temperature Sensing. New J. Chem. 2010, 34, 2097–2100. [Google Scholar] [CrossRef]
- Leung, K.C.-F.; Wong, W.-Y.; Aricó, F.; Haussmann, P.C.; Stoddart, J.F. The Stability of Imine-containing Dynamic [2]Rotaxane to Hydrolysis. Org. Biomol. Chem. 2010, 8, 83–89. [Google Scholar] [CrossRef] [PubMed]
- Wong, W.-Y.; Leung, K.C.-F.; Stoddart, J.F. Self-assembly, Stability Quantification, Controlled Molecular Switching, and Sensing Properties of an Anthracene-containing Dynamic [2]Rotaxane. Org. Biomol. Chem. 2010, 8, 2332–2343. [Google Scholar] [CrossRef] [PubMed]
- Leung, K.C.-F.; Nguyen, T.D.; Stoddart, J.F.; Zink, J.I. Supramolecular Nanovalves Controlled by Proton Abstraction and Competitive Binding. Chem. Mater. 2006, 18, 5919–5928. [Google Scholar] [CrossRef]
- Saha, S.; Leung, K.C.-F.; Nguyen, T.D.; Stoddart, J.F.; Zink, J.I. Nanovalves. Adv. Funct. Mater. 2007, 17, 685–693. [Google Scholar] [CrossRef]
- Cotí, K.K.; Belowich, M.E.; Liong, M.; Ambrogio, M.W.; Lau, Y.A.; Khatib, H.A.; Zink, J.I.; Khashab, N.M.; Stoddart, J.F. Mechanised Nanoparticles for Drug Delivery. Nanoscale 2009, 1, 16–39. [Google Scholar] [CrossRef] [PubMed]
- Rowan, S.J.; Cantrill, S.J.; Cousins, G.R.L.; Sanders, J.K.M.; Stoddart, J.F. Dynamic Covalent Chemistry. Angew. Chem. Int. Ed. 2002, 41, 898–952. [Google Scholar] [CrossRef]
- Grigoras, M.; Catanescu, C.O. Imine Oligomers and Polymers. J. Macromol. Sci. Polym. Rev. 2004, C44, 131–173. [Google Scholar] [CrossRef]
- Meyer, C.D.; Joiner, C.S.; Stoddart, J.F. Template-directed Synthesis Employing Reversible Imine Bond Formation. Chem. Soc. Rev. 2007, 36, 1705–1723. [Google Scholar] [CrossRef] [PubMed]
- Haussmann, P.C.; Stoddart, J.F. Synthesizing Interlocked Molecules Dynamically. Chem. Rec. 2009, 9, 136–154. [Google Scholar] [CrossRef] [PubMed]
- Herrmann, A. Dynamic Mixtures and Combinatorial Libraries: Imines as Probes for Molecular Evolution at the Interface between Chemistry and Biology. Org. Biomol. Chem. 2009, 7, 3195–3204. [Google Scholar] [CrossRef] [PubMed]
- Maeda, T.; Otsuka, H.; Takahara, A. Dynamic Covalent Polymers: Reorganizable Polymers with Dynamic Covalent Bonds. Prog. Polym. Sci. 2009, 34, 581–604. [Google Scholar] [CrossRef]
- Klivansky, L.M.; Koshkakaryan, G.; Cao, D.; Liu, Y. Linear pi-acceptor-templated Dynamic Clipping to Macrobicycles and [2]Rotaxanes. Angew. Chem. Int. Ed. 2009, 48, 4185–4189. [Google Scholar] [CrossRef]
- Yin, J.; Dasgupta, S.; Wu, J. Template-directed Synthesis of Rotaxanes by Clipping: The Effect of the Dialkylammonium Recognition Sites. Org. Lett. 2010, 12, 1712–1715. [Google Scholar] [CrossRef] [PubMed]
- Leung, K.C.-F.; Lau, K.-N. Self-assembly and Thermodynamic Synthesis of Rotaxane Dendrimers and Related Structures. Polym. Chem. 2010, 1, 988–1000. [Google Scholar] [CrossRef]
- Ho, W.K.-W.; Lee, S.-F.; Wong, C.-H.; Zhu, X.-M.; Kwan, C.-S.; Chak, C.-P.; Mendes, P.M.; Cheng, C.H.K.; Leung, K.C.-F. Type III-B Rotaxane Dendrimers. Chem. Commun. 2013, 49, 10781–10783. [Google Scholar] [CrossRef]
- Harrison, I.T. The Effect of Ring Size on Threading Reactions of Macrocycles. J. Chem. Soc. Chem. Commun. 1972, 231, 231–232. [Google Scholar] [CrossRef]
- Raymo, F.M.; Stoddart, J.F. Slippage–A Simple and Efficient Way to Self-assemble [n]Rotaxanes. Pure Appl. Chem. 1997, 69, 1987–1997. [Google Scholar] [CrossRef]
- Elizarov, A.M.; Chang, T.; Chiu, S.-H.; Stoddart, J.F. Self-assembly of Dendrimers by Slippage. Org. Lett. 2002, 4, 3565–3568. [Google Scholar] [CrossRef] [PubMed]
- Hsueh, S.-Y.; Lai, C.-C.; Liu, Y.-H.; Wang, Y.; Peng, S.-M.; Chiu, S.-H. Protecting a Squaraine Near-IR Dye Through Its Incorporation in a Slippage-derived [2]Rotaxane. Org. Lett. 2007, 9, 4523–4526. [Google Scholar]
- Schill, G. Catenanes, Rotaxanes, and Knots; Academic Press: New York, NY, USA, 1971; p. 3. [Google Scholar]
- McConnell, A.J.; Beer, P.D. Kinetic Studies Exploring the Role of Anion Templation in the Slippage Formation of Rotaxane-Like Structures. Chem. Eur. J. 2011, 17, 2724–2733. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Xu, D.; Yan, X.; Chen, J.; Dong, S.; Zheng, B.; Huang, F. Self-Healing Supramolecular Gels Formed by Crown Ether Based Host-Guest Interactions. Angew. Chem. Int. Ed. 2012, 51, 7011–7015. [Google Scholar] [CrossRef]
- Wang, F.; Han, C.; He, C.; Zhou, Q.; Zhang, J.; Wang, C.; Li, N.; Huang, F. Self-sorting Organization of Two Heteroditopic Monomers to Supramolecular Alternating Copolymers. J. Am. Chem. Soc. 2008, 130, 11254–11255. [Google Scholar] [CrossRef] [PubMed]
- Ji, X.; Yao, Y.; Li, J.; Yan, X.; Huang, F. A Supramolecular Cross-linked Conjugated Polymer Network for Multiple Fluorescent Sensing. J. Am. Chem. Soc. 2013, 135, 74–77. [Google Scholar] [CrossRef] [PubMed]
- Dong, S.; Zheng, B.; Xu, D.; Yan, X.; Zhang, M.; Huang, F. A Crown Ether Appended Super Gelator with Multiple Stimulus Responsiveness. Adv. Mater. 2012, 24, 3191–3195. [Google Scholar] [CrossRef] [PubMed]
- Dong, S.; Luo, Y.; Yan, X.; Zheng, B.; Ding, X.; Yu, Y.; Ma, Z.; Zhao, Q.; Huang, F. A Dual-Responsive Supramolecular Polymer Gel Formed by Crown Ether Based Molecular Recognition. Angew. Chem. Int. Ed. 2011, 50, 1905–1909. [Google Scholar] [CrossRef]
- Cantrill, S.J.; Fulton, D.A.; Heiss, A.M.; Pease, A.R.; Stoddart, J.F.; White, A.J.P.; Williams, D.J. The Influence of Macrocyclic Polyether Constitution Upon Ammonium Ion/Crown Ether Recognition Processes. Chem. Eur. J. 2000, 6, 2274–2287. [Google Scholar] [CrossRef] [PubMed]
- Bolla, M.A.; Tiburcio, J.; Loeb, S.J. Characterization of a Slippage Stopper for the 1,2-Bis(pyridinium)ethane-[24]crown-8 ether [2]Pseudorotaxane Motif. Tetrahedron 2008, 64, 8423–8428. [Google Scholar] [CrossRef]
- Linnartz, P.; Bitter, S.; Schalley, C.A. Deslipping of Ester Rotaxanes: A Cooperative Interplay of Hydrogen Bonding with Rotational Barriers. Eur. J. Org. Chem. 2003, 24, 4819–4829. [Google Scholar] [CrossRef]
- Bauta, W.E.; Lovett, D.P.; Cantrell, W.R.; Burke, B.D. Formal Synthesis of Angiogenesis Inhibitor NM-3. J. Org. Chem. 2003, 68, 5967–5973. [Google Scholar] [CrossRef] [PubMed]
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Leung, K.C.-F.; Lau, K.-N.; Wong, W.-Y. Revisiting the Formation and Tunable Dissociation of a [2]Pseudorotaxane Formed by Slippage Approach. Int. J. Mol. Sci. 2015, 16, 8254-8265. https://doi.org/10.3390/ijms16048254
Leung KC-F, Lau K-N, Wong W-Y. Revisiting the Formation and Tunable Dissociation of a [2]Pseudorotaxane Formed by Slippage Approach. International Journal of Molecular Sciences. 2015; 16(4):8254-8265. https://doi.org/10.3390/ijms16048254
Chicago/Turabian StyleLeung, Ken Cham-Fai, Kwun-Ngai Lau, and Wing-Yan Wong. 2015. "Revisiting the Formation and Tunable Dissociation of a [2]Pseudorotaxane Formed by Slippage Approach" International Journal of Molecular Sciences 16, no. 4: 8254-8265. https://doi.org/10.3390/ijms16048254
APA StyleLeung, K. C.-F., Lau, K.-N., & Wong, W.-Y. (2015). Revisiting the Formation and Tunable Dissociation of a [2]Pseudorotaxane Formed by Slippage Approach. International Journal of Molecular Sciences, 16(4), 8254-8265. https://doi.org/10.3390/ijms16048254