Mutation Breeding of Extracellular Polysaccharide-Producing Microalga Crypthecodinium cohnii by a Novel Mutagenesis with Atmospheric and Room Temperature Plasma
Abstract
:1. Introduction
2. Results
2.1. Determination of the Optimal Sampling Time
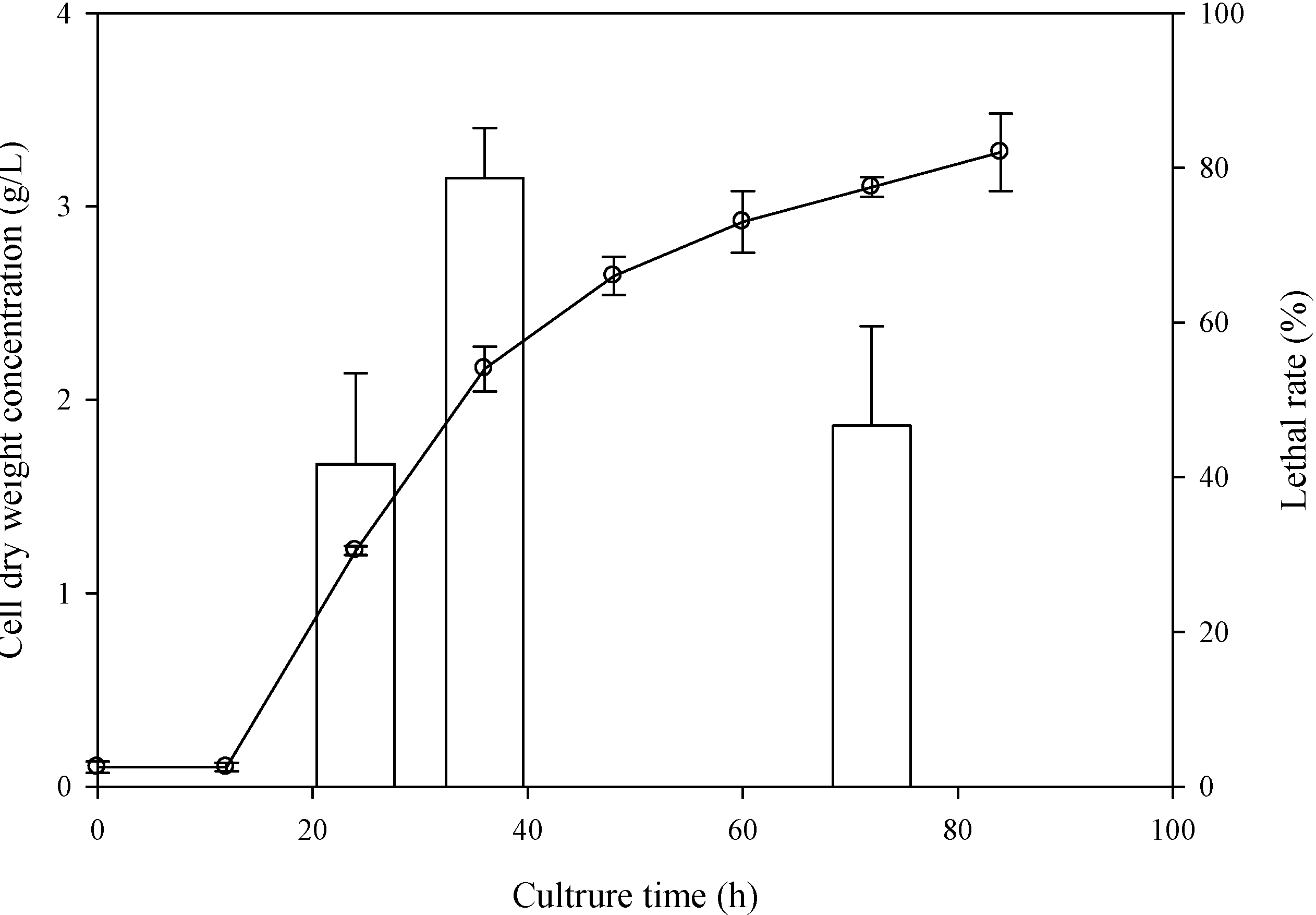
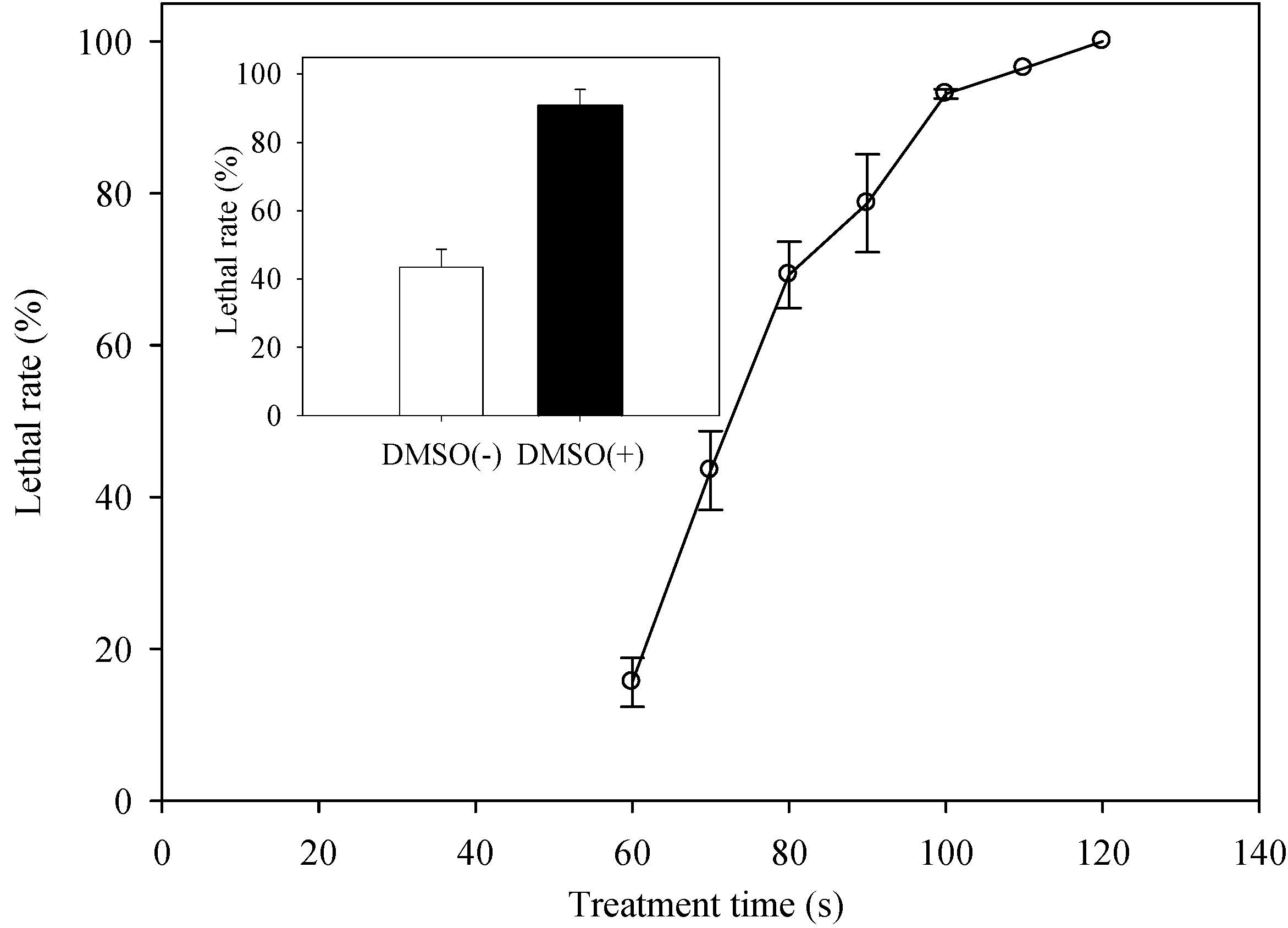
2.2. Determination of the Optimal ARTP Treatment Duration
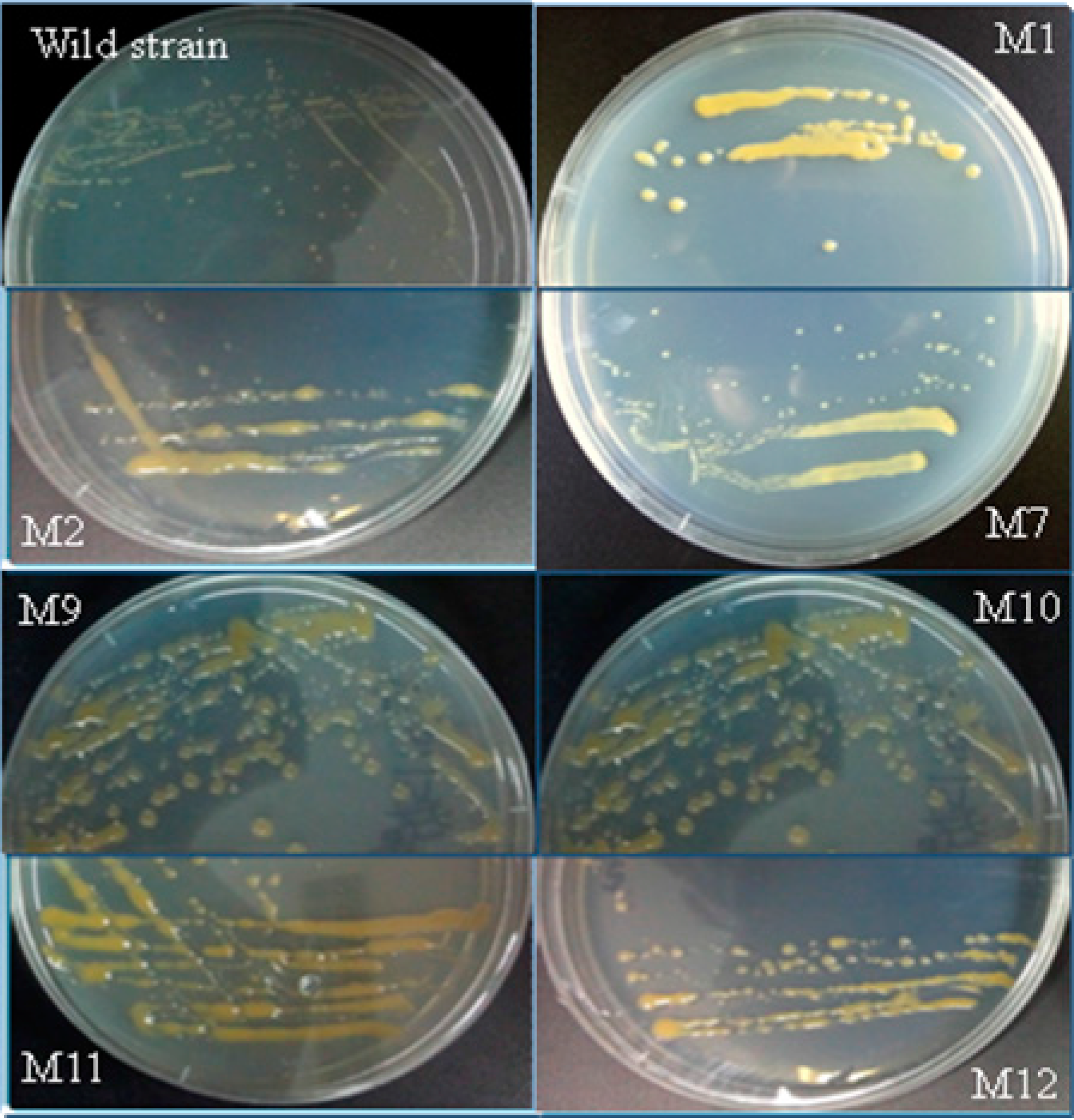
2.3. Screening for Mutants with Enhanced EPS Content
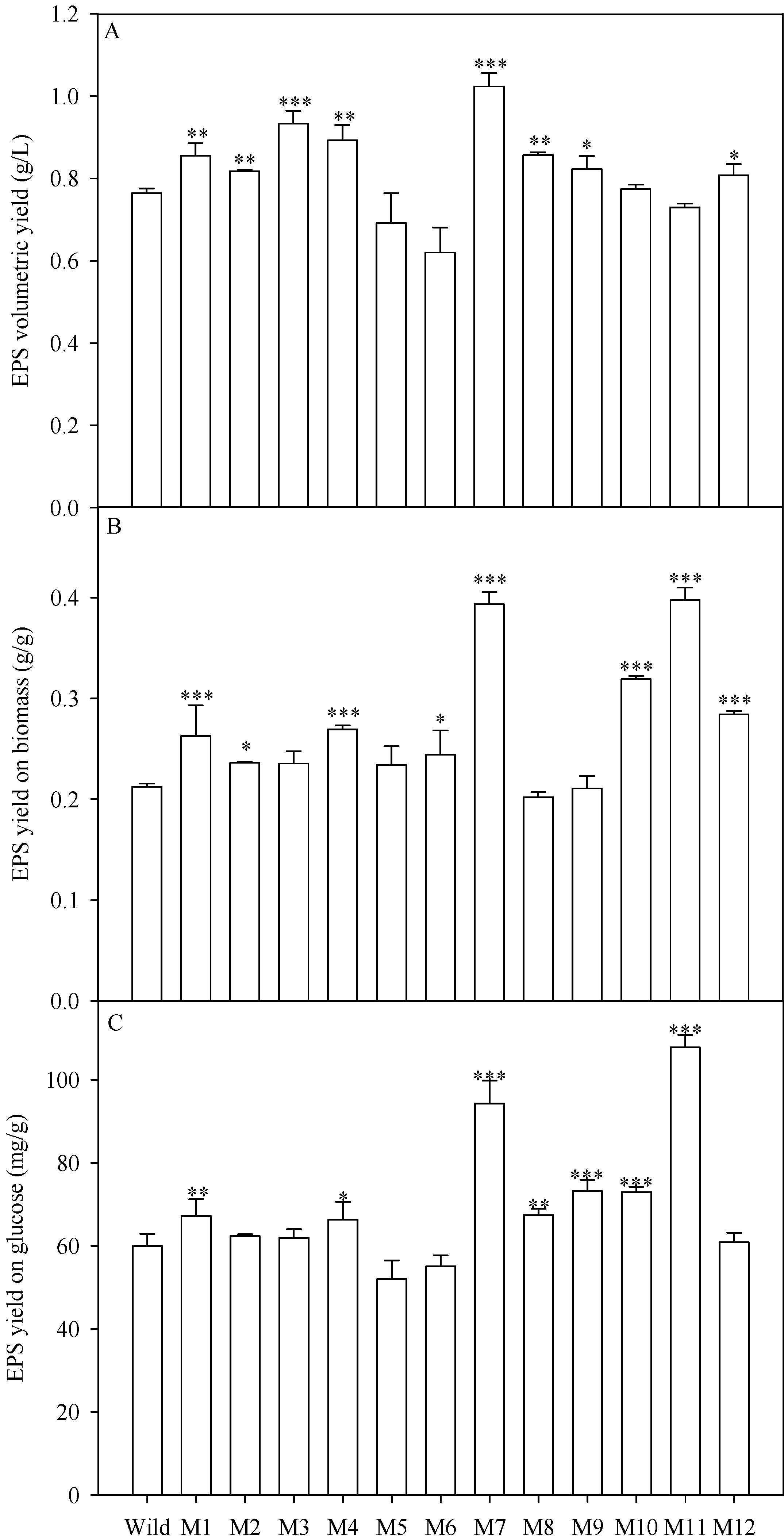
2.4. EPS Production of the Mutants
| Species | Cell Dry Weight Concentration (g/L) | Growth Yield on Glucose (g/g) |
|---|---|---|
| wild type | 3.60 ± 0.09 | 0.28 ± 0.02 |
| M1 | 3.25 ± 0.29 | 0.26 ± 0.02 |
| M2 | 3.46 ± 0.22 | 0.26 ± 0.00 |
| M3 | 3.96 ± 0.32 *** | 0.27 ± 0.01 |
| M4 | 3.31 ± 0.10 | 0.25 ± 0.02 |
| M5 | 2.95 ± 0.11 | 0.22 ± 0.01 |
| M6 | 2.54 ± 0.02 | 0.23 ± 0.01 |
| M7 | 2.60 ± 0.02 | 0.24 ± 0.01 |
| M8 | 4.24 ± 0.09 *** | 0.33 ± 0.00 *** |
| M9 | 3.90 ± 0.12 ** | 0.35 ± 0.02 *** |
| M10 | 2.43 ± 0.05 | 0.23 ± 0.01 |
| M11 | 1.83 ± 0.08 | 0.27 ± 0.02 |
| M12 | 2.84 ± 0.07 | 0.21 ± 0.01 |
2.5. Monosaccharide Composition
| Species | Mannose | Ribose | Glucose | Galactose | Fucose | Unknown |
|---|---|---|---|---|---|---|
| Wild | 6.53 ± 0.15 | 5.99 ± 0.51 | 61.73 ± 0.59 | 20.15 ± 0.06 | 3.48 ± 0.08 | 2.13 ± 0.05 |
| M7 | 6.05 ± 0.01 | 4.72 ± 0.12 | 63.77 ± 0.12 | 19.61 ± 0.07 | 3.65 ± 0.28 | 2.2 ± 0.04 |
3. Discussion
4. Experimental Section
4.1. Algal Species and Culture Conditions
4.2. Mutation by ARTP
4.3. Isolation of Extracellular Polysaccharides
4.4. Analysis of Monosaccharide Composition
4.5. Determination of Residual Glucose Concentration and Cell Dry Weight
4.6. Statistical Analysis
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Boddohi, S.; Kipper, M.J. Engineering nanoassemblies of polysaccharides. Adv. Mater. 2010, 22, 2998–3016. [Google Scholar] [CrossRef] [PubMed]
- Laurienzo, P. Marine polysaccharides in pharmaceutical applications: An overview. Mar. Drugs 2010, 8, 2435–2465. [Google Scholar] [PubMed]
- Wu, J.Y.; Chen, X.; Siu, K.C. Isolation and structure characterization of an antioxidative glycopeptide from mycelial culture broth of a medicinal fungus. Int. J. Mol. Sci. 2014, 15, 17318–17332. [Google Scholar] [CrossRef] [PubMed]
- Yao, Y.; Shi, Z.X.; Ren, G.X. Antioxidant and immunoregulatory activity of polysaccharides from quinoa (Chenopodium quinoa Willd.). Int. J. Mol. Sci. 2014, 15, 19307–19318. [Google Scholar] [CrossRef] [PubMed]
- Sun, L.Q.; Wang, L.; Li, J.; Liu, H.H. Characterization and antioxidant activities of degraded polysaccharides from two marine Chrysophyta. Food Chem. 2014, 160, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Guzman, S.; Gato, A.; Lamela, M.; Freire-Garabal, M.; Calleja, J.M. Anti-inflammatory and immunomodulatory activities of polysaccharide from Chlorella stigmatophora and Phaeodactylum tricomutum. Phytother. Res. 2003, 17, 665–670. [Google Scholar] [PubMed]
- Samarakoon, K.W.; Ko, J.Y.; Shah, M.M.R.; Lee, J.H.; Kang, M.C.; O-Nam, K.; Lee, J.B.; Jeon, Y.J. In vitro studies of anti-inflammatory and anticancer activities of organic solvent extracts from cultured marine microalgae. Algae 2013, 28, 111–119. [Google Scholar] [CrossRef]
- Gardeva, E.; Toshkova, R.; Yossifova, L.; Minkova, K.; Gigova, L. Cytotoxic and apoptogenic potential of red microalgal polysaccharides. Biotechnol. Biotechnol. Equip. 2012, 26, 3167–3172. [Google Scholar] [CrossRef]
- Raposo, M.F.D.; de Morais, A.; de Morais, R. Influence of sulphate on the composition and antibacterial and antiviral properties of the exopolysaccharide from Porphyridium cruentum. Life Sci. 2014, 101, 56–63. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.; Yim, J.H.; Kim, S.Y.; Kim, H.S.; Lee, W.G.; Kim, S.J.; Kang, P.S.; Lee, C.K. In vitro inhibition of influenza A virus infection by marine microalga-derived sulfated polysaccharide p-KG03. Antivir. Res. 2012, 93, 253–259. [Google Scholar] [CrossRef] [PubMed]
- Raposo, M.F.D.; de Morais, R.; de Morais, A. Health applications of bioactive compounds from marine microalgae. Life Sci. 2013, 93, 479–486. [Google Scholar] [CrossRef] [PubMed]
- Pignolet, O.; Jubeau, S.; Vaca-Garcia, C.; Michaud, P. Highly valuable microalgae: Biochemical and topological aspects. J. Ind. Microbiol. Biotechnol. 2013, 40, 781–796. [Google Scholar] [CrossRef] [PubMed]
- Dewapriya, P.; Kim, S.-K. Marine microorganisms: An emerging avenue in modern nutraceuticals and functional foods. Food Res. Int. 2014, 56, 115–125. [Google Scholar] [CrossRef]
- Zhang, X.; Zhang, X.F.; Li, H.P.; Wang, L.Y.; Zhang, C.; Xing, X.H.; Bao, C.Y. Atmospheric and room temperature plasma (ARTP) as a new powerful mutagenesis tool. Appl. Microbiol. Biotechnol. 2014, 98, 5387–5396. [Google Scholar] [CrossRef] [PubMed]
- Fang, M.Y.; Jin, L.H.; Zhang, C.; Tan, Y.Y.; Jiang, P.X.; Ge, N.; Li, H.P.; Xing, X.H. Rapid mutation of Spirulina platensis by a new mutagenesis system of atmospheric and room temperature plasmas (ARTP) and generation of a mutant library with diverse phenotypes. PLoS ONE 2013, 8, e77046. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Feng, L.R.; Wei, L.; Li, H.G.; Wang, L.; Zhou, Y.; Yu, X.B. Mutation breeding of lycopene-producing strain Blakeslea trispora by a novel atmospheric and room temperature plasma (ARTP). Appl. Biochem. Biotechnol. 2014, 174, 452–460. [Google Scholar] [CrossRef] [PubMed]
- Chang, K.J.L.; Nichols, C.M.; Blackburn, S.I.; Dunstan, G.A.; Koutoulis, A.; Nichols, P.D. Comparison of thraustochytrids Aurantiochytrium sp., Schizochytrium sp., Thraustochytrium sp., and Ulkenia sp. for production of biodiesel, long-chain omega-3 oils, and exopolysaccharide. Mar. Biotechnol. 2014, 16, 396–411. [Google Scholar] [CrossRef] [PubMed]
- De Swaaf, M.E.; Grobben, G.J.; Eggink, G.; de Rijk, T.C.; van der Meer, P.; Sijtsma, L. Characterisation of extracellular polysaccharides produced by Crypthecodinium cohnii. Appl. Microbiol. Biotechnol. 2001, 57, 395–400. [Google Scholar] [CrossRef] [PubMed]
- Mohamed, S.; Hashim, S.N.; Rahman, H.A. Seaweeds: A sustainable functional food for complementary and alternative therapy. Trends Food Sci. Technol. 2012, 23, 83–96. [Google Scholar] [CrossRef]
- Mendes, A.; Reis, A.; Vasconcelos, R.; Guerra, P.; da Silva, T.L. Crypthecodinium cohnii with emphasis on DHA production: A review. J. Appl. Phycol. 2009, 21, 199–214. [Google Scholar] [CrossRef]
- Hazen, K.C. Influence of DMSO on antifungal activity during susceptibility testing in vitro. Diagn. Microbiol. Infect. Dis. 2013, 75, 60–63. [Google Scholar] [CrossRef] [PubMed]
- Laroussi, M.; Lu, X. Room-temperature atmospheric pressure plasma plume for biomedical applications. Appl. Phys. Lett. 2005, 87. [Google Scholar] [CrossRef]
- Leduc, M.; Guay, D.; Coulombe, S.; Leask, R.L. Effects of non-thermal plasmas on DNA and mammalian cells. Plasma Process. Polym. 2010, 7, 899–909. [Google Scholar] [CrossRef]
- Lu, Y.; Wang, L.Y.; Ma, K.; Li, G.; Zhang, C.; Zhao, H.X.; Lai, Q.H.; Li, H.P.; Xing, X.H. Characteristics of hydrogen production of an Enterobacter aerogenes mutant generated by a new atmospheric and room temperature plasma (ARTP). Biochem. Eng. J. 2011, 55, 17–22. [Google Scholar] [CrossRef]
- Degeest, B.; de Vuyst, L. Correlation of activities of the enzymes alpha-phosphoglucomutase, UDP-galactose 4-epimerase, and UDP-glucose pyrophosphorylase with exopolysaccharide biosynthesis by Streptococcus thermophilus LY03. Appl. Environ. Microbiol. 2000, 66, 3519–3527. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.W.; Ji, S.L.; Li, H.J.; Zhou, J.S.; Duan, Y.Q.; Dang, L.Z.; Mo, M.H. Increased polysaccharide production and biosynthetic gene expressions in a submerged culture of Ganoderma lucidum by the overexpression of the homologous alpha-phosphoglucomutase gene. Bioprocess Biosyst. Eng. 2015, 38, 399–405. [Google Scholar] [CrossRef] [PubMed]
- Grobben, G.; van Casteren, W.; Schols, H.; Oosterveld, A.; Sala, G.; Smith, M.; Sikkema, J.; de Bont, J. Analysis of the exopolysaccharides produced by Lactobacillus delbrueckii subsp. bulgaricus NCFB 2772 grown in continuous culture on glucose and fructose. Appl. Microbiol. Biotechnol. 1997, 48, 516–521. [Google Scholar] [CrossRef]
- Dai, J.; Zhu, S.; Tang, J.; Wang, M.; Yin, H.P.; Chen, S.W. Analysis of monosaccharide composition in polysaccharides from D. salina by pre-column derivatization high performance liquid chromtography. J. Instrum. Anal. 2007, 26, 206–210. [Google Scholar]
- Miller, G.L. Use of dinitrosalicylic acid reagent for determination of reducing sugar. Anal. Chem. 1959, 31, 426–428. [Google Scholar] [CrossRef]
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, B.; Sun, Z.; Ma, X.; Yang, B.; Jiang, Y.; Wei, D.; Chen, F. Mutation Breeding of Extracellular Polysaccharide-Producing Microalga Crypthecodinium cohnii by a Novel Mutagenesis with Atmospheric and Room Temperature Plasma. Int. J. Mol. Sci. 2015, 16, 8201-8212. https://doi.org/10.3390/ijms16048201
Liu B, Sun Z, Ma X, Yang B, Jiang Y, Wei D, Chen F. Mutation Breeding of Extracellular Polysaccharide-Producing Microalga Crypthecodinium cohnii by a Novel Mutagenesis with Atmospheric and Room Temperature Plasma. International Journal of Molecular Sciences. 2015; 16(4):8201-8212. https://doi.org/10.3390/ijms16048201
Chicago/Turabian StyleLiu, Bin, Zheng Sun, Xiaonian Ma, Bo Yang, Yue Jiang, Dong Wei, and Feng Chen. 2015. "Mutation Breeding of Extracellular Polysaccharide-Producing Microalga Crypthecodinium cohnii by a Novel Mutagenesis with Atmospheric and Room Temperature Plasma" International Journal of Molecular Sciences 16, no. 4: 8201-8212. https://doi.org/10.3390/ijms16048201
APA StyleLiu, B., Sun, Z., Ma, X., Yang, B., Jiang, Y., Wei, D., & Chen, F. (2015). Mutation Breeding of Extracellular Polysaccharide-Producing Microalga Crypthecodinium cohnii by a Novel Mutagenesis with Atmospheric and Room Temperature Plasma. International Journal of Molecular Sciences, 16(4), 8201-8212. https://doi.org/10.3390/ijms16048201







