The Relevance of miRNA-21 in HSV-Induced Inflammation in a Mouse Model
Abstract
:1. Introduction
2. Results
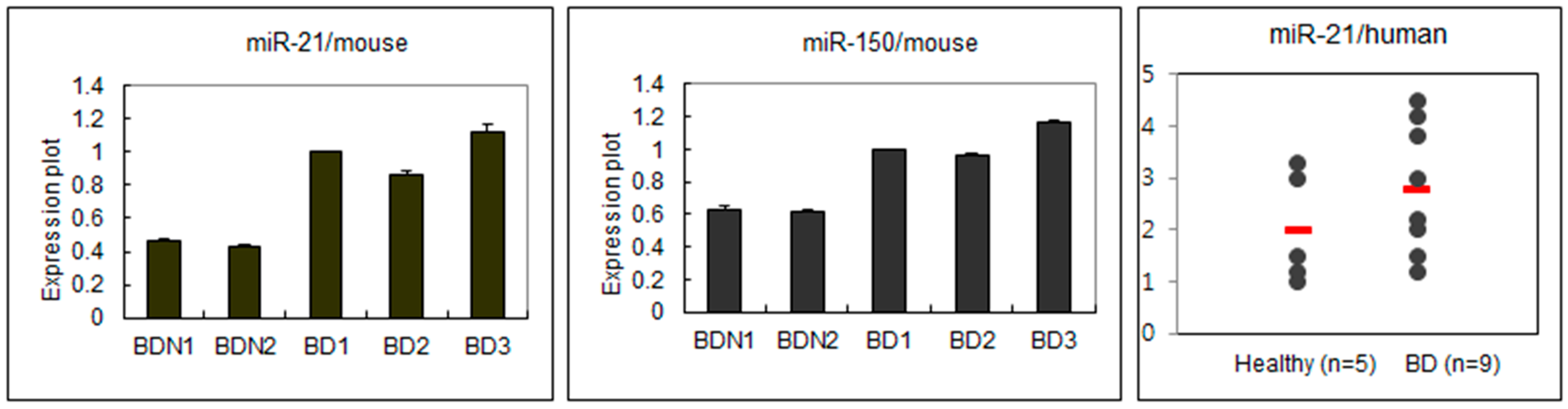
2.1. Different Expressions of miRNA Regarding BD Symptoms
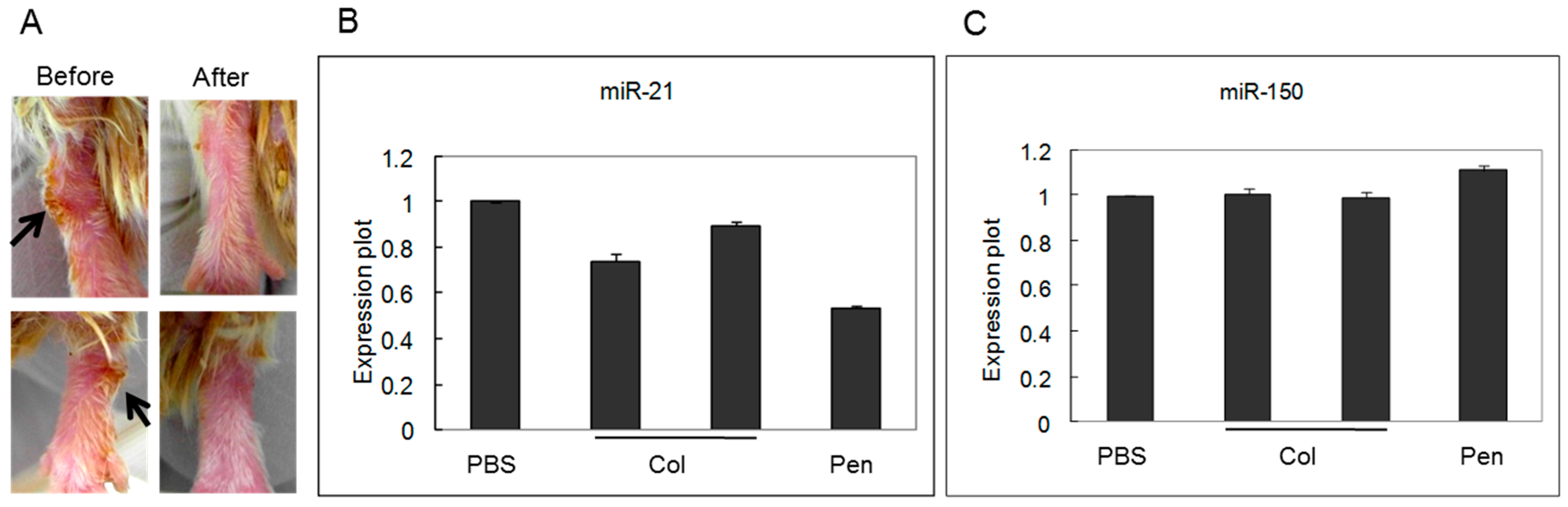
2.2. miRNA Expression Was Regulated by Medication
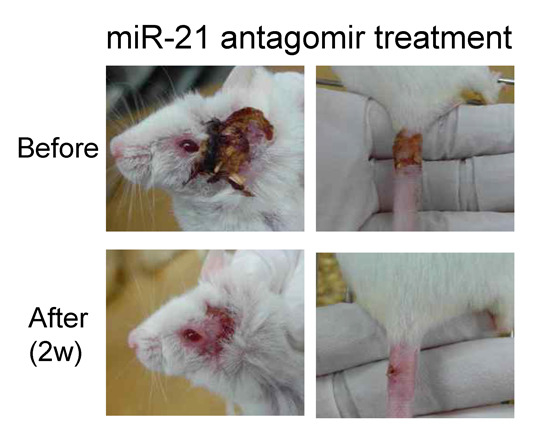
2.3. Correlation of miR-21 Inhibition with BD Symptoms
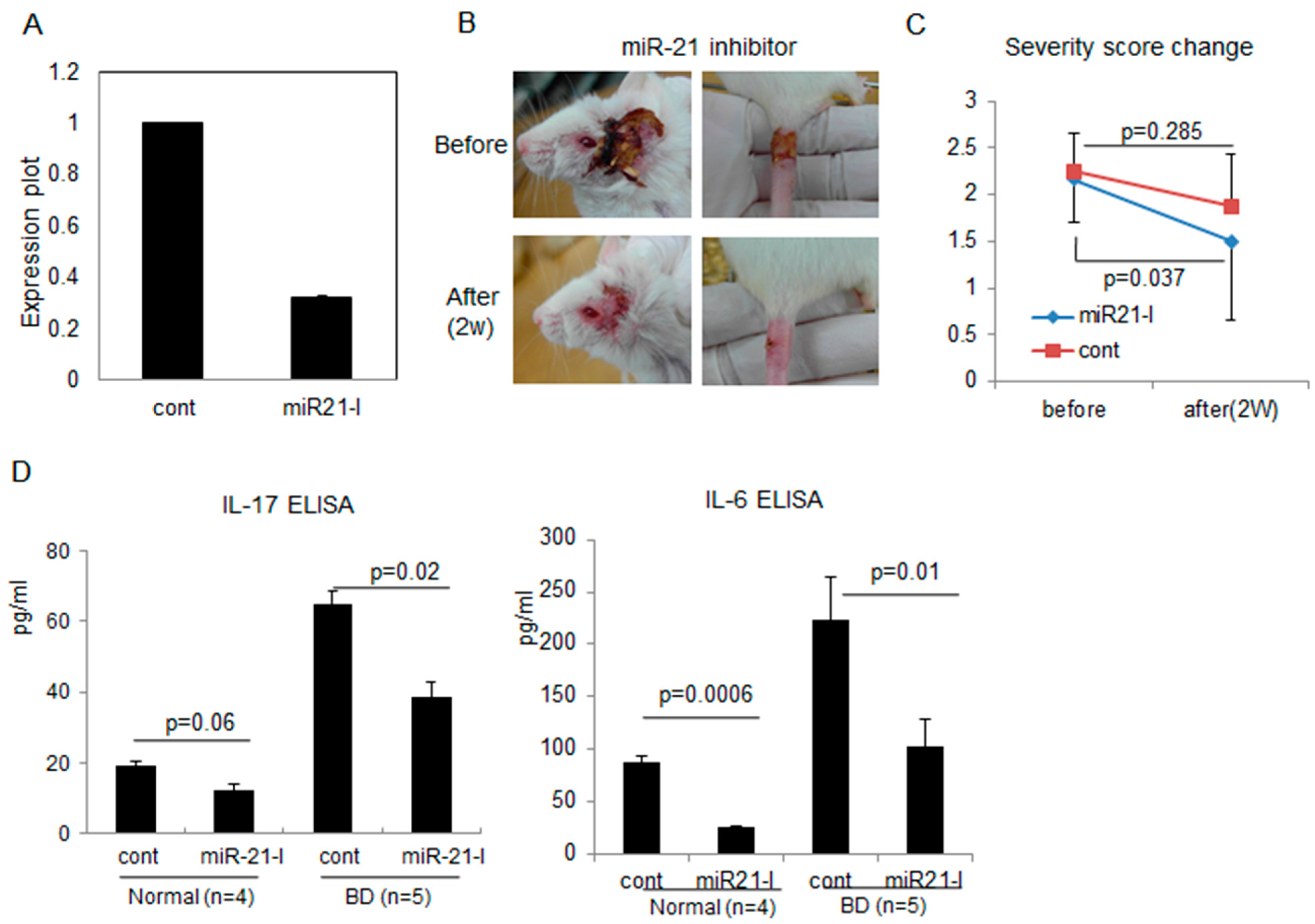
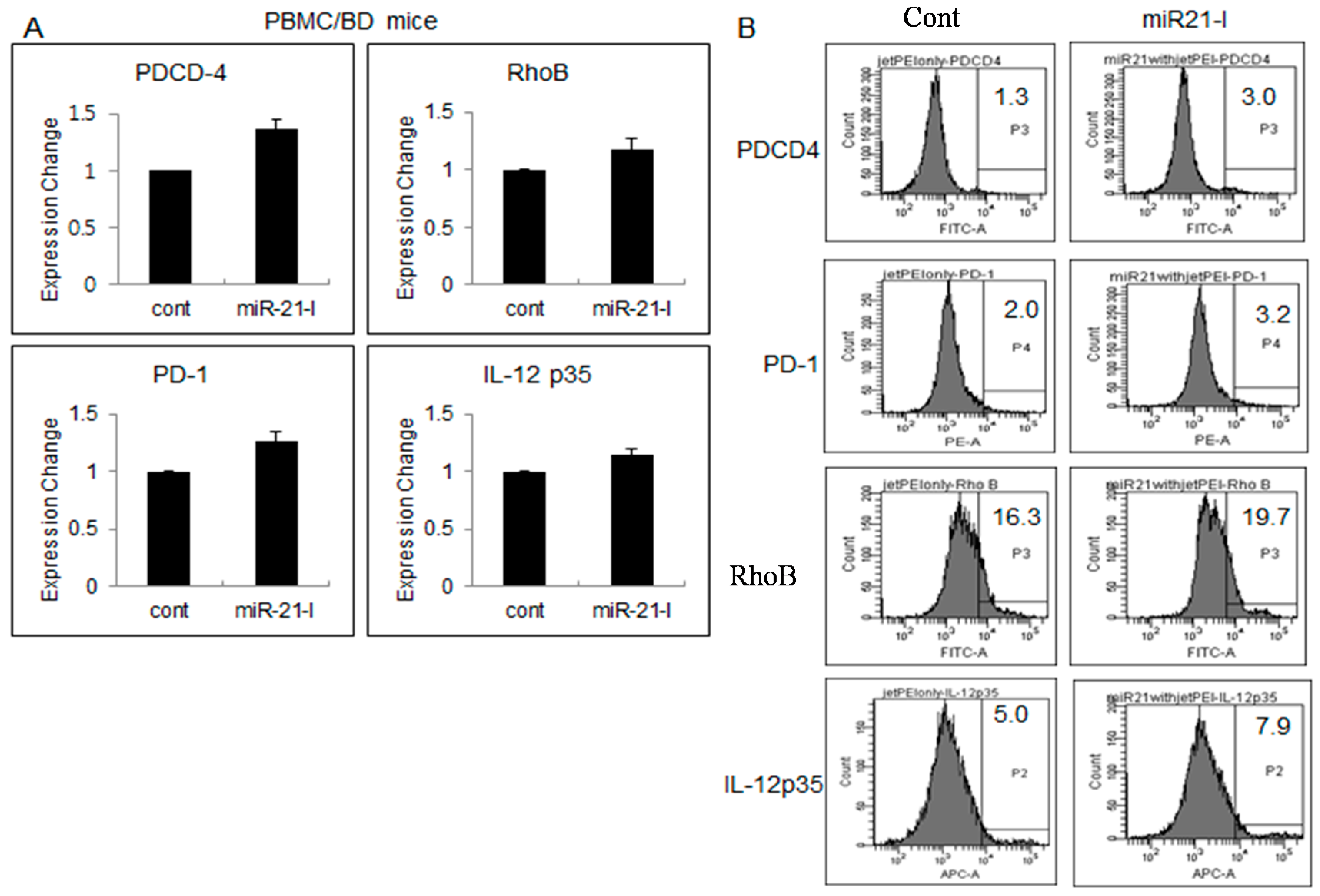
2.4. Up-Regulated Genes after miR-21 Inhibition
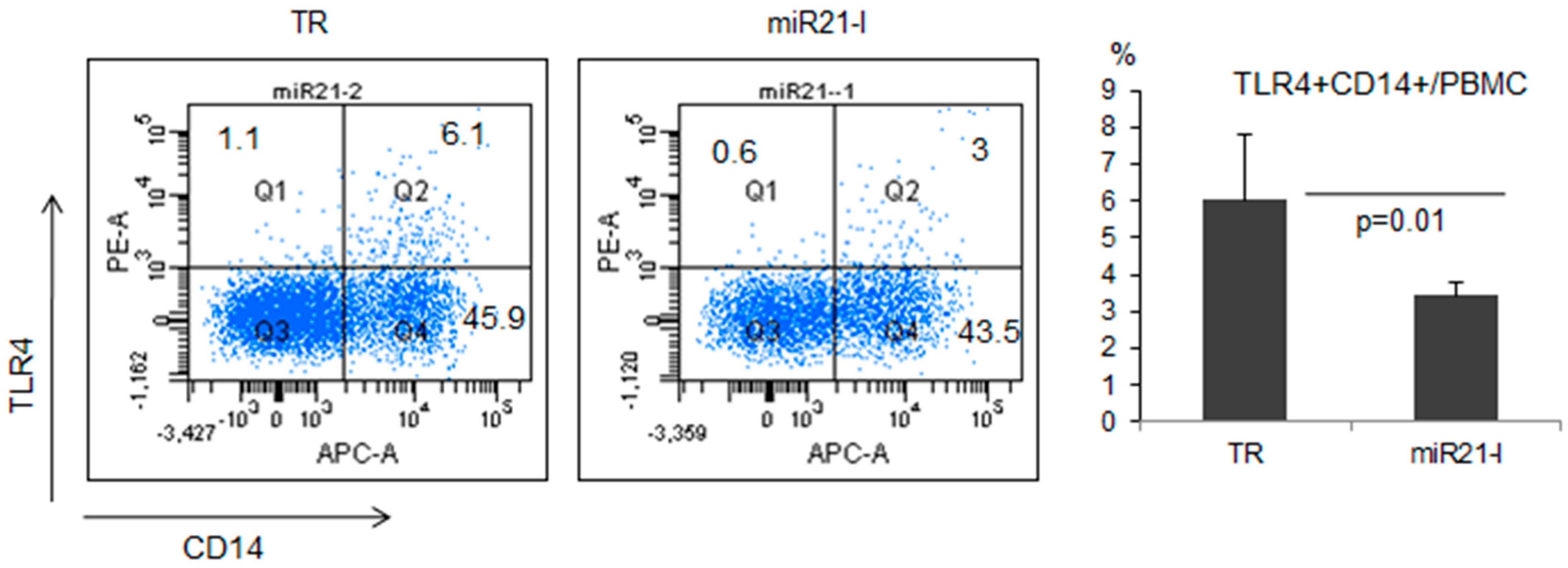
2.5. miR-21 Inhibition Down-Regulated TLR4 in Normal Healthy Mice
3. Discussion
4. Experimental Section
4.1. Human Materials
| Patient | Age | Sex | OU | GU | Arthritis | GI | NEUR | VAS | OL | Pathergy | HLA-B51 | EN | ESR | CRP |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| KSY | 63 | F | + | + | + | − | − | − | − | − | − | 25 | 0.03 | |
| CMR | 36 | F | + | + | + | + | − | − | − | − | + | 55 | 0.5 | |
| HHK | 51 | F | + | + | + | − | − | − | − | − | + | 71 | 2.93 | |
| JYS | 32 | F | + | + | + | − | − | − | − | − | + | + | 70 | 0.14 |
| LJH | 42 | F | + | + | + | − | − | − | − | − | + | + | 18 | 0.07 |
| SJO | 55 | F | + | − | + | − | − | − | − | − | + | + | 21 | 0.52 |
| SKH | 53 | M | + | − | + | − | + | − | − | − | + | + | 20 | 0.09 |
| KSM | 35 | F | + | − | + | − | − | − | − | − | + | + | 24 | 0.05 |
| LMS | 49 | F | + | − | + | − | − | − | − | − | + | + | 24 | 0.7 |
| Patient | Colchicine | Steroid | Azathioprin | Bucillamine | HCQ | Minocycline | NSAIDs | SZP |
|---|---|---|---|---|---|---|---|---|
| KSY | + | + | + | + | + | − | + | + |
| CMR | + | + | + | − | − | + | − | + |
| HHK | + | + | − | − | − | + | + | + |
| JYS | + | + | + | − | − | + | + | + |
| LJH | + | + | − | − | + | − | + | + |
| SJO | + | + | − | + | + | − | + | + |
| SKH | + | + | − | + | − | − | + | + |
| KSM | + | + | − | − | + | − | + | + |
| LMS | + | + | − | − | − | − | + | + |
4.2. Animals
4.3. Clarification of BD, BDN, and Normal Mouse
4.4. Severity Score of BD Mouse
4.5. Medication of BD-Like Symptoms
4.6. miRNA Inhibition
4.7. Reverse Transcriptase (RT)-PCR
| Genes | Primers |
|---|---|
| PD-1 | (F) TCGTGGTAACAGAGAGAATCCT (R) TTCAGAGTGTCGTCCTTGCTT |
| PDCD4 | (F) TTGGCAGTGTCCTTAGCCTT (R) GGCTAGCTCAGGGAGATCCT |
| RhoB | (F) CCCAGTGTCTGTGTGTGTCC (R) TGAGGCCTGGCTCTTTAGAA |
| IL-12p35 | (F) TAGATGCTACCAAGGCAC (R) ATCACGCTACCTCCTCTT |
4.8. Real-Time Quantitative PCR (qPCR)
4.9. Flow Cytometry
4.10. Measurement of Cytokines by ELISA
4.11. Statistical Analysis
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Chugh, P.; Dittmer, D.P. Potential pitfalls in microrna profiling. Wiley Interdiscip. Rev. RNA 2012, 3, 601–616. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Q.; Xiao, X.; Wang, C.; Zhang, X.; Li, F.; Zhou, Y.; Kijlstra, A.; Yang, P. Decreased microRNA-155 expression in ocular Behcet’s disease but not in Vogt Koyanagi Harada syndrome. Investig. Ophthalmol. Vis. Sci. 2012, 53, 5665–5674. [Google Scholar] [CrossRef]
- Zhou, Q.; Hou, S.; Liang, L.; Li, X.; Tan, X.; Wei, L.; Lei, B.; Kijlstra, A.; Yang, P. MicroRNA-146a and Ets-1 gene polymorphisms in ocular Behcet’s disease and Vogt-Koyanagi-Harada syndrome. Ann. Rheum. Dis. 2014, 73, 170–176. [Google Scholar] [CrossRef] [PubMed]
- Lagos-Quintana, M.; Rauhut, R.; Lendeckel, W.; Tuschl, T. Identification of novel genes coding for small expressed RNAs. Science 2001, 294, 853–858. [Google Scholar] [CrossRef] [PubMed]
- Si, M.L.; Zhu, S.; Wu, H.; Lu, Z.; Wu, F.; Mo, Y.Y. MiR-21-mediated tumor growth. Oncogene 2007, 26, 2799–2803. [Google Scholar] [CrossRef] [PubMed]
- Dahiya, N.; Sherman-Baust, C.A.; Wang, T.L.; Davidson, B.; Shih Ie, M.; Zhang, Y.; Wood, W., 3rd.; Becker, K.G.; Morin, P.J. MicroRNA expression and identification of putative miRNA targets in ovarian cancer. PLoS ONE 2008, 3, e2436. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Tang, S.; Le, S.Y.; Lu, R.; Rader, J.S.; Meyers, C.; Zheng, Z.M. Aberrant expression of oncogenic and tumor-suppressive microRNAs in cervical cancer is required for cancer cell growth. PLoS ONE 2008, 3, e2557. [Google Scholar] [CrossRef] [PubMed]
- Chan, J.A.; Krichevsky, A.M.; Kosik, K.S. MicroRNA-21 is an antiapoptotic factor in human glioblastoma cells. Cancer Res. 2005, 65, 6029–6033. [Google Scholar] [CrossRef] [PubMed]
- Cui, L.; Zhang, X.; Ye, G.; Zheng, T.; Song, H.; Deng, H.; Xiao, B.; Xia, T.; Yu, X.; Le, Y.; et al. Gastric juice microRNAs as potential biomarkers for the screening of gastric cancer. Cancer 2013, 119, 1618–1626. [Google Scholar] [CrossRef]
- Zhang, H.; Guo, Y.; Shang, C.; Song, Y.; Wu, B. miR-21 downregulated TCF21 to inhibit KISS1 in renal cancer. Urology 2012, 80, 1298–1302.e1. [Google Scholar] [CrossRef] [PubMed]
- Kurashige, J.; Kamohara, H.; Watanabe, M.; Tanaka, Y.; Kinoshita, K.; Saito, S.; Hiyoshi, Y.; Iwatsuki, M.; Baba, Y.; Baba, H. Serum microRNA-21 is a novel biomarker in patients with esophageal squamous cell carcinoma. J. Surg. Oncol. 2012, 106, 188–192. [Google Scholar] [CrossRef] [PubMed]
- Feng, Y.H.; Wu, C.L.; Tsao, C.J.; Chang, J.G.; Lu, P.J.; Yeh, K.T.; Uen, Y.H.; Lee, J.C.; Shiau, A.L. Deregulated expression of sprouty2 and microRNA-21 in human colon cancer: Correlation with the clinical stage of the disease. Cancer Biol. Ther. 2011, 11, 111–121. [Google Scholar] [CrossRef] [PubMed]
- Salim, H.; Akbar, N.S.; Zong, D.; Vaculova, A.H.; Lewensohn, R.; Moshfegh, A.; Viktorsson, K.; Zhivotovsky, B. miRNA-214 modulates radiotherapy response of non-small cell lung cancer cells through regulation of p38MAPK, apoptosis and senescence. Br. J. Cancer 2012, 107, 1361–1373. [Google Scholar] [CrossRef] [PubMed]
- Dillhoff, M.; Liu, J.; Frankel, W.; Croce, C.; Bloomston, M. MicroRNA-21 is overexpressed in pancreatic cancer and a potential predictor of survival. J. Gastrointest. Surg. 2008, 12, 2171–2176. [Google Scholar] [CrossRef] [PubMed]
- Tao, Y.J.; Ren, Y.; Dong, J.B.; Zhang, L.; Cheng, J.P.; Zhou, X. Anti-sense miRNA-21 oligonucleotide inhibits Tb 3.1 human tongue squamous cell carcinoma growth in vitro (in Chinese). Zhonghua Kou Qiang Yi Xue Za Zhi 2011, 46, 79–83. [Google Scholar] [PubMed]
- Zhang, H.L.; Yang, L.F.; Zhu, Y.; Yao, X.D.; Zhang, S.L.; Dai, B.; Zhu, Y.P.; Shen, Y.J.; Shi, G.H.; Ye, D.W. Serum miRNA-21: Elevated levels in patients with metastatic hormone-refractory prostate cancer and potential predictive factor for the efficacy of docetaxel-based chemotherapy. Prostate 2011, 71, 326–331. [Google Scholar] [CrossRef] [PubMed]
- Wang, F.; Zheng, Z.; Guo, J.; Ding, X. Correlation and quantitation of microRNA aberrant expression in tissues and sera from patients with breast tumor. Gynecol. Oncol. 2010, 119, 586–593. [Google Scholar] [CrossRef] [PubMed]
- Conti, A.; Aguennouz, M.; La Torre, D.; Tomasello, C.; Cardali, S.; Angileri, F.F.; Maio, F.; Cama, A.; Germano, A.; Vita, G.; et al. miR-21 and 221 upregulation and miR-181b downregulation in human grade II-IV astrocytic tumors. J. Neurooncol. 2009, 93, 325–332. [Google Scholar] [CrossRef] [PubMed]
- Lawrie, C.H.; Soneji, S.; Marafioti, T.; Cooper, C.D.; Palazzo, S.; Paterson, J.C.; Cattan, H.; Enver, T.; Mager, R.; Boultwood, J.; et al. MicroRNA expression distinguishes between germinal center B cell-like and activated B cell-like subtypes of diffuse large B cell lymphoma. Int. J. Cancer 2007, 121, 1156–1161. [Google Scholar] [CrossRef]
- Gibcus, J.H.; Tan, L.P.; Harms, G.; Schakel, R.N.; de Jong, D.; Blokzijl, T.; Moller, P.; Poppema, S.; Kroesen, B.J.; van den Berg, A. Hodgkin lymphoma cell lines are characterized by a specific miRNA expression profile. Neoplasia 2009, 11, 167–176. [Google Scholar] [PubMed]
- Wu, H.; Neilson, J.R.; Kumar, P.; Manocha, M.; Shankar, P.; Sharp, P.A.; Manjunath, N. miRNA profiling of naive, effector and memory CD8 T cells. PLoS ONE 2007, 2, e1020. [Google Scholar] [CrossRef] [PubMed]
- Svrcek, M.; El-Murr, N.; Wanherdrick, K.; Dumont, S.; Beaugerie, L.; Cosnes, J.; Colombel, J.F.; Tiret, E.; Flejou, J.F.; Lesuffleur, T.; et al. Overexpression of microRNAs-155 and 21 targeting mismatch repair proteins in inflammatory bowel diseases. Carcinogenesis 2013, 34, 828–834. [Google Scholar] [CrossRef]
- Takagi, T.; Naito, Y.; Mizushima, K.; Hirata, I.; Yagi, N.; Tomatsuri, N.; Ando, T.; Oyamada, Y.; Isozaki, Y.; Hongo, H.; et al. Increased expression of microRNA in the inflamed colonic mucosa of patients with active ulcerative colitis. J. Gastroenterol. Hepatol. 2010, 25 (Suppl. 1), S129–S133. [Google Scholar] [CrossRef]
- Wu, F.; Zhang, S.; Dassopoulos, T.; Harris, M.L.; Bayless, T.M.; Meltzer, S.J.; Brant, S.R.; Kwon, J.H. Identification of microRNAs associated with ileal and colonic crohn’s disease. Inflamm. Bowel Dis. 2010, 16, 1729–1738. [Google Scholar] [CrossRef] [PubMed]
- Sonkoly, E.; Wei, T.; Janson, P.C.; Saaf, A.; Lundeberg, L.; Tengvall-Linder, M.; Norstedt, G.; Alenius, H.; Homey, B.; Scheynius, A.; et al. MicroRNAs: Novel regulators involved in the pathogenesis of psoriasis? PLoS ONE 2007, 2, e610. [Google Scholar] [CrossRef]
- Moschos, S.A.; Williams, A.E.; Perry, M.M.; Birrell, M.A.; Belvisi, M.G.; Lindsay, M.A. Expression profiling in vivo demonstrates rapid changes in lung microRNA levels following lipopolysaccharide-induced inflammation but not in the anti-inflammatory action of glucocorticoids. BMC Genomics 2007, 8. [Google Scholar] [CrossRef] [PubMed]
- Frassanito, M.A.; Dammacco, R.; Cafforio, P.; Dammacco, F. Th1 polarization of the immune response in behcet’s disease: A putative pathogenetic role of interleukin-12. Arthritis Rheumatol. 1999, 42, 1967–1974. [Google Scholar] [CrossRef]
- Kim, E.S.; Kim, S.W.; Moon, C.M.; Park, J.J.; Kim, T.I.; Kim, W.H.; Cheon, J.H. Interactions between IL17a, IL23r, and STAT4 polymorphisms confer susceptibility to intestinal behcet’s disease in Korean population. Life Sci. 2012, 90, 740–746. [Google Scholar] [CrossRef] [PubMed]
- Remmers, E.F.; Cosan, F.; Kirino, Y.; Ombrello, M.J.; Abaci, N.; Satorius, C.; Le, J.M.; Yang, B.; Korman, B.D.; Cakiris, A.; et al. Genome-wide association study identifies variants in the MHC class I, IL10, and IL23R-IL12RB2 regions associated with behcet’s disease. Nat. Genet. 2010, 42, 698–702. [Google Scholar] [CrossRef]
- Mizuki, N.; Meguro, A.; Ota, M.; Ohno, S.; Shiota, T.; Kawagoe, T.; Ito, N.; Kera, J.; Okada, E.; Yatsu, K.; et al. Genome-wide association studies identify IL23R-IL12RB2 and IL10 as behcet’s disease susceptibility loci. Nat. Genet. 2010, 42, 703–706. [Google Scholar] [CrossRef]
- Behcet, H. Über rezidivierende, aphthöse, durch ein virus verursachte geschwüre am mund, am auge und an den genitalien (in German). Dermatol. Wochenschr. 1937, 105, 1152–1157. [Google Scholar]
- Lee, S.; Bang, D.; Cho, Y.H.; Lee, E.S.; Sohn, S. Polymerase chain reaction reveals herpes simplex virus DNA in saliva of patients with Behcet’s disease. Arch. Dermatol. Res. 1996, 288, 179–183. [Google Scholar] [CrossRef] [PubMed]
- Bang, D.; Cho, Y.H.; Choi, H.J.; Lee, S.; Sohn, S.; Lee, E.S. Detection of herpes simplex virus DNA by polymerase chain reaction in genital ulcer of patients with Behçet’s disease. In Proceedings of the 7th International Conference on Behçet’s Disease, Tunis, Tunisia, 10–11 October 1996; pp. 74–76.
- Nakano, H.; Tamura, T.; Yoshimoto, T.; Yagita, H.; Miyasaka, M.; Butcher, E.C.; Nariuchi, H.; Kakiuchi, T.; Matsuzawa, A. Genetic defect in T lymphocyte-specific homing into peripheral lymph nodes. Eur. J. Immunol. 1997, 27, 215–221. [Google Scholar] [CrossRef] [PubMed]
- Sohn, S.; Lee, E.S.; Bang, D.; Lee, S. Behcet’s disease-like symptoms induced by the herpes simplex virus in ICR mice. Eur. J. Dermatol. 1998, 8, 21–23. [Google Scholar] [PubMed]
- Sohn, S.; Lee, E.S.; Bang, D. Learning from HSV-infected mice as a model of Behcet’s disease. Clin. Exp. Rheumatol. 2012, 30, S96–S103. [Google Scholar] [PubMed]
- Sohn, S.; Bang, D.; Lee, E.S.; Kwon, H.J.; Lee, S.I.; Lee, S. Experimental studies on the antiviral agent famciclovir in Behcet’s disease symptoms in ICR mice. Br. J. Dermatol. 2001, 145, 799–804. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.S.; Knies, J.L.; Stark, C.; Colburn, N.H. Pdcd4 suppresses tumor phenotype in JB6 cells by inhibiting AP-1 transactivation. Oncogene 2003, 22, 3712–3720. [Google Scholar] [CrossRef] [PubMed]
- Frankel, L.B.; Christoffersen, N.R.; Jacobsen, A.; Lindow, M.; Krogh, A.; Lund, A.H. Programmed cell death 4 (PDCD4) is an important functional target of the microRNA miR-21 in breast cancer cells. J. Biol. Chem. 2008, 283, 1026–1033. [Google Scholar] [CrossRef] [PubMed]
- Connolly, E.C.; van Doorslaer, K.; Rogler, L.E.; Rogler, C.E. Overexpression of miR-21 promotes an in vitro metastatic phenotype by targeting the tumor suppressor Rhob. Mol. Cancer Res. 2010, 8, 691–700. [Google Scholar] [CrossRef] [PubMed]
- Lu, T.X.; Hartner, J.; Lim, E.J.; Fabry, V.; Mingler, M.K.; Cole, E.T.; Orkin, S.H.; Aronow, B.J.; Rothenberg, M.E. MicroRNA-21 limits in vivo immune response-mediated activation of the IL-12/IFN-γ pathway, th1 polarization, and the severity of delayed-type hypersensitivity. J. Immunol. 2011, 187, 3362–3373. [Google Scholar] [CrossRef] [PubMed]
- Kim, T.W.; Staschke, K.; Bulek, K.; Yao, J.; Peters, K.; Oh, K.H.; Vandenburg, Y.; Xiao, H.; Qian, W.; Hamilton, T.; et al. A critical role for IRAK4 kinase activity in Toll-like receptor-mediated innate immunity. J. Exp. Med. 2007, 204, 1025–1036. [Google Scholar] [CrossRef] [PubMed]
- Takeda, K.; Akira, S. TLR signaling pathways. Semin. Immunol. 2004, 16, 3–9. [Google Scholar] [CrossRef] [PubMed]
- Do, J.E.; Kwon, S.Y.; Park, S.; Lee, E.S. Effects of vitamin D on expression of Toll-like receptors of monocytes from patients with Behcet’s disease. Rheumatology 2008, 47, 840–848. [Google Scholar] [CrossRef] [PubMed]
- Choi, B.; Lee, E.S.; Sohn, S. Vitamin D3 ameliorates herpes simplex virus-induced Behcet’s disease-like inflammation in a mouse model through down-regulation of Toll-like receptors. Clin. Exp. Rheumatol. 2011, 29, S13–S19. [Google Scholar] [PubMed]
- Xiao, C.; Rajewsky, K. MicroRNA control in the immune system: Basic principles. Cell 2009, 136, 26–36. [Google Scholar] [CrossRef] [PubMed]
- Lindsay, M.A. MicroRNAs and the immune response. Trends Immunol. 2008, 29, 343–351. [Google Scholar] [CrossRef] [PubMed]
- Ventura, A.; Young, A.G.; Winslow, M.M.; Lintault, L.; Meissner, A.; Erkeland, S.J.; Newman, J.; Bronson, R.T.; Crowley, D.; Stone, J.R.; et al. Targeted deletion reveals essential and overlapping functions of the miR-17 through 92 family of miRNA clusters. Cell 2008, 132, 875–886. [Google Scholar] [CrossRef] [PubMed]
- Koralov, S.B.; Muljo, S.A.; Galler, G.R.; Krek, A.; Chakraborty, T.; Kanellopoulou, C.; Jensen, K.; Cobb, B.S.; Merkenschlager, M.; Rajewsky, N.; et al. Dicer ablation affects antibody diversity and cell survival in the B lymphocyte lineage. Cell 2008, 132, 860–874. [Google Scholar] [CrossRef] [PubMed]
- Xiao, C.; Srinivasan, L.; Calado, D.P.; Patterson, H.C.; Zhang, B.; Wang, J.; Henderson, J.M.; Kutok, J.L.; Rajewsky, K. Lymphoproliferative disease and autoimmunity in mice with increased miR-17–92 expression in lymphocytes. Nat. Immunol. 2008, 9, 405–414. [Google Scholar] [CrossRef] [PubMed]
- Lu, T.X.; Munitz, A.; Rothenberg, M.E. MicroRNA-21 is up-regulated in allergic airway inflammation and regulates IL-12p35 expression. J. Immunol. 2009, 182, 4994–5002. [Google Scholar] [CrossRef] [PubMed]
- Shimizu, J.; Takai, K.; Fujiwara, N.; Arimitsu, N.; Ueda, Y.; Wakisaka, S.; Yoshikawa, H.; Kaneko, F.; Suzuki, T.; Suzuki, N. Excessive CD4+ T cells co-expressing interleukin-17 and interferon-γ in patients with Behcet’s disease. Clin. Exp. Immunol. 2012, 168, 68–74. [Google Scholar] [CrossRef] [PubMed]
- Chi, W.; Zhu, X.; Yang, P.; Liu, X.; Lin, X.; Zhou, H.; Huang, X.; Kijlstra, A. Upregulated IL-23 and IL-17 in Behcet patients with active uveitis. Investig. Ophthalmol. Vis. Sci. 2008, 49, 3058–3064. [Google Scholar] [CrossRef]
- Hatley, M.E.; Patrick, D.M.; Garcia, M.R.; Richardson, J.A.; Bassel-Duby, R.; van Rooij, E.; Olson, E.N. Modulation of K-Ras-dependent lung tumorigenesis by microRNA-21. Cancer Cell 2010, 18, 282–293. [Google Scholar] [CrossRef] [PubMed]
- Sabatel, C.; Malvaux, L.; Bovy, N.; Deroanne, C.; Lambert, V.; Gonzalez, M.L.; Colige, A.; Rakic, J.M.; Noel, A.; Martial, J.A.; et al. MicroRNA-21 exhibits antiangiogenic function by targeting Rhob expression in endothelial cells. PLoS ONE 2011, 6, e16979. [Google Scholar] [CrossRef] [PubMed]
- Iliopoulos, D.; Kavousanaki, M.; Ioannou, M.; Boumpas, D.; Verginis, P. The negative costimulatory molecule PD-1 modulates the balance between immunity and tolerance via miR-21. Eur. J. Immunol. 2011, 41, 1754–1763. [Google Scholar] [CrossRef] [PubMed]
- Merline, R.; Moreth, K.; Beckmann, J.; Nastase, M.V.; Zeng-Brouwers, J.; Tralhao, J.G.; Lemarchand, P.; Pfeilschifter, J.; Schaefer, R.M.; Iozzo, R.V.; et al. Signaling by the matrix proteoglycan decorin controls inflammation and cancer through PDCD4 and microRNA-21. Sci. Signal. 2011, 4. [Google Scholar] [CrossRef]
- Liu, C.; Yu, J.; Yu, S.; Lavker, R.M.; Cai, L.; Liu, W.; Yang, K.; He, X.; Chen, S. MicroRNA-21 acts as an oncomir through multiple targets in human hepatocellular carcinoma. J. Hepatol. 2010, 53, 98–107. [Google Scholar] [CrossRef] [PubMed]
- Shim, J.; Lee, E.S.; Park, S.; Bang, D.; Sohn, S. CD4+ CD25+ regulatory T cells ameliorate Behcet’s disease-like symptoms in a mouse model. Cytotherapy 2011, 13, 835–847. [Google Scholar] [CrossRef] [PubMed]
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Choi, B.; Kim, H.-A.; Suh, C.-H.; Byun, H.O.; Jung, J.-Y.; Sohn, S. The Relevance of miRNA-21 in HSV-Induced Inflammation in a Mouse Model. Int. J. Mol. Sci. 2015, 16, 7413-7427. https://doi.org/10.3390/ijms16047413
Choi B, Kim H-A, Suh C-H, Byun HO, Jung J-Y, Sohn S. The Relevance of miRNA-21 in HSV-Induced Inflammation in a Mouse Model. International Journal of Molecular Sciences. 2015; 16(4):7413-7427. https://doi.org/10.3390/ijms16047413
Chicago/Turabian StyleChoi, Bunsoon, Hyoun-Ah Kim, Chang-Hee Suh, Hae Ok Byun, Ju-Yang Jung, and Seonghyang Sohn. 2015. "The Relevance of miRNA-21 in HSV-Induced Inflammation in a Mouse Model" International Journal of Molecular Sciences 16, no. 4: 7413-7427. https://doi.org/10.3390/ijms16047413
APA StyleChoi, B., Kim, H.-A., Suh, C.-H., Byun, H. O., Jung, J.-Y., & Sohn, S. (2015). The Relevance of miRNA-21 in HSV-Induced Inflammation in a Mouse Model. International Journal of Molecular Sciences, 16(4), 7413-7427. https://doi.org/10.3390/ijms16047413