Preservation of Renal Blood Flow by the Antioxidant EUK-134 in LPS-Treated Pigs
Abstract
:1. Introduction
2. Results and Discussion
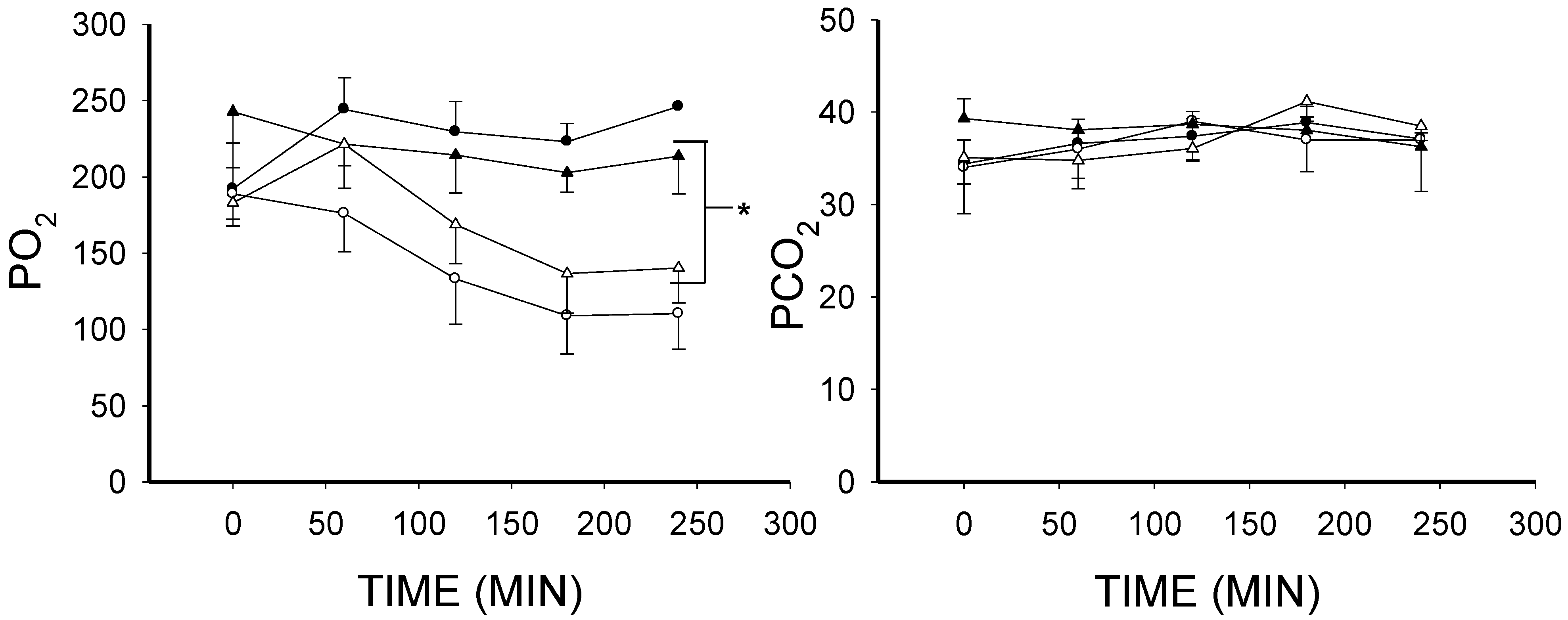
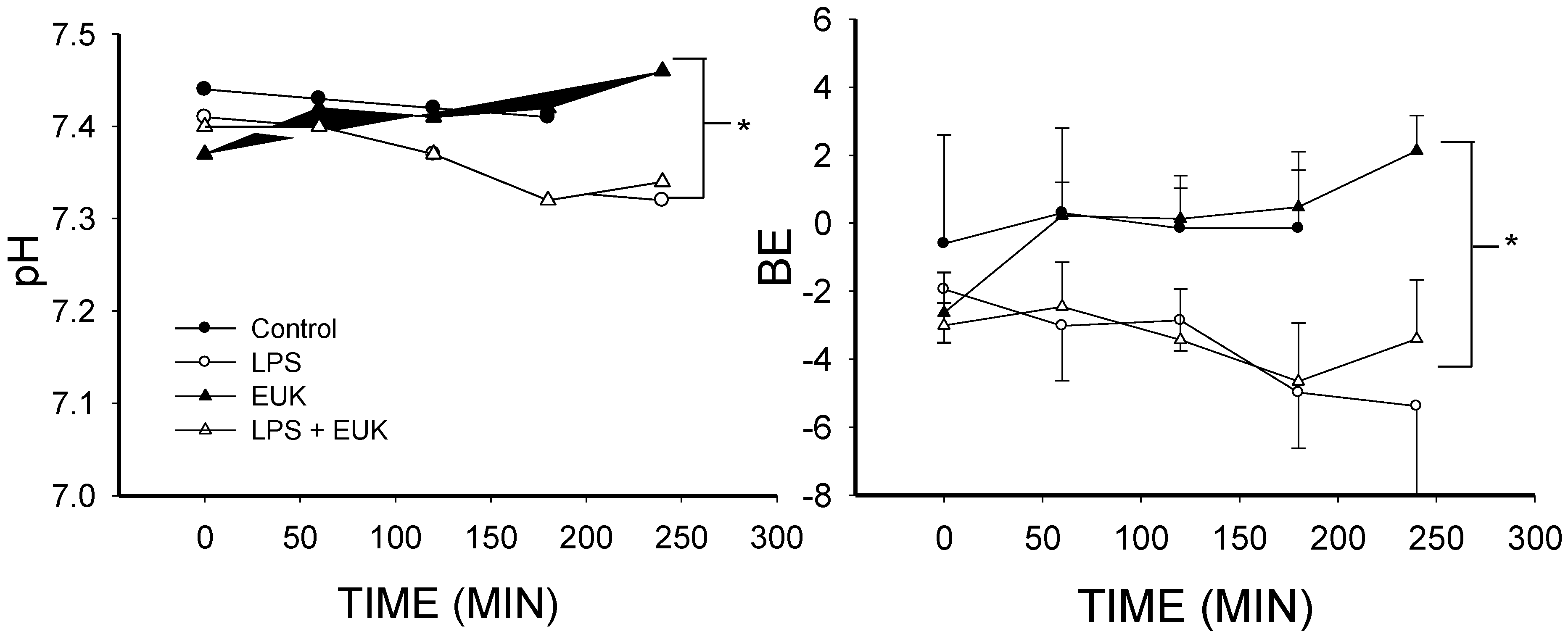
2.1. The Effect of Lipopolysaccharide (LPS) on Blood Gases
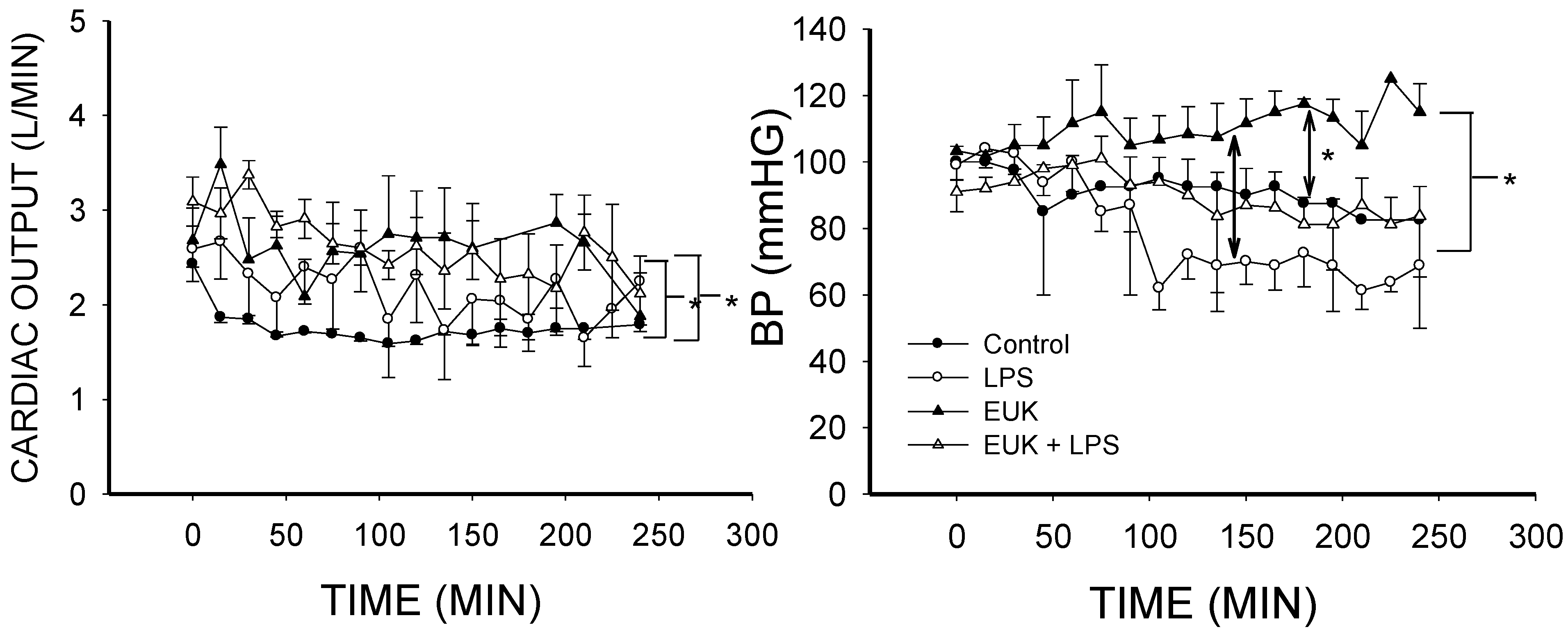
2.2. EUK-134 Effect on LPS-Induced Hemodynamic Changes
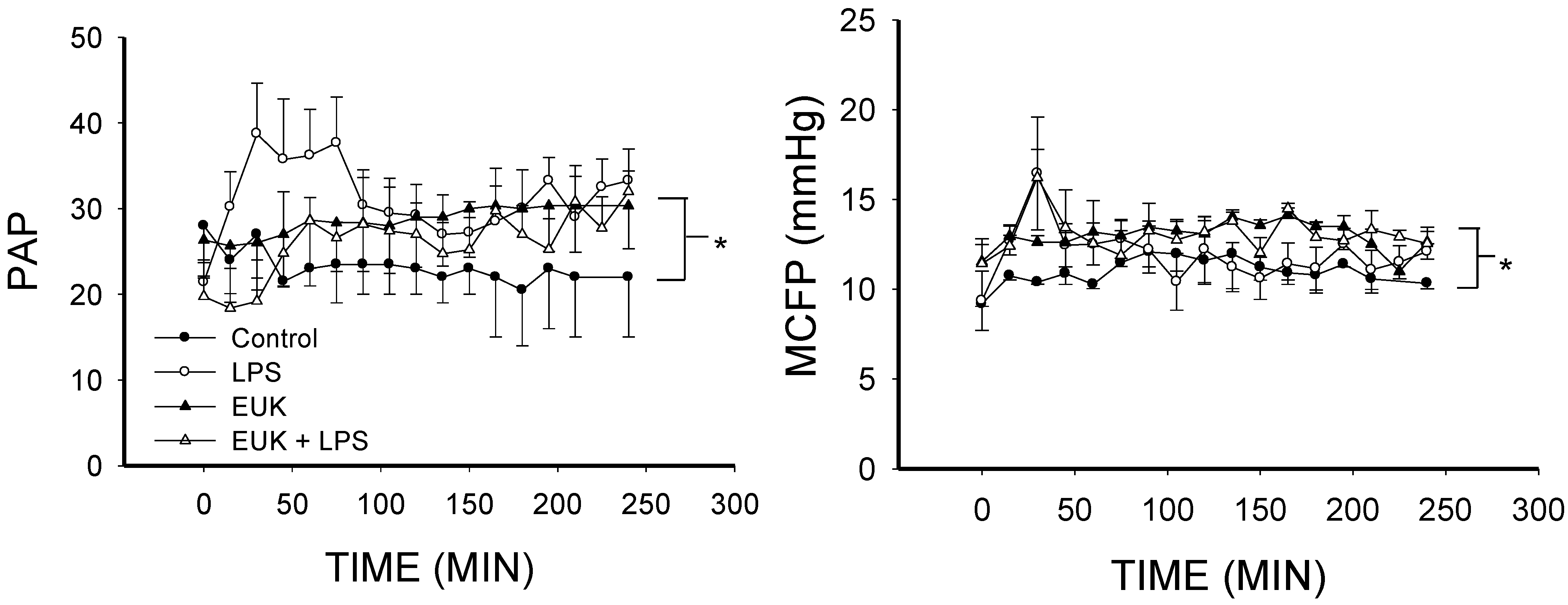

2.3. EUK-134 Attenuation of LPS-Induced Vascular Flow
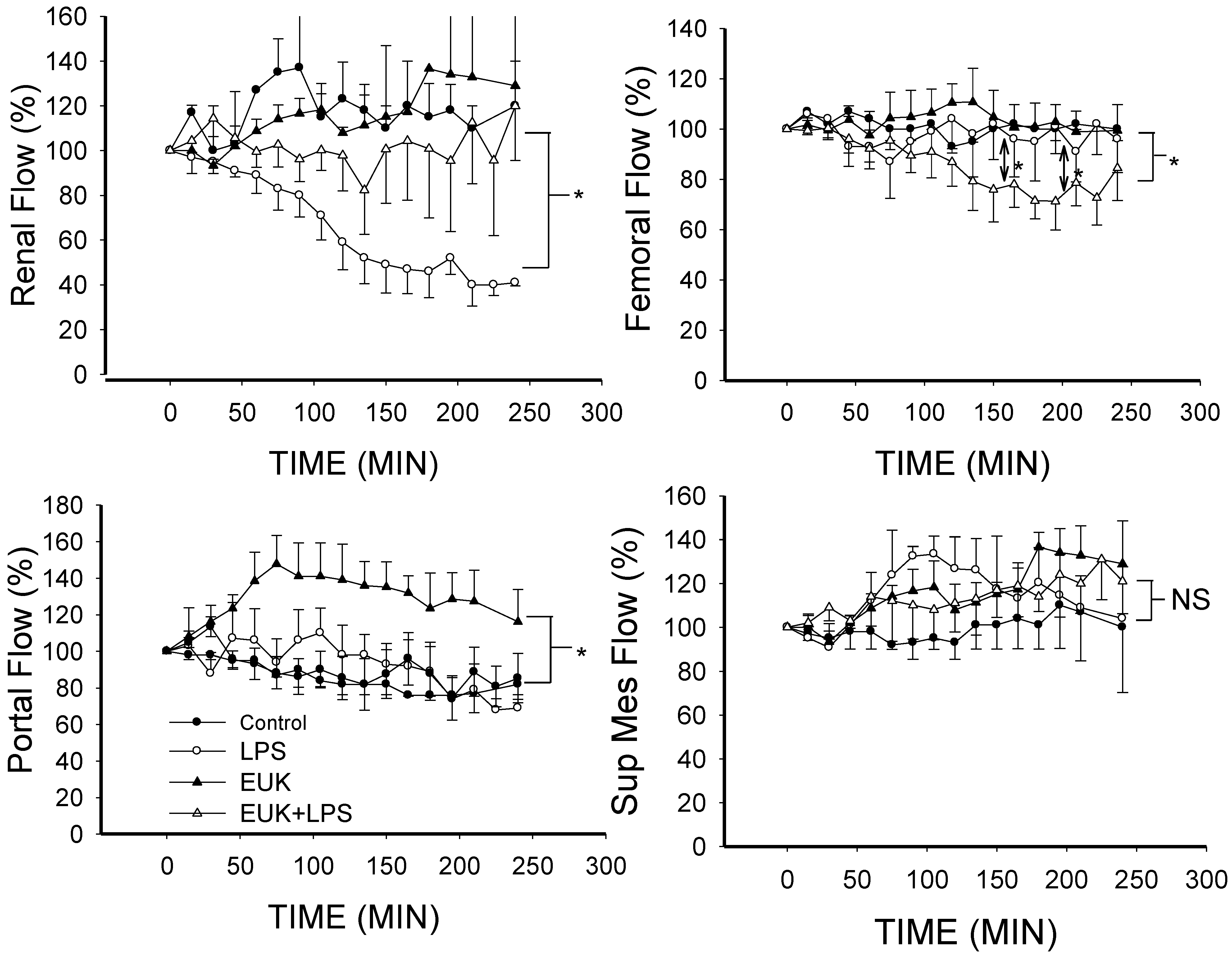
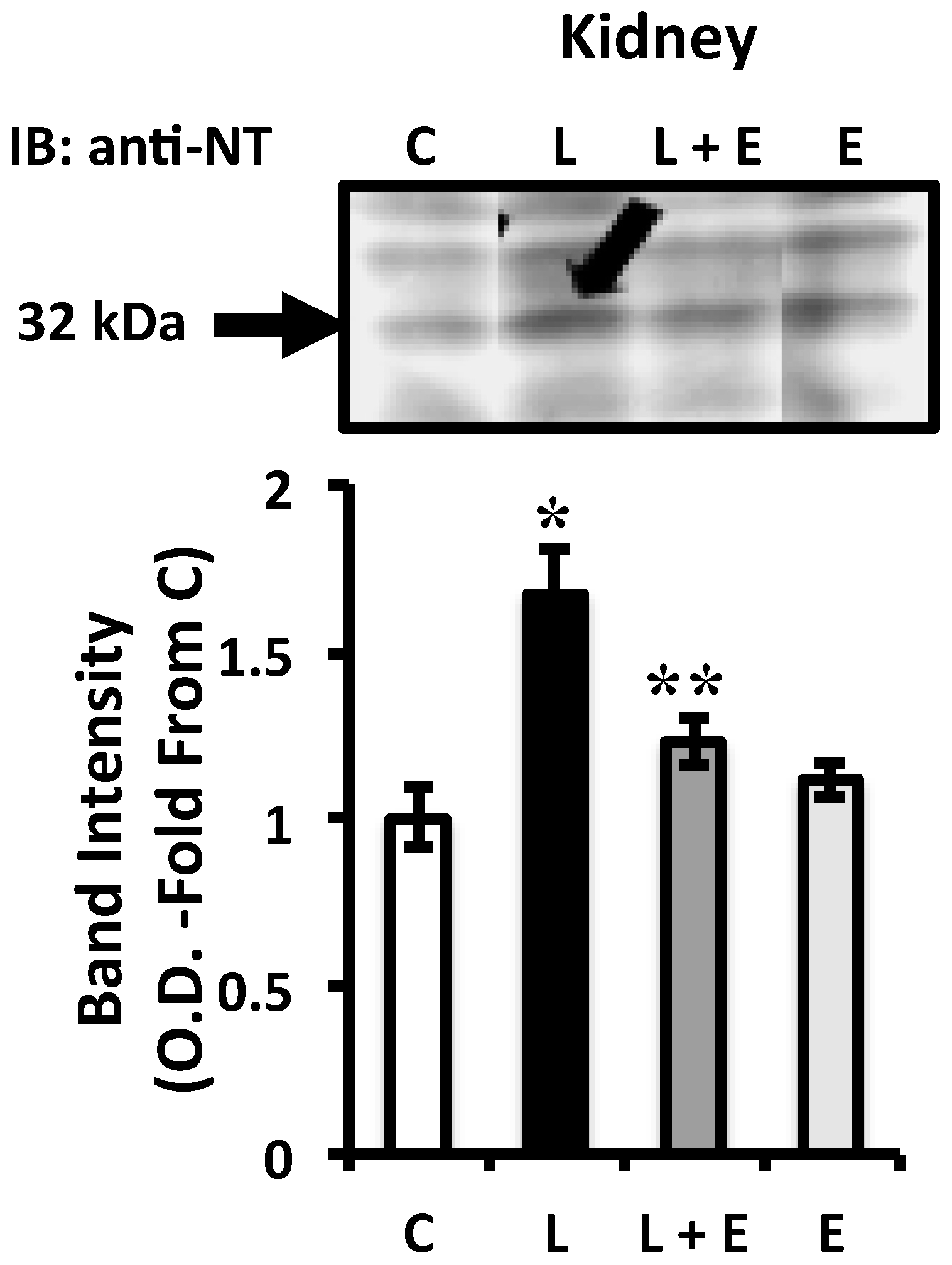
2.4. Attenuation of LPS-Induced Renal Oxidative Stress by EUK-134 Treatment
2.5. General Discussion
3. Experimental Section
3.1. General Procedure
3.2. Measurement of MCFP
3.3. Flow-Measurement
3.4. Protocol
3.5. Hemodynamic Calculations
3.6. Western Blot Analysis for Nitrotyrosine
3.7. Statistics
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Deutschman, C.S.; Tracey, K.J. Sepsis: Current dogma and new perspectives. Immunity 2014, 40, 463–475. [Google Scholar] [CrossRef] [PubMed]
- Russell, J.A. Management of sepsis. N. Engl. J. Med. 2006, 355, 1699–1713. [Google Scholar] [CrossRef] [PubMed]
- Salvemini, D.; Cuzzocrea, S. Oxidative stress in septic shock and disseminated intravascular coagulation. Free Radic. Biol. Med. 2002, 33, 1173–1185. [Google Scholar] [CrossRef] [PubMed]
- Wilson, J.X. Evaluation of vitamin C for adjuvant sepsis therapy. Antioxid. Redox Signal. 2013, 19, 2129–2140. [Google Scholar] [CrossRef] [PubMed]
- De Backer, D.; Scolletta, S. Clinical management of the cardiovascular failure in sepsis. Curr. Vasc. Pharmacol. 2013, 11, 222–242. [Google Scholar] [PubMed]
- Goode, H.F.; Webster, N.R. Free radicals and antioxidants in sepsis. Crit. Care Med. 1993, 21, 1770–1776. [Google Scholar] [CrossRef] [PubMed]
- Warner, B.W.; Hasselgren, P.O.; Fischer, J.E. Effect of allopurinol and superoxide dismutase on survival rate in rats with sepsis. Curr. Surg. 1986, 43, 292–293. [Google Scholar] [PubMed]
- Kunimoto, F.; Morita, T.; Ogawa, R.; Fujita, T. Inhibition of lipid peroxidation improves survival rate of endotoxemic rats. Circ. Shock 1987, 21, 15–22. [Google Scholar] [PubMed]
- Schneider, J.; Friderichs, E.; Giertz, H. Protection by recombinant human superoxide dismutase in lethal rat endotoxemia. Prog. Clin. Biol. Res. 1989, 308, 913–917. [Google Scholar] [PubMed]
- Supinski, G.S.; Callahan, L.A. Polyethylene glycol-superoxide dismutase prevents endotoxin-induced cardiac dysfunction. Am. J. Respir. Crit. Care Med. 2006, 173, 1240–1247. [Google Scholar] [CrossRef] [PubMed]
- Traber, D.L.; Adams, T., Jr.; Sziebert, L.; Stein, M.; Traber, L. Potentiation of lung vascular response to endotoxin by superoxide dismutase. J. Appl. Physiol. 1985, 58, 1005–1009. [Google Scholar] [PubMed]
- Olson, N.C.; Grizzle, M.K.; Anderson, D.L. Effect of polyethylene glycol-superoxide dismutase and catalase on endotoxemia in pigs. J. Appl. Physiol. 1987, 63, 1526–1532. [Google Scholar] [PubMed]
- Novotny, M.J.; Laughlin, M.H.; Adams, H.R. Evidence for lack of importance of oxygen free radicals in Escherichia coli endotoxemia in dogs. Am. J. Physiol. 1988, 254, H954–H962. [Google Scholar] [PubMed]
- Broner, C.W.; Shenep, J.L.; Stidham, G.L.; Stokes, D.C.; Fairclough, D.; Schonbaum, G.R.; Rehg, J.E.; Hildner, W.K. Effect of antioxidants in experimental Escherichia coli septicemia. Circ. Shock 1989, 29, 77–92. [Google Scholar] [PubMed]
- Olson, N.C.; Anderson, D.L.; Grizzle, M.K. Dimethylthiourea attenuates endotoxin-induced acute respiratory failure in pigs. J. Appl. Physiol. 1987, 63, 2426–2432. [Google Scholar] [PubMed]
- Seekamp, A.; Lalonde, C.; Zhu, D.G.; Demling, R. Catalase prevents prostanoid release and lung lipid peroxidation after endotoxemia in sheep. J. Appl. Physiol. 1988, 65, 1210–1216. [Google Scholar] [PubMed]
- Petrone, W.F.; English, D.K.; Wong, K.; McCord, J.M. Free radicals and inflammation: Superoxide-dependent activation of a neutrophil chemotactic factor in plasma. Proc. Natl. Acad. Sci. USA 1980, 77, 1159–1163. [Google Scholar] [CrossRef] [PubMed]
- Bernard, G.R.; Lucht, W.D.; Niedermeyer, M.E.; Snapper, J.R.; Ogletree, M.L.; Brigham, K.L. Effect of N-acetylcysteine on the pulmonary response to endotoxin in the awake sheep and upon in vitro granulocyte function. J. Clin. Investig. 1984, 73, 1772–1784. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez, P.K.; Zhuang, J.; Doctrow, S.R.; Malfroy, B.; Benson, P.F.; Menconi, M.J.; Fink, M.P. EUK-8, a synthetic superoxide dismutase and catalase mimetic, ameliorates acute lung injury in endotoxemic swine. J. Pharmacol. Exp. Ther. 1995, 275, 798–806. [Google Scholar] [PubMed]
- Galvao, A.M.; Wanderley, M.S.; Silva, R.A.; Filho, C.A.; Melo-Junior, M.R.; Silva, L.A.; Streck, E.L.; Dornelas de Andrade, A.F.; Souza Maia, M.B.; Barbosa de Castro, C.M. Intratracheal co-administration of antioxidants and ceftriaxone reduces pulmonary injury and mortality rate in an experimental model of sepsis. Respirology 2014, 19, 1080–1087. [Google Scholar] [CrossRef] [PubMed]
- Campos, R.; Shimizu, M.H.; Volpini, R.A.; de Braganca, A.C.; Andrade, L.; Lopes, F.D.; Olivo, C.; Canale, D.; Seguro, A.C. N-acetylcysteine prevents pulmonary edema and acute kidney injury in rats with sepsis submitted to mechanical ventilation. Am. J. Physiol. Lung Cell. Mol. Physiol. 2012, 302, L640–L650. [Google Scholar] [CrossRef]
- Saetre, T.; Hoiby, E.A.; Aspelin, T.; Lermark, G.; Egeland, T.; Lyberg, T. Aminoethyl-isothiourea, a nitric oxide synthase inhibitor and oxygen radical scavenger, improves survival and counteracts hemodynamic deterioration in a porcine model of streptococcal shock. Crit. Care Med. 2000, 28, 2697–2706. [Google Scholar] [CrossRef] [PubMed]
- Brandes, R.P.; Koddenberg, G.; Gwinner, W.; Kim, D.; Kruse, H.J.; Busse, R.; Mugge, A. Role of increased production of superoxide anions by NAD(P)H oxidase and xanthine oxidase in prolonged endotoxemia. Hypertension 1999, 33, 1243–1249. [Google Scholar] [CrossRef] [PubMed]
- Tsao, C.M.; Jhang, J.G.; Chen, S.J.; Ka, S.M.; Wu, T.C.; Liaw, W.J.; Huang, H.C.; Wu, C.C. Adjuvant potential of selegiline in attenuating organ dysfunction in septic rats with peritonitis. PLoS ONE 2014, 9, e108455. [Google Scholar] [CrossRef] [PubMed]
- Maurya, H.; Mangal, V.; Gandhi, S.; Prabhu, K.; Ponnudurai, K. Prophylactic antioxidant potential of gallic acid in murine model of sepsis. Int. J. Inflamm. 2014, 2014, 580320. [Google Scholar] [CrossRef]
- Cowley, H.C.; Bacon, P.J.; Goode, H.F.; Webster, N.R.; Jones, J.G.; Menon, D.K. Plasma antioxidant potential in severe sepsis: A comparison of survivors and nonsurvivors. Crit. Care Med. 1996, 24, 1179–1183. [Google Scholar] [CrossRef] [PubMed]
- Heller, A.R.; Groth, G.; Heller, S.C.; Breitkreutz, R.; Nebe, T.; Quintel, M.; Koch, T. N-acetylcysteine reduces respiratory burst but augments neutrophil phagocytosis in intensive care unit patients. Crit. Care Med. 2001, 29, 272–276. [Google Scholar] [CrossRef]
- Ortolani, O.; Conti, A.; de Gaudio, A.R.; Moraldi, E.; Cantini, Q.; Novelli, G. The effect of glutathione and N-acetylcysteine on lipoperoxidative damage in patients with early septic shock. Am. J. Respir. Crit. Care Med. 2000, 161, 1907–1911. [Google Scholar] [CrossRef]
- Suter, P.M.; Domenighetti, G.; Schaller, M.D.; Laverriere, M.C.; Ritz, R.; Perret, C. N-acetylcysteine enhances recovery from acute lung injury in man. A randomized, double-blind, placebo-controlled clinical study. Chest 1994, 105, 190–194. [Google Scholar] [CrossRef] [PubMed]
- Rank, N.; Michel, C.; Haertel, C.; Lenhart, A.; Welte, M.; Meier-Hellmann, A.; Spies, C. N-acetylcysteine increases liver blood flow and improves liver function in septic shock patients: Results of a prospective, randomized, double-blind study. Crit. Care Med. 2000, 28, 3799–3807. [Google Scholar] [CrossRef] [PubMed]
- Bernard, G.R.; Wheeler, A.P.; Arons, M.M.; Morris, P.E.; Paz, H.L.; Russell, J.A.; Wright, P.E. A trial of antioxidants N-acetylcysteine and procysteine in ARDS. The Antioxidant in ARDS Study Group. Chest 1997, 112, 164–172. [Google Scholar] [CrossRef] [PubMed]
- Jepsen, S.; Herlevsen, P.; Knudsen, P.; Bud, M.I.; Klausen, N.O. Antioxidant treatment with N-acetylcysteine during adult respiratory distress syndrome: A prospective, randomized, placebo-controlled study. Crit. Care Med. 1992, 20, 918–923. [Google Scholar] [CrossRef] [PubMed]
- Crimi, E.; Liguori, A.; Condorelli, M.; Cioffi, M.; Astuto, M.; Bontempo, P.; Pignalosa, O.; Vietri, M.T.; Molinari, A.M.; Sica, V.; et al. The beneficial effects of antioxidant supplementation in enteral feeding in critically ill patients: A prospective, randomized, double-blind, placebo-controlled trial. Anesth. Analg. 2004, 99, 857–863. [Google Scholar] [CrossRef] [PubMed]
- Torraco, A.; Carrozzo, R.; Piemonte, F.; Pastore, A.; Tozzi, G.; Verrigni, D.; Assenza, M.; Orecchioni, A.; D’Egidio, A.; Marraffa, E.; et al. Effects of levosimendan on mitochondrial function in patients with septic shock: A randomized trial. Biochimie 2014, 102, 166–173. [Google Scholar] [CrossRef] [PubMed]
- Galley, H.F. Bench-to-bedside review: Targeting antioxidants to mitochondria in sepsis. Crit. Care 2010, 14, 230. [Google Scholar] [PubMed]
- McDonald, M.C.; di Villa Bianca, R.E.; Wayman, N.S.; Pinto, A.; Sharpe, M.A.; Cuzzocrea, S.; Chatterjee, P.K.; Thiemermann, C. A superoxide dismutase mimetic with catalase activity (EUK-8) reduces the organ injury in endotoxic shock. Eur. J. Pharmacol. 2003, 466, 181–189. [Google Scholar] [CrossRef] [PubMed]
- Bianca, R.; Wayman, N.S.; McDonald, M.C.; Pinto, A.; Shape, M.A.; Chatterjee, P.K.; Thiemermann, C. Superoxide dismutase mimetic with catalase activity, EUK-134, attenuates the multiple organ injury and dysfunction caused by endotoxin in the rat. Med. Sci. Monit. 2002, 8, BR1–BR7. [Google Scholar] [PubMed]
- Rosenthal, R.A.; Fish, B.; Hill, R.P.; Huffman, K.D.; Lazarova, Z.; Mahmood, J.; Medhora, M.; Molthen, R.; Moulder, J.E.; Sonis, S.T.; et al. Salen Mn complexes mitigate radiation injury in normal tissues. Anticancer Agents Med. Chem. 2011, 11, 359–372. [Google Scholar] [CrossRef] [PubMed]
- Chatterjee, P.K.; Patel, N.S.; Kvale, E.O.; Brown, P.A.; Stewart, K.N.; Mota-Filipe, H.; Sharpe, M.A.; di Paola, R.; Cuzzocrea, S.; Thiemermann, C. EUK-134 reduces renal dysfunction and injury caused by oxidative and nitrosative stress of the kidney. Am. J. Nephrol. 2004, 24, 165–177. [Google Scholar] [CrossRef] [PubMed]
- Gianello, P.; Saliez, A.; Bufkens, X.; Pettinger, R.; Misseleyn, D.; Hori, S.; Malfroy, B. EUK-134, a synthetic superoxide dismutase and catalase mimetic, protects rat kidneys from ischemia-reperfusion-induced damage. Transplantation 1996, 62, 1664–1666. [Google Scholar] [CrossRef] [PubMed]
- Rong, Y.; Doctrow, S.R.; Tocco, G.; Baudry, M. EUK-134, a synthetic superoxide dismutase and catalase mimetic, prevents oxidative stress and attenuates kainate-induced neuropathology. Proc. Natl. Acad. Sci. USA 1999, 96, 9897–9902. [Google Scholar] [CrossRef] [PubMed]
- Lawler, J.M.; Kunst, M.; Hord, J.M.; Lee, Y.; Joshi, K.; Botchlett, R.E.; Ramirez, A.; Martinez, D.A. EUK-134 ameliorates nNOSμ translocation and skeletal muscle fiber atrophy during short-term mechanical unloading. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2014, 306, R470–R482. [Google Scholar] [CrossRef] [PubMed]
- Ni, X.; Yang, Z.J.; Carter, E.L.; Martin, L.J.; Koehler, R.C. Striatal neuroprotection from neonatal hypoxia-ischemia in piglets by antioxidant treatment with EUK-134 or edaravone. Dev. Neurosci. 2011, 33, 299–311. [Google Scholar] [CrossRef] [PubMed]
- Javeshghani, D.; Hussain, S.N.A.; Scheidel, J.; Quinn, M.T.; Magder, S.A. Superoxide production in the vasculature of lipopolysaccharide treated rats and pigs. Shock 2003, 19, 486–493. [Google Scholar] [CrossRef] [PubMed]
- Al Ghouleh, I.; Magder, S. Nicotinamide adenine dinucleotide phosphate (reduced form) oxidase is important for LPS-induced endothelial cell activation. Shock 2008, 29, 553–559. [Google Scholar]
- Al Ghouleh, I.; Magder, S. NADPH oxidase-derived superoxide destabilizes lipopolysaccharide-induced interleukin 8 mRNA via p38, extracellular signal-regulated kinase mitogen-activated protein kinase, and the destabilizing factor tristetraprolin. Shock 2012, 37, 433–440. [Google Scholar] [CrossRef] [PubMed]
- Gattinoni, L.; Carlesso, E. Supporting hemodynamics: What should we target? What treatments should we use? Crit. Care 2013, 17 (Suppl. 1), S4. [Google Scholar] [CrossRef] [PubMed]
- Garcia, X.; Pinsky, M.R. Clinical applicability of functional hemodynamic monitoring. Ann. Intensive Care 2011, 1, 35. [Google Scholar] [CrossRef] [PubMed]
- Kellum, J.A. Disorders of acid-base balance. Crit. Care Med. 2007, 35, 2630–2636. [Google Scholar] [CrossRef] [PubMed]
- Magder, S. Bench-to-bedside review: Ventilatory abnormalities in sepsis. Crit. Care 2009, 13, 202. [Google Scholar] [CrossRef] [PubMed]
- Javeshghani, D.; Magder, S. Presence of nitrotyrosine with minimal iNOS induction in LPS treated pigs. Shock 2001, 16, 304–311. [Google Scholar] [CrossRef] [PubMed]
- Magder, S.; Vanelli, G. Circuit factors in the high cardiac output of sepsis. J. Crit. Care 1996, 11, 155–166. [Google Scholar] [CrossRef] [PubMed]
- Izumi, M.; McDonald, M.C.; Sharpe, M.A.; Chatterjee, P.K.; Thiemermann, C. Superoxide dismutase mimetics with catalase activity reduce the organ injury in hemorrhagic shock. Shock 2002, 18, 230–235. [Google Scholar] [CrossRef] [PubMed]
- Hatib, F.; Jansen, J.R.; Pinsky, M.R. Peripheral vascular decoupling in porcine endotoxic shock. J. Appl. Physiol. 2011, 111, 853–860. [Google Scholar] [CrossRef] [PubMed]
- Szabo, C.; Saunders, C.; O’Connor, M.; Salzman, A.L. Peroxynitrite causes energy depletion and increases permeability via activation of poly (ADP-ribose) synthetase in pulmonary epithelial cells. Am. J. Respir. Cell Mol. Biol. 1997, 16, 105–109. [Google Scholar] [CrossRef] [PubMed]
- Mehta, S.; Javeshghani, D.; Datta, P.; Levy, R.D.; Magder, S. Porcine endotoxaemic shock is associated with increased expired nitric oxide. Crit. Care Med. 1999, 27, 385–393. [Google Scholar] [CrossRef] [PubMed]
- Salvemini, D.; Riley, D.P.; Lennon, P.J.; Wang, Z.Q.; Currie, M.G.; Macarthur, H.; Misko, T.P. Protective effects of a superoxide dismutase mimetic and peroxynitrite decomposition catalysts in endotoxin-induced intestinal damage. Br. J. Pharmacol. 1999, 127, 685–692. [Google Scholar] [CrossRef] [PubMed]
- Javesghani, D.; Magder, S.A.; Barreiro, E.; Quinn, M.T.; Hussain, S.N. Molecular characterization of a superoxide-generating NAD(P)H oxidase in the ventilatory muscles. Am. J. Respir. Crit. Care Med. 2002, 165, 412–418. [Google Scholar] [CrossRef] [PubMed]
- Javeshghani, D.; Magder, S. Regional changes in constitutive nitric oxide synthase and the hemodynamic consequences of its inhibition in lipopolysaccharide-treated pigs. Shock 2001, 16, 232–238. [Google Scholar] [CrossRef] [PubMed]
- Doctrow, S.R.; Huffman, K.; Marcus, C.B.; Tocco, G.; Malfroy, E.; Adinolfi, C.A.; Kruk, H.; Baker, K.; Lazarowych, N.; Mascarenhas, J.; et al. Salen-manganese complexes as catalytic scavengers of hydrogen peroxide and cytoprotective agents: Structure-activity relationship studies. J. Med. Chem. 2002, 45, 4549–4558. [Google Scholar] [CrossRef] [PubMed]
- Magder, S. Central venous pressure: A useful but not so simple measurement. Crit. Care Med. 2006, 34, 2224–2227. [Google Scholar] [CrossRef] [PubMed]
- Marik, P.E.; Baram, M.; Vahid, B. Does central venous pressure predict fluid responsiveness? A systematic review of the literature and the tale of seven mares. Chest 2008, 134, 172–178. [Google Scholar] [CrossRef] [PubMed]
- Al Ghouleh, I.; Khoo, N.K.; Knaus, U.G.; Griendling, K.K.; Touyz, R.M.; Thannickal, V.J.; Barchowsky, A.; Nauseef, W.M.; Kelley, E.E.; Bauer, P.M.; et al. Oxidases and peroxidases in cardiovascular and lung disease: New concepts in reactive oxygen species signaling. Free Radic. Biol. Med. 2011, 51, 1271–1288. [Google Scholar]
- Bedard, K.; Krause, K.H. The NOX family of ROS-generating NADPH oxidases: Physiology and pathophysiology. Physiol. Rev. 2007, 87, 245–313. [Google Scholar] [CrossRef] [PubMed]
- Bressack, M.A.; Morton, N.S.; Hortop, J. Group B streptococcal sepsis in the piglet: Effects of fluid therapy on venous return, organ edema, and organ blood flow. Circ. Res. 1987, 61, 659–669. [Google Scholar] [CrossRef] [PubMed]
- Hussain, S.N.; Roussos, C. Distribution of respiratory muscle and organ blood flow during endotoxic shock in dogs. J. Appl. Physiol. 1985, 59, 1802–1808. [Google Scholar] [PubMed]
- Van Lambalgen, A.A.; Bronsveld, W.; van den Bos, G.C.; Thijs, L.G. Distribution of cardiac output, oxygen consumption and lactate production in canine endotoxin shock. Cardiovasc Res. 1984, 18, 195–205. [Google Scholar]
- Lang, C.H.; Bagby, G.J.; Ferguson, J.L.; Spitzer, J.J. Cardiac output and redistribution of organ blood flow in hypermetabolic sepsis. Am. J. Physiol. 1984, 246, R331–R337. [Google Scholar] [PubMed]
- Breslow, M.J.; Miller, C.F.; Parker, S.D.; Walman, A.T.; Traystman, R.J. Effect of vasopressors on organ blood flow during endotoxin shock in pigs. Am. J. Physiol. 1987, 252, H291–H300. [Google Scholar] [PubMed]
- Wyler, F.; Forsyth, R.P.; Nies, A.S.; Neutze, J.M.; Melmon, K.L. Endotoxin-induced regional circulatory changes in the unanesthetized monkey. Circ. Res. 1969, 24, 777–786. [Google Scholar] [CrossRef] [PubMed]
- Ruokonen, E.; Takala, J.; Kari, A.; Saxen, H.; Mertsola, J.; Hansen, E.J. Regional blood flow and oxygen transport in septic shock. Crit. Care Med. 1993, 21, 1296–1303. [Google Scholar] [CrossRef] [PubMed]
- Hussain, S.N.; Chatillon, A.; Comtois, A.; Roussos, C.; Magder, S. Chemical activation of thin-fiber phrenic afferents. 2. Cardiovascular responses. J. Appl. Physiol. 1991, 70, 77–86. [Google Scholar] [PubMed]
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Magder, S.; Parthenis, D.G.; Ghouleh, I.A. Preservation of Renal Blood Flow by the Antioxidant EUK-134 in LPS-Treated Pigs. Int. J. Mol. Sci. 2015, 16, 6801-6817. https://doi.org/10.3390/ijms16046801
Magder S, Parthenis DG, Ghouleh IA. Preservation of Renal Blood Flow by the Antioxidant EUK-134 in LPS-Treated Pigs. International Journal of Molecular Sciences. 2015; 16(4):6801-6817. https://doi.org/10.3390/ijms16046801
Chicago/Turabian StyleMagder, Sheldon, Dimitrios G. Parthenis, and Imad Al Ghouleh. 2015. "Preservation of Renal Blood Flow by the Antioxidant EUK-134 in LPS-Treated Pigs" International Journal of Molecular Sciences 16, no. 4: 6801-6817. https://doi.org/10.3390/ijms16046801
APA StyleMagder, S., Parthenis, D. G., & Ghouleh, I. A. (2015). Preservation of Renal Blood Flow by the Antioxidant EUK-134 in LPS-Treated Pigs. International Journal of Molecular Sciences, 16(4), 6801-6817. https://doi.org/10.3390/ijms16046801




