ATR-FTIR Spectroscopy for the Assessment of Biochemical Changes in Skin Due to Cutaneous Squamous Cell Carcinoma
Abstract
:1. Introduction
2. Results
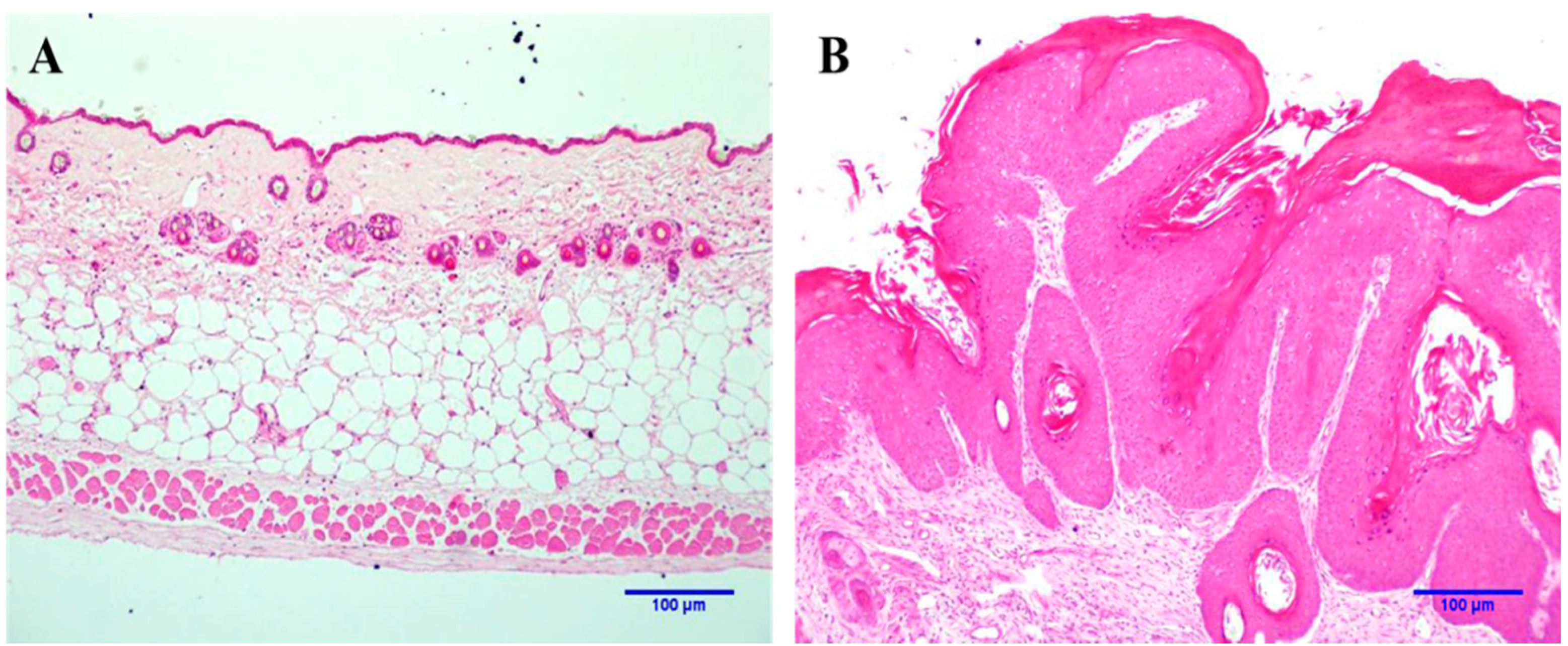
2.1. Histopathological Analysis
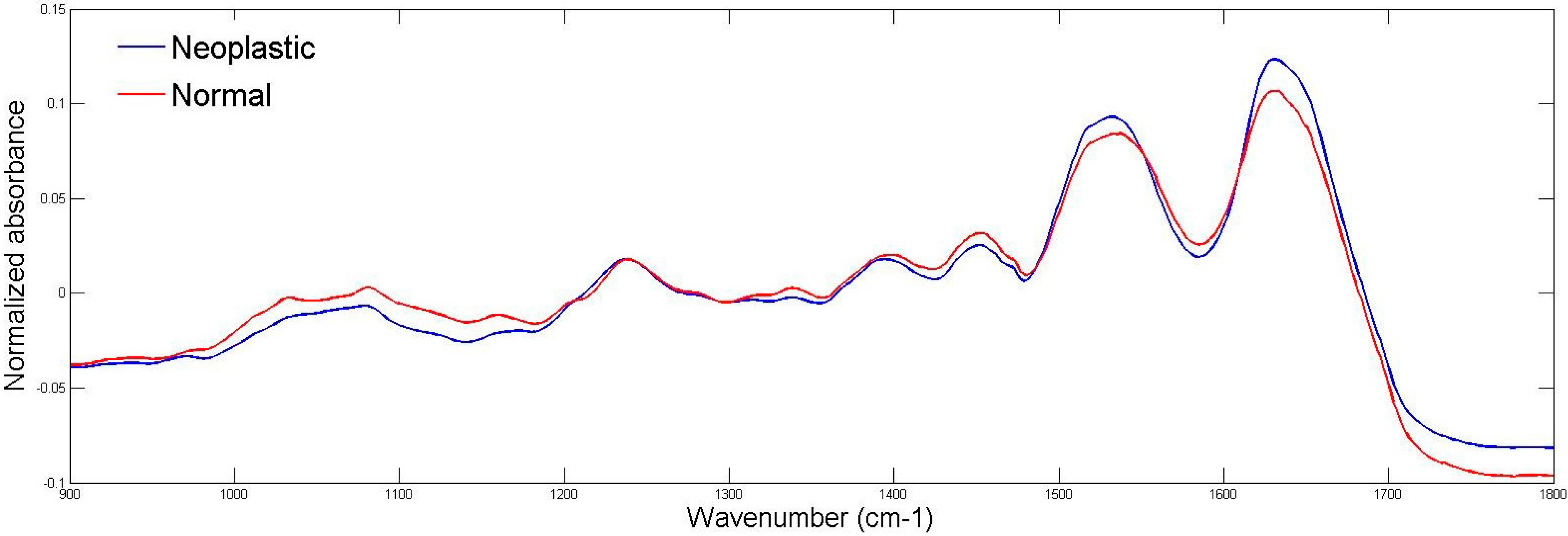
2.2. ATR-FTIR Spectroscopy
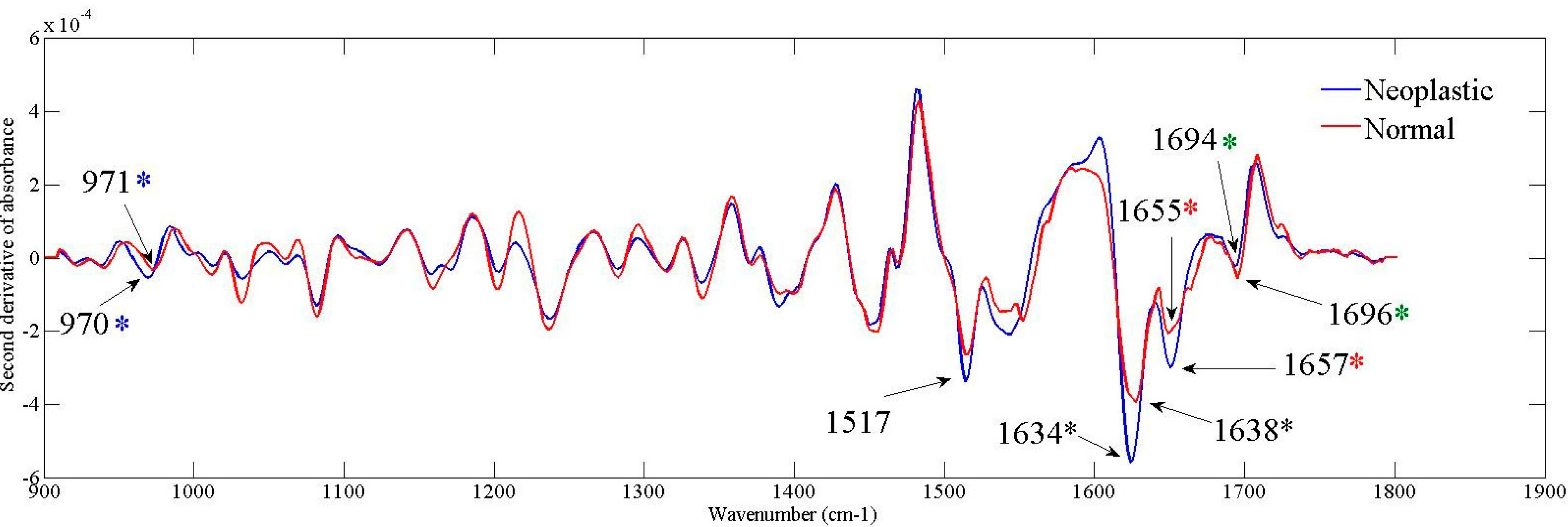
| ATR-FTIR Band Positions (cm−1) | Band Intensity | p Value | ||
|---|---|---|---|---|
| Normal | Neoplastic | Normal | Neoplastic | |
| 971 | 970 | −3.919 × 10−5 ± 3.853 × 10−6 | −5.8 × 10−5 ± 3.536 × 10−6 | 0.0007 |
| 1517 | 1517 | −2.68 × 10−4 ± 5.0 × 10−6 | −3.49 × 10−4 ± 8.726 × 10−6 | 0.0001 |
| 1638 | 1634 | −4.1 × 10−4 ± 9.918 × 10−6 | −5.64 × 10−4 ± 1.393 × 10−5 | 0.0001 |
| 1655 | 1657 | −2.14 × 10−4 ± 5.333 × 10−6 | −3.07 × 10−4 ± 1.430 × 10−5 | 0.0001 |
| 1694 | 1696 | −5.641 × 10−5 ± 2.538 × 10−6 | −2.407 × 10−5 ± 2.270 × 10−6 | 0.0001 |
2.3. Cluster Analysis
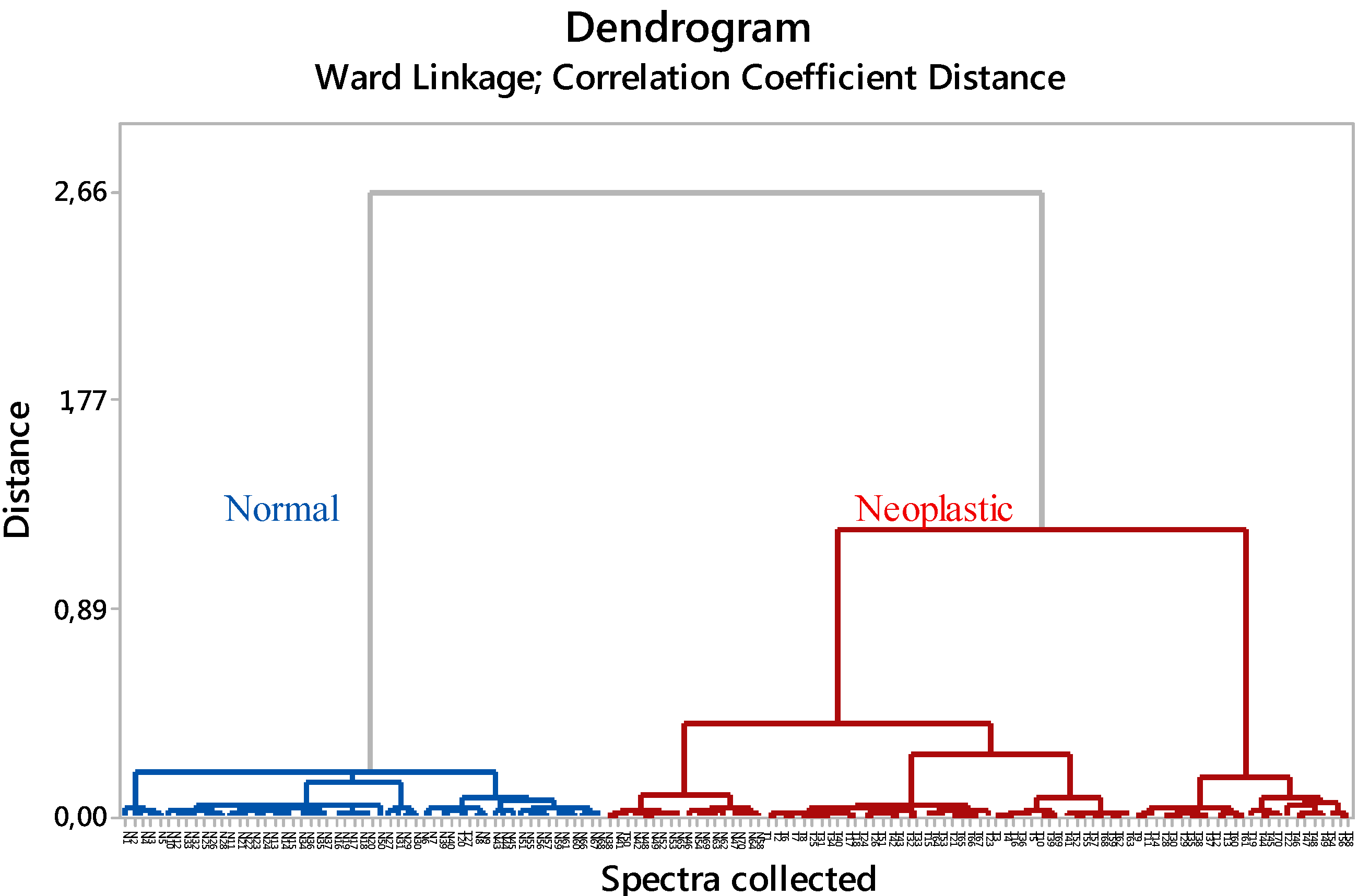
| True Positive (TP) | True Negative (TN) | False Positive (FP) | False Negative (FN) | Accuracy (%) | Sensitivity (%) | Specificity (%) | |
|---|---|---|---|---|---|---|---|
| Spectral data | 68 | 53 | 17 | 2 | 86.4 | 97.1 | 75.7 |
3. Discussion
4. Experimental Section
4.1. Chemical Carcinogenesis
| Group | Treatment | Description |
|---|---|---|
| Group 1 (n = 13) | Normal | Normal skin |
| Group 2 (n = 13) | Neoplasia | Neoplastic skin |
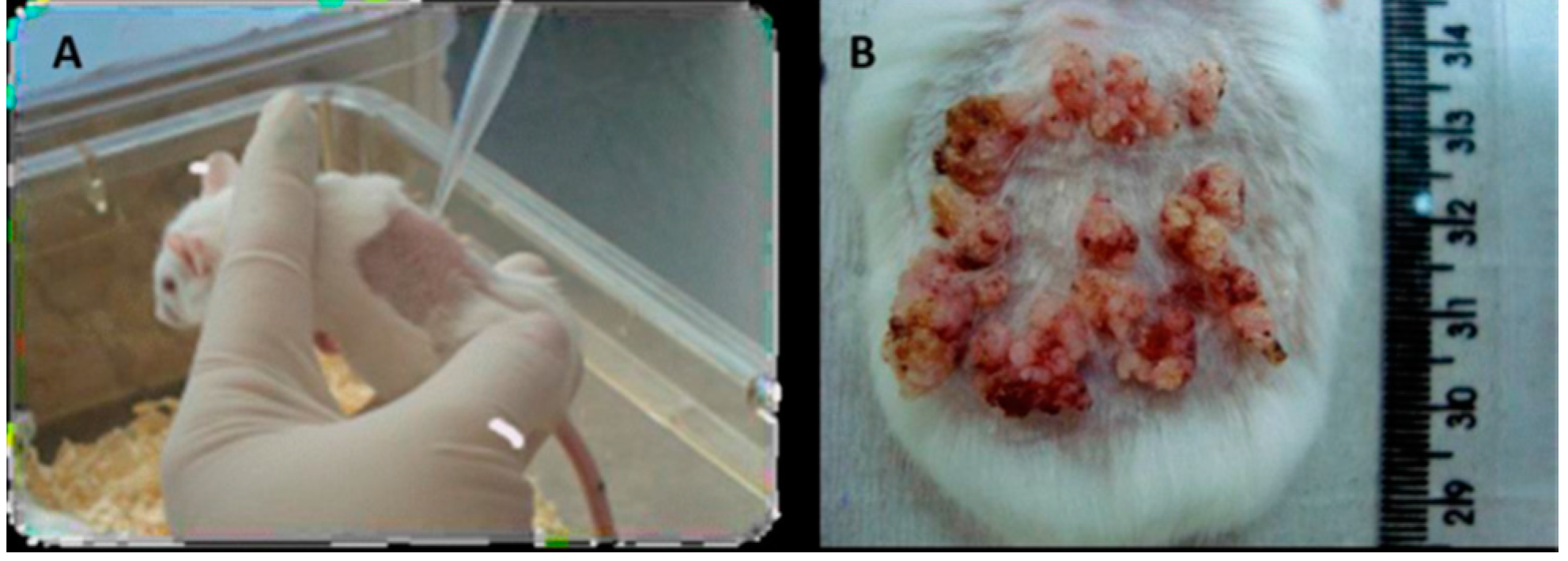
4.2. ATR-FTIR Spectroscopy
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of interest
References
- World Health Organization, WHO. Available online: http://www.iarc.fr/en/publications/pdfs-online/wcr/2008/wcr_2008.pdf (accessed on 12 December 2014).
- Dubas, L.; Ingraffea, A. Nonmelanoma skin cancer. Facial Plast. Surg. Clin. N. Am. 2013, 21, 43–53. [Google Scholar] [CrossRef]
- Instituto Nacional de câncer, INCA. Available online: http://www.inca.gov.br/estimativa/2014/ (accessed on 12 December 2014).
- Lee, D.; Miller, S. Nonmelanoma skin cancer. Facial Plast. Surg. Clin. N. Am. 2009, 17, 309–324. [Google Scholar] [CrossRef]
- Kim, R.H.; Armstrong, A.W. Nonmelanoma skin cancer. Dermatol. Clin. 2012, 30, 125–139. [Google Scholar] [CrossRef] [PubMed]
- Diem, M.; Milijkovic, M; Bird, B.; Chernenko, T.; Schubert, J.; Marcsisin, E.; Mazur, A.; Kingston, E.; Zuser, E.; Papamarkakis, K.; et al. Applications of Infrared and Raman microspectroscopy of cells and tissue in medical diagnosis :Present status and future promises. Spectroscopy 2012, 27, 463–496. [Google Scholar]
- Baker, M.J.; Trevisan, J.; Bassan, P.; Bhargava, R.; Butler, H.J.; Dorling, K.M.; Fielden, P.R.; Fogarty, S.W.; Fullwood, N.J.; Heys, K.A. Using Fourier transform IR spectroscopy to analyze biological materials. Nat. Protoc. 2014, 9, 1771–1791.
- Petrich, W. MID-Infrared and Raman spectroscopy for medical diagnostics. Appl. Spectrosc. Rev. 2011, 36, 181–237. [Google Scholar] [CrossRef]
- Dorling, M.K.; Baker, M.J. Highlighting attenuated total reflection Fourier transform infrared spectroscopy for rapid serum analysis. Trends Biotechnol. 2013, 31, 327–328. [Google Scholar] [CrossRef] [PubMed]
- Kazarian, S.; Chan, K.L. ATR-FTIR spectroscopic imaging: Recent advances and applications to biological systems. Analyst 2013, 138, 1940–1951. [Google Scholar] [CrossRef] [PubMed]
- Greenler, R. Infrared study of adsorbed molecules on metal surfaces by reflection techniques. J. Chem. Phys. 1966, 44, 310–315. [Google Scholar] [CrossRef]
- Filik, J.; Frogley, M.D.; Pijanka, J.K.; Wehbe, K.; Cinque, G. Electric field standing wave artefacts in FTIR micro-spectroscopy of biological materials. Analyst 2012, 137, 853–861. [Google Scholar]
- Bassan, P.; Sachdeva, A.; Lee, J.; Gardner, P. Substrate contributions in micro ATR of thin samples: Implications for analysis of cells, tissue and biological fluids. Analyst 2013, 38, 4139–4146. [Google Scholar]
- Bassan, P.; Kohler, A.; Martens, H.; Lee, J.; Byrne, H.J.; Dumas, P.; Gazi, E.; Brown, M.; Clarke, N.; Gardner, P. Resonant Mie scattering (RMieS) correction of infrared spectra from highly scattering biological samples. Analyst 2010, 135, 268–277. [Google Scholar]
- Chan, K.L.A.; Kazarian, S.G. Correcting the effect of refraction and dispersion of light in FT-IR spectroscopic imaging in transmission through thick infrared windows. Anal. Chem. 2013, 85, 1029–1036. [Google Scholar]
- King, S.W.; Milosevic, M. A method to extract absorption coefficient of thin films from transmission spectra of the films on thick substrates. J. Appl. Phys. 2012, 111, 073109. [Google Scholar] [CrossRef]
- Sukuta, S.; Bruch, R. Factor analysis of cancer Fourier transform infrared evanescent wave fiberoptical (FTIR-FEW) spectra. Lasers Surg. Med. 1999, 24, 382–388. [Google Scholar] [CrossRef] [PubMed]
- Afanasyeva, N.I.; Kolyakov, S.F.; Letokhov, V.S.; Sokolov, V.V.; Frank, G.A. Diagnostics of cancer by fiber optic evanescent wave FTIR (FEW-FTIR) spectroscopy. Proc. SPIE 1996, 2928, 154. [Google Scholar]
- Spielvogel, J.; Lobik, L.; Nissencorn, I.; Hibst, R.; Gotshal, Y.; Katzir, A. Cancer diagnostics using Fourier transform fiber optic infrared evanescent wave spectroscopy (FTIR-FEWS). Proc. SPIE 1998, 3262, 185. [Google Scholar]
- Movasaghi, Z.; Rehman, S.; ur Rehman, D.I. Fourier transform infrared (FTIR) spectroscopy of biological tissues. Appl. Spectrosc. Rev. 2008, 43, 134–179. [Google Scholar] [CrossRef]
- Wood, B.R.; Quinn, M.A.; Burden, F.R.; McNaughton, D. An investigation into FT-IR spectroscopy as a bio-diagnostic tool for cervical cancer. Biospectroscopy 1996, 2, 143–153. [Google Scholar] [CrossRef]
- Argov, S.; Sahu, R.K.; Bernshtain, E.; Salam, A.; Shohat, G.; Zelig, U.; Mordechai, S. Inflammatory bowel diseases as an intermediate stage between normal and cancer: A FTIR-microspectroscopy approach. Biopolymers 2004, 75, 384–392. [Google Scholar] [CrossRef] [PubMed]
- Barth, A. Infrared spectroscopy of proteins. Biochim. Biophys. Acta 2007, 1767, 1073–1101. [Google Scholar] [CrossRef] [PubMed]
- Khurana, R.; Fink, A. Do parallel-helix proteins have a unique Fourier transform infrared spectrum? Biophys. J. 2000, 78, 994–1000. [Google Scholar] [CrossRef] [PubMed]
- Cohen, P. The role of protein phosphorylation in human health and disease. Eur. J. Biochem. 2001, 268, 5001–5010. [Google Scholar] [CrossRef] [PubMed]
- Reimand, J.; Wagih, O.; Bader, G.D. The mutational landscape of phosphorylation signaling in cancer. Sci. Rep. 2013, 3, 2651. [Google Scholar] [CrossRef] [PubMed]
- Zanivan, S.; Cox, J.; Meves, A.; Fässler, R.; Neilson, L.J.; Tang, H.R.; Kalna, G.; Machesky, L.M.; van Ree, J.H.; van Deursen, J.M. In vivo SILAC-based proteomics reveals phosphoproteome changes during mouse skin carcinogenesis. Cell Rep. 2013, 3, 552–566. [Google Scholar]
- Abel, E.; Angel, J.; Kiguchi, K. Multi-stage chemical carcinogenesis in mouse skin: Fundamentals and applications. Nat. Protoc. 2009, 4, 1350–1362. [Google Scholar] [CrossRef] [PubMed]
- Mian, S.; Colley, H.; Thornhill, M.; Rehman, I. Development of a dewaxing protocol for tissue-engineered models of the oral mucosa used for Raman spectroscopic analysis. Appl. Spectrosc. Rev. 2014, 49, 614–617. [Google Scholar] [CrossRef]
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lima, C.A.; Goulart, V.P.; Côrrea, L.; Pereira, T.M.; Zezell, D.M. ATR-FTIR Spectroscopy for the Assessment of Biochemical Changes in Skin Due to Cutaneous Squamous Cell Carcinoma. Int. J. Mol. Sci. 2015, 16, 6621-6630. https://doi.org/10.3390/ijms16046621
Lima CA, Goulart VP, Côrrea L, Pereira TM, Zezell DM. ATR-FTIR Spectroscopy for the Assessment of Biochemical Changes in Skin Due to Cutaneous Squamous Cell Carcinoma. International Journal of Molecular Sciences. 2015; 16(4):6621-6630. https://doi.org/10.3390/ijms16046621
Chicago/Turabian StyleLima, Cássio A., Viviane P. Goulart, Luciana Côrrea, Thiago M. Pereira, and Denise M. Zezell. 2015. "ATR-FTIR Spectroscopy for the Assessment of Biochemical Changes in Skin Due to Cutaneous Squamous Cell Carcinoma" International Journal of Molecular Sciences 16, no. 4: 6621-6630. https://doi.org/10.3390/ijms16046621
APA StyleLima, C. A., Goulart, V. P., Côrrea, L., Pereira, T. M., & Zezell, D. M. (2015). ATR-FTIR Spectroscopy for the Assessment of Biochemical Changes in Skin Due to Cutaneous Squamous Cell Carcinoma. International Journal of Molecular Sciences, 16(4), 6621-6630. https://doi.org/10.3390/ijms16046621







