DNA Synthesis during Endomitosis Is Stimulated by Insulin via the PI3K/Akt and TOR Signaling Pathways in the Silk Gland Cells of Bombyx mori
Abstract
:1. Introduction
2. Results
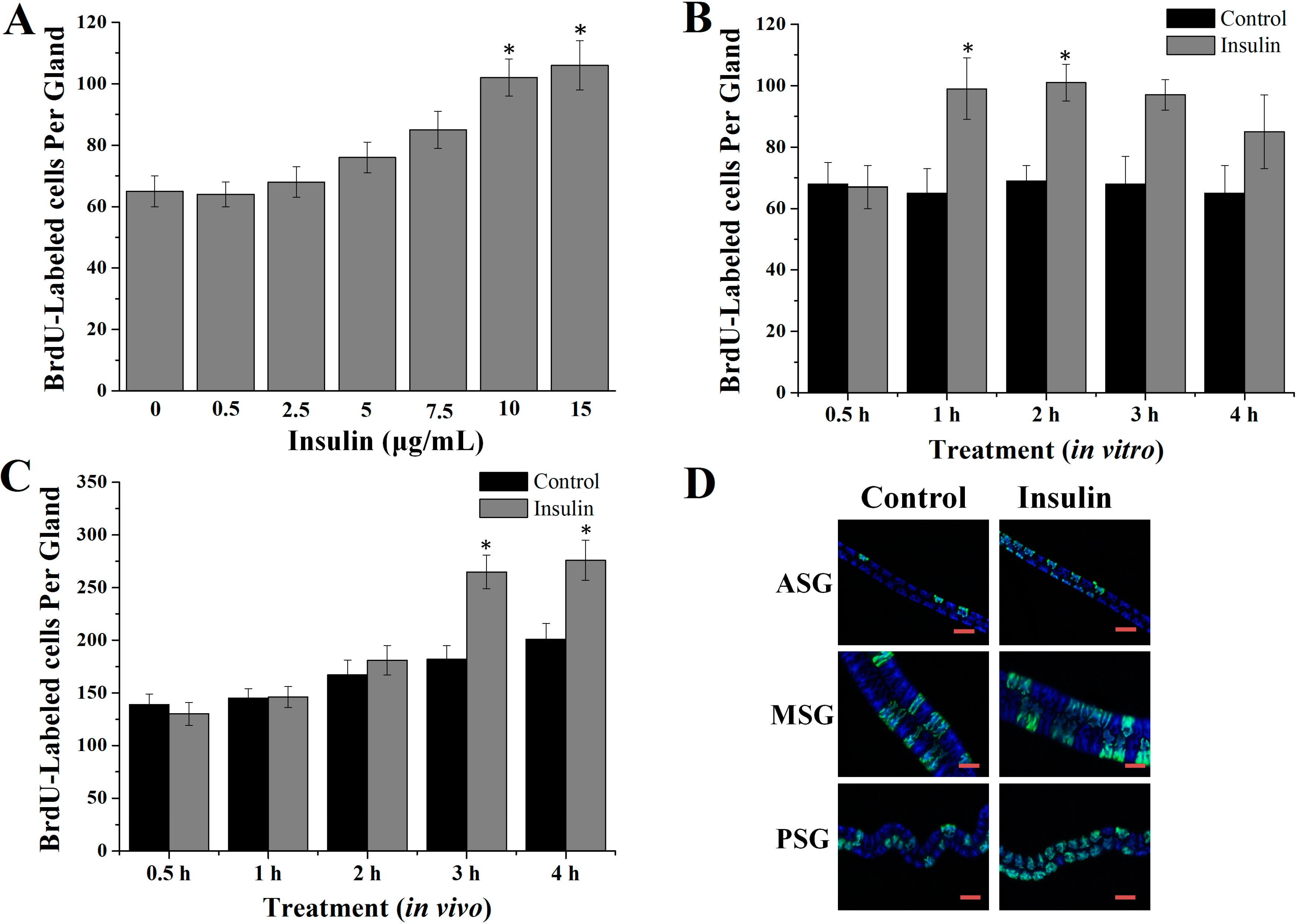
2.1. Insulin Activated Endomitotic DNA Synthesis of Silk Gland Cells
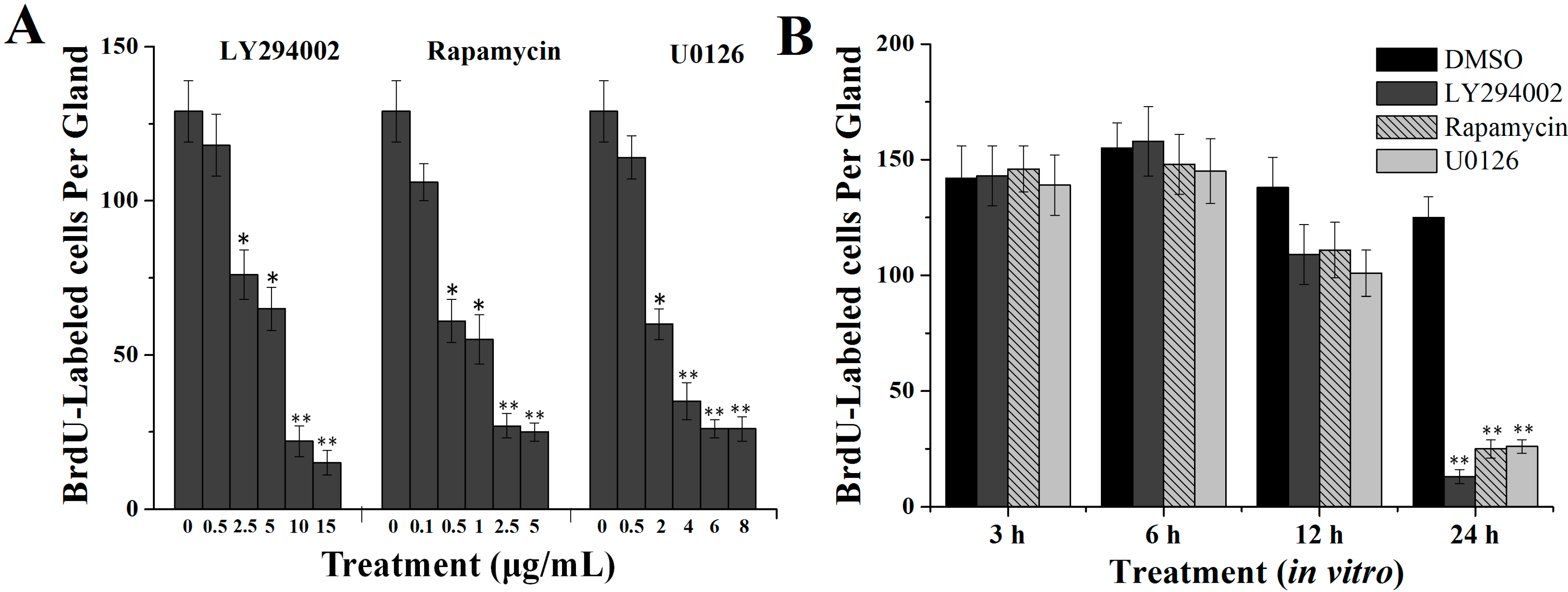
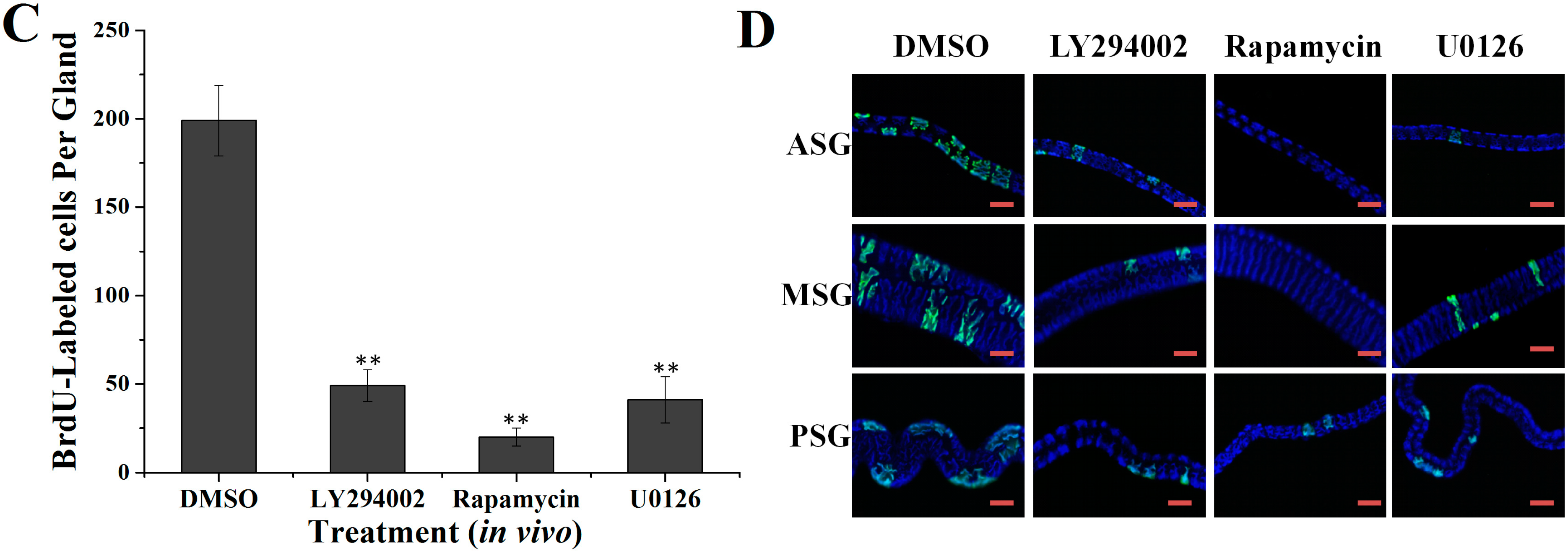
2.2. Effects of Specific Inhibitors LY294002, Rapamycin and U0126 on DNA Synthesis of Silk Gland Cells
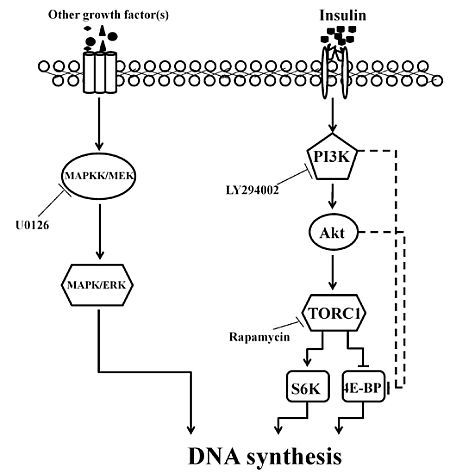
2.3. Involvement of the PI3K/Akt and TOR Signaling Pathway in Insulin-Stimulated Endomitotic DNA Synthesis
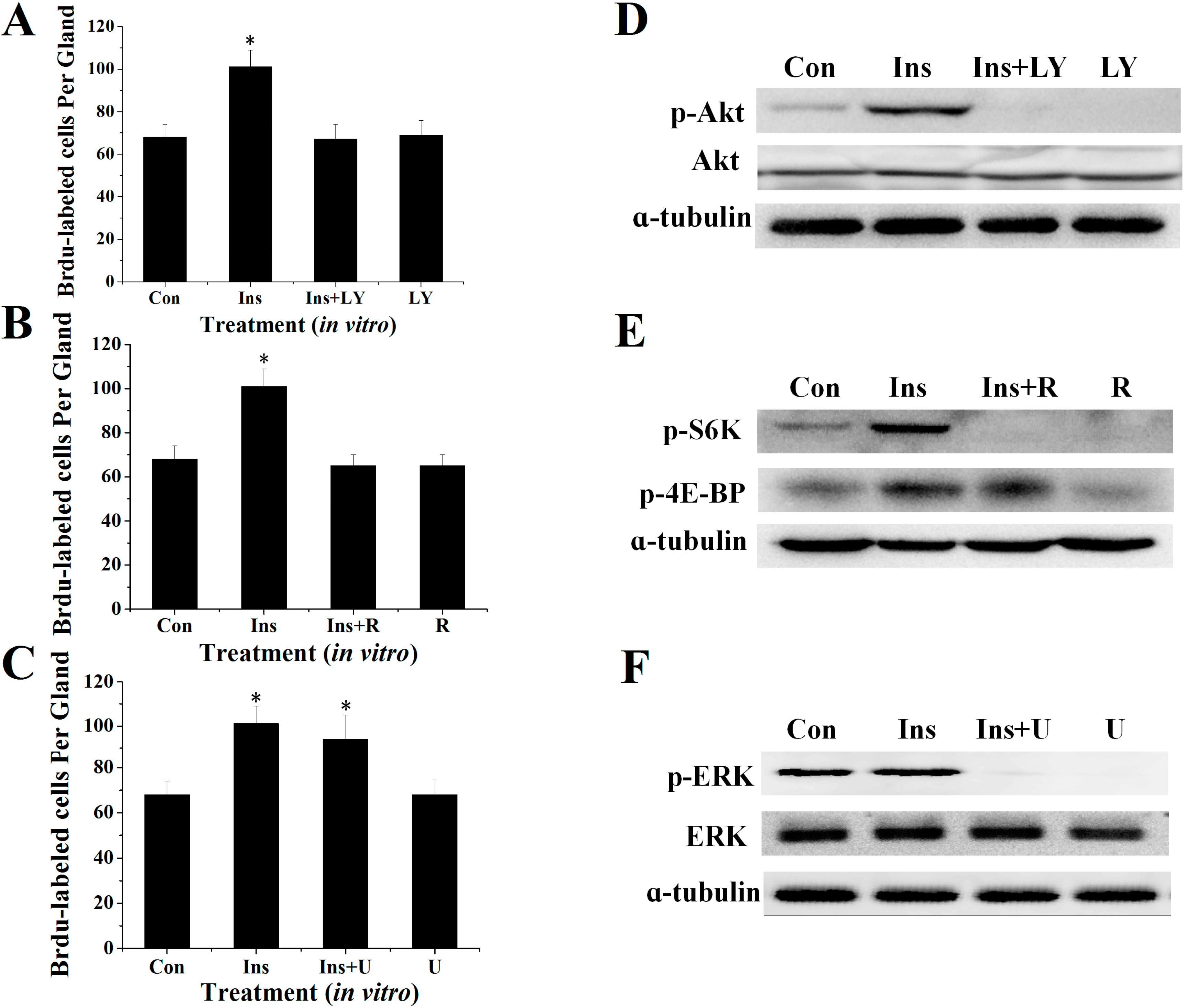
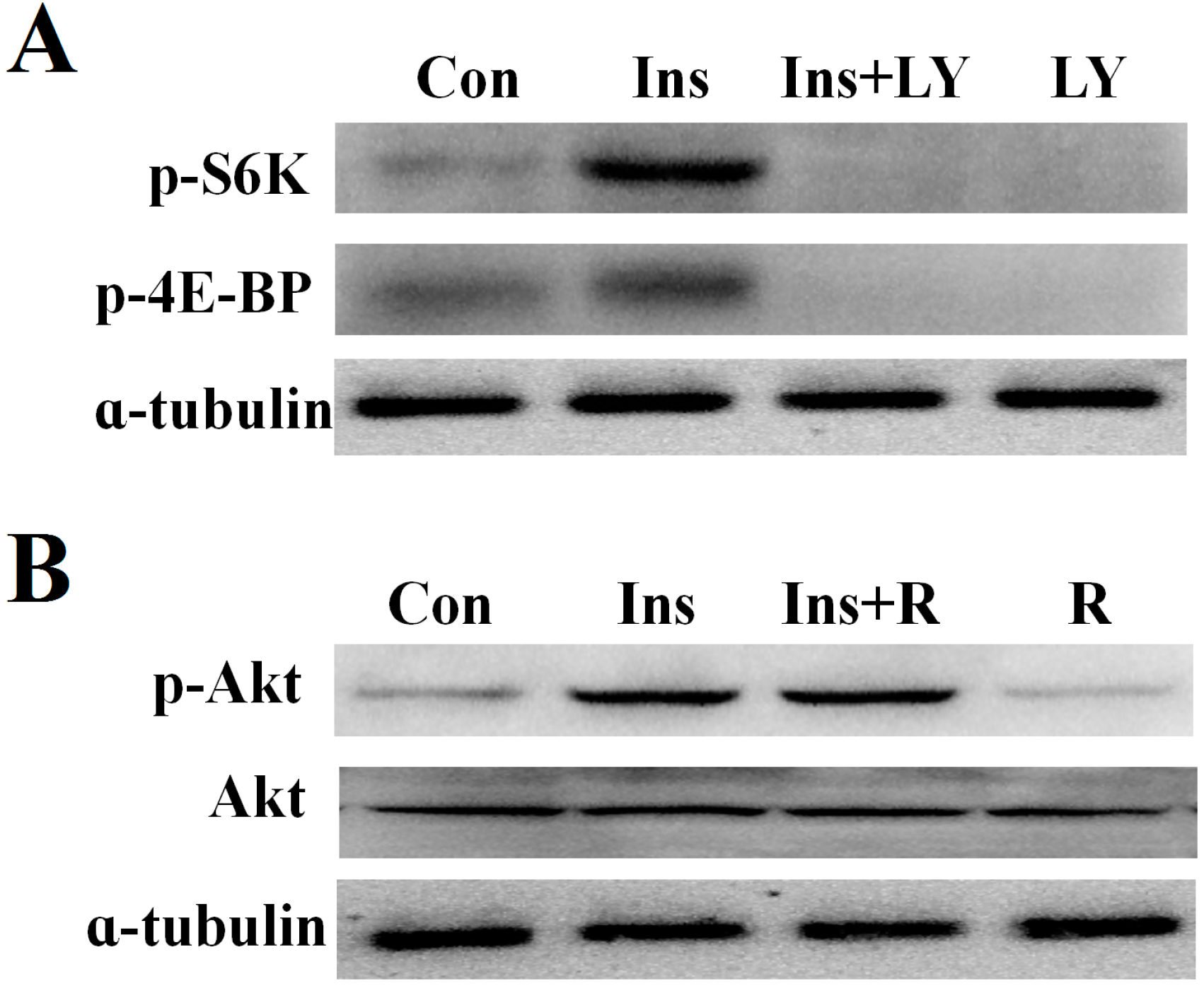
2.4. PI3K/Akt Signaling Is an Upstream Signaling Pathway for Insulin-Activated TOR Signaling
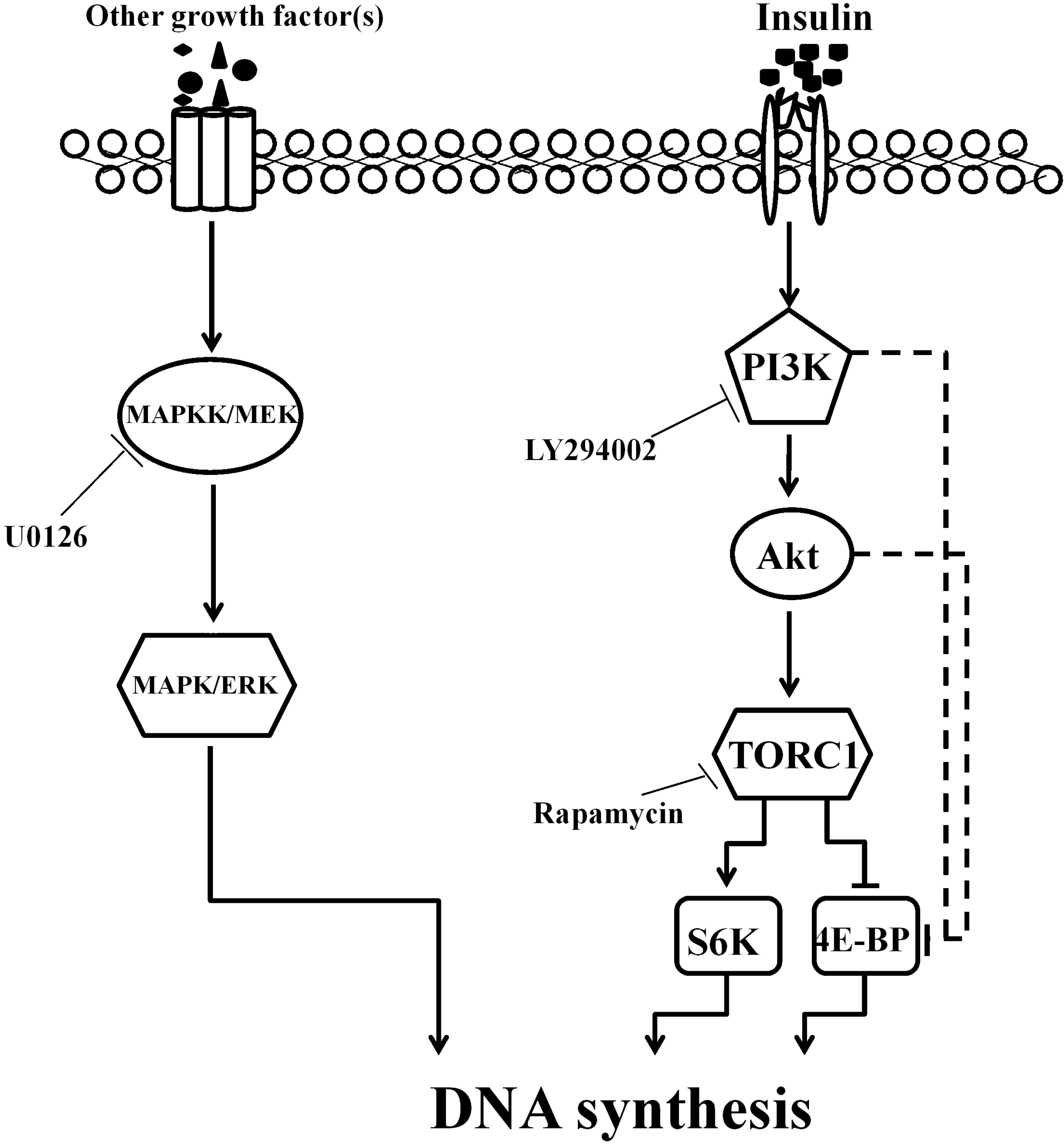
3. Discussion
4. Experimental Section
4.1. Experimental Animals
4.2. Reagents and Antibodies
4.3. In Vitro Culture of Silk Glands
4.4. Effects of Insulin on DNA Synthesis in Silk Gland Cells
4.4.1. Dose-Dependent Effects in Vitro
4.4.2. Time-Dependent Effects in Vitro
4.4.3. Effects of the Insulin in Vivo
4.5. Effects of the Specific Inhibitors on DNA Synthesis in Silk Gland Cells
4.5.1. Dose-Dependent Effects in Vitro
4.5.2. Time-Dependent Effects in Vitro
4.5.3. Effects of Inhibitors in Vivo
4.6. BrdU Incorporation and Immunocytochemical Staining
4.7. Western Blot Analysis
4.8. Statistical Analysis
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Shigematsu, H.; Kurata, K.; Takeshita, H. Nucleic acids accumulation of silk gland of Bombyx mori in relation to silk protein. Comp. Biochem. Physiol. B 1978, 61, 237–242. [Google Scholar]
- Zhang, C.D.; Li, F.F.; Chen, X.Y.; Huang, M.H.; Zhang, J.; Cui, H.J.; Pan, M.H.; Lu, C. DNA replication events during larval silk gland development in the silkworm. Bombyx mori. J. Insect Physiol. 2012, 58, 974–978. [Google Scholar] [CrossRef]
- Wullschleger, S.; Loewith, R.; Hall, M.N. TOR signaling in growth and metabolism. Cell 2006, 124, 471–484. [Google Scholar] [CrossRef] [PubMed]
- Britton, J.S.; Lockwood, W.K.; Li, L.; Cohen, S.M.; Edgar, B.A. Drosophila’s Insulin/PI3-kinase pathway coordinates cellular metabolism with nutritional conditions. Dev. Cell 2002, 2, 239–249. [Google Scholar] [CrossRef] [PubMed]
- Johnston, L.A.; Gallant, P. Control of growth and organ size in Drosophila. Bioessays 2002, 24, 54–64. [Google Scholar] [CrossRef] [PubMed]
- Hafen, E.; Stocker, H. How are the sizes of cells, organs, and bodies controlled? PLoS Biol. 2003, 1, E86. [Google Scholar] [CrossRef] [PubMed]
- Saucedo, L.J.; Gao, X.S.; Chiarelli, D.A.; Li, L.; Pan, D.; Edgar, B.A. Rheb promotes cell growth as a component of the insulin/TOR signalling network. Nat. Cell Biol. 2003, 5, 566–571. [Google Scholar] [CrossRef] [PubMed]
- Stocker, H.; Radimerski, T.; Schindelholz, B.; Wittwer, F.; Belawat, P.; Daram, P.; Breuer, S.; Thomas, G.; Hafen, E. Rheb is an essential regulator of S6K in controlling cell growth in Drosophila. Nat. Cell Biol. 2003, 5, 559–565. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Gao, X.; Saucedo, L.J.; Ru, B.; Edgar, B.A.; Pan, D. Rheb is a direct target of the tuberous sclerosis tumour suppressor proteins. Nat. Cell Biol. 2003, 5, 578–581. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Zhou, S.; Ma, L.; Tian, L.; Wang, S.; Sheng, Z.T.; Jiang, R.J.; William, G.; Bendena, B.; Li, S. Transcriptional regulation of the insulin signaling pathway genes by starvation and 20-hydroxyecdysone in the Bombyx fat body. J. Insect Physiol. 2010, 56, 1436–1444. [Google Scholar] [CrossRef] [PubMed]
- Zhou, S.; Zhou, Q.; Liu, Y.; Wang, S.; Wen, D.; He, Q.; Wang, W.; Bendena, W.G.; Li, S. Two Tor genes in the silkworm Bombyx mori. Insect Mol. Biol. 2010, 19, 727–735. [Google Scholar] [CrossRef] [PubMed]
- Claeys, I.; Simonet, G.; Poels, J.; van Loy, T.; Vercammen, L.; de Loof, A.; Vanden Broeck, J. Insulin related peptides and their conserved signal transduction pathway. Peptides 2002, 23, 807–816. [Google Scholar] [CrossRef] [PubMed]
- Garofalo, R.S. Genetic analysis of insulin signaling in Drosophila. Trends Endocrinol. Metab. 2002, 13, 156–162. [Google Scholar] [CrossRef] [PubMed]
- Leevers, S.J.; Weinkove, D.; MacDougall, L.K.; Hafen, E.; Waterfield, M.D. The Drosophila phosphoinositide 3-kinase Dp110 promotes cell growth. EMBO J. 1996, 15, 6584–6594. [Google Scholar] [PubMed]
- Teleman, A.A. Molecular mechanisms of metabolic regulation by insulin in Drosophila. Biochem. J. 2010, 425, 13–26. [Google Scholar] [CrossRef]
- Fingar, D.C.; Salama, S.; Tsou, C.; Harlow, E.; Blenis, J. Mammalian cell size is controlled by mTOR and its downstream targets S6K1 and 4EBP1/eIF4E. Genes Dev. 2002, 16, 1472–1487. [Google Scholar] [CrossRef] [PubMed]
- Avruch, J. Insulin signal transduction through protein kinase cascades. Mol. Cell. Biochem. 1998, 182, 31–48. [Google Scholar] [CrossRef] [PubMed]
- Adachi, T.; Takiya, S.; Suzuki, Y.; Iwami, M.; Kawakami, A.; Takahashi, S.Y.; Ishizaki, H.; Nagasawa, H.; Suzuki, A. cDNA structure and expression of bombyxin, an insulin-like brain secretory peptide of the silkmoth Bombyx mori. J. Biol. Chem. 1989, 264, 7681–7685. [Google Scholar] [PubMed]
- Nagasawa, H.; Maruyama, K.; Sato, B.; Hietter, H.; Kataoka, H.; Isogai, A.; Tamura, S.; Ishizaki, H.; Senba, T.; Suzuki, A. Structure and synthesis of bombyxin from the silkworm, Bombyx mori. Peptide Chem. 1988, 1987, 123–126. [Google Scholar]
- Kawakami, A.; Iwami, M.; Nagasawa, H.; Suzuki, A.; Ishizaki, H. Structure and organization of four clustered genes that encode bombyxin, an insulin-related brain secretory peptide of the silkmoth Bombyx mori. Proc. Natl. Acad. Sci. USA 1989, 86, 6843–6847. [Google Scholar] [CrossRef] [PubMed]
- Iwami, M.; Kawakami, A.; Ishizaki, H.; Takahashi, S.Y.; Adachi, T.; Suzuki, Y.; Nagasawa, H.; Suzuki, A. Cloning of a gene encoding bombyxin, an insulin-like brain secretory peptide of the silkmoth Bombyx mori with prothoracicotropic activity. Dev. Growth Differ. 1989, 31, 31–37. [Google Scholar] [CrossRef]
- Iwami, M.; Tanaka, A.; Hano, N.; Sakurai, S. Bombyxin gene expression in tissues other than brain detected by reverse transcription polymerase chain reaction (RT-PCR) and in situ hybridization. Experientia 1996, 52, 882–887. [Google Scholar] [CrossRef] [PubMed]
- Gu, S.H.; Lin, J.L.; Lin, P.L.; Chen, C.H. Insulin stimulates ecdysteroidogenesis by prothoracic glands in the silkworm, Bombyx mori. Insect Biochem. Mol. Biol. 2009, 39, 171–179. [Google Scholar] [CrossRef]
- Nakahara, Y.; Matsumoto, H.; Kanamori, Y.; Kataoka, H.; Mizoguchi, A.; Kiuchi, M.; Kamimura, M. Insulin signaling is involved in hematopoietic regulation in an insect hematopoietic organ. J. Insect Physiol. 2006, 52, 105–111. [Google Scholar] [CrossRef] [PubMed]
- Salto, T.; Iwabuchi, K. Effect of bombyxin-II, an insulin-related peptide of insects, on Bombyxmorihemocyte division in single-cell culture. Appl. Entomol. Zool. 2003, 38, 583–588. [Google Scholar] [CrossRef]
- Baumann, C.A.; Saltiel, A.R. Spatial compartmentalization of signal transduction in insulin action. Bioessays 2001, 23, 215–222. [Google Scholar] [CrossRef] [PubMed]
- Saltiel, A.R.; Kahn, C.R. Insulin signalling and the regulation of glucose and lipid metabolism. Nature 2001, 414, 799–806. [Google Scholar] [CrossRef] [PubMed]
- Vlahos, C.J.; Matter, W.F.; Hui, K.Y.; Brown, R.F. A specific inhibitor of phosphatidylinositol 3-kinase, 2-(4-morpholinyl)-8-phenyl-4H-1-benzopyran-4-one (LY294002). J. Biol. Chem. 1994, 269, 5241–5248. [Google Scholar] [PubMed]
- Sehgal, S.N. Rapamune® (RAPA, rapamycin, sirolimus): Mechanism of action immunosuppressive effect results from blockade of signal transduction and inhibition of cell cycle progression. Clin. Biochem. 1998, 31, 335–340. [Google Scholar] [CrossRef] [PubMed]
- Duncia, J.V.; Santella, J.B., III; Higley, C.A.; Pitts, W.J.; Wityak, J.; Frietze, W.E.; Rankin, F.W.; Sun, J.H.; Earl, R.A.; Tabaka, A.C.; et al. MEK inhibitors: The chemistry and biological activity of U0126, its analogs, and cyclization products. Bioorg. Med. Chem. Lett. 1998, 8, 2839–2844. [Google Scholar] [CrossRef]
- Hietakangas, V.; Cohen, S.M. Re-evaluating AKT regulation: Role of TOR complex 2 in tissue growth. Genes Dev. 2007, 21, 632–637. [Google Scholar] [CrossRef] [PubMed]
- Oldham, S.; Montagne, J.; Radimerski, T.; Thomas, G.; Hafen, E. Genetic and biochemical haracterization of dTOR, the Drosophila homolog of the target of rapamycin. Genes Dev. 2000, 14, 2689–2694. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Stallock, J.P.; Ng, J.C.; Reinhard, C.; Neufeld, T.P. Regulation of cellular growth by the Drosophila target of rapamycin dTOR. Genes Dev. 2000, 14, 2712–2724. [Google Scholar] [CrossRef] [PubMed]
- Riehle, M.A.; Brown, M.R. Insulin stimulates ecdysteroid production through a conserved signaling cascade in the mosquito Aedes aegypti. Insect Biochem. Mol. Biol. 1999, 29, 855–860. [Google Scholar] [CrossRef] [PubMed]
- Fingar, D.C.; Blenis, J. Target of rapamycin (TOR): An integrator of nutrient and growth factor signals and coordinator of cell growth and cell cycle progression. Oncogene 2004, 23, 3151–3171. [Google Scholar] [CrossRef] [PubMed]
- Inoki, K.; Li, Y.; Zhu, T.; Wu, J.; Guan, K.L. TSC2 is phosphorylated and inhibited by Akt and suppresses mTOR signaling. Nat. Cell Biol. 2002, 4, 648–657. [Google Scholar] [CrossRef]
- Oldham, S.; Hafen, E. Insulin/IGF and target of rapamycin signaling: A TOR de force in growth control. Trends Cell Biol. 2003, 13, 79–85. [Google Scholar] [CrossRef] [PubMed]
- Chaussade, C.; Rewcastle, G.W.; Kendall, J.D.; Denny, W.A.; Cho, K.; Gronning, L.M.; Chong, M.L.; Anagnostou, S.H.; Jackson, S.P.; Daniele, N.; et al. Evidence for functional redundancy of class IA PI3K isoforms in insulin signaling. Biochem. J. 2007, 404, 449–458. [Google Scholar] [CrossRef] [PubMed]
- Brunn, G.J.; Williams, J.; Sabers, C.; Wiederrecht, G.; Lawrence, J.C., Jr.; Abraham, R.T. Direct inhibition of the signaling functions of the mammalian target of rapamycin by the phosphoinositide 3-kinase inhibitors, wortmannin and LY294002. EMBO J. 1996, 15, 5256–5267. [Google Scholar] [PubMed]
- Gharbi, S.; Zvelebil, M.; Shuttleworth, S.; Hancox, T.; Saghir, N.; Timms, J.; Waterfield, M. Exploring the specificity of the PI3K family inhibitor LY294002. Biochem. J. 2007, 404, 15–21. [Google Scholar] [CrossRef] [PubMed]
- Miron, M.; Paul, L.; Sonenberg, N. Signaling from Akt to FRAP/TOR targets both 4E-BP and S6K in Drosophila melanogaster. Mol. Cell. Biol. 2003, 23, 9117–9126. [Google Scholar] [CrossRef] [PubMed]
- Cao, Z.X.; Liu, L.Z.; Dixon, D.A.; Zheng, J.Z.; Chandran, B.; Jiang, B.H. Insulin-like growth factor-I induces cyclooxygenase-2 expression via PI3K, MAPK and PKC signaling pathways in human ovarian cancer cells. Cell Signal. 2007, 19, 1542–1553. [Google Scholar] [CrossRef] [PubMed]
- Kumar, V.; Zhang, M.X.; Swank, M.W.; Kunz, J.; Wu, G.Y. Regulation of dendritic morphogenesis by Ras-PI3K-Akt-mTOR and Ras-MAPK signaling pathways. J. Neurosci. 2005, 25, 11288–11299. [Google Scholar] [CrossRef] [PubMed]
- Satake, S.I.; Masumura, M.; Ishizaki, H.; Nagata, K.; Kataoka, H.; Suzuki, A.; Mizoguchi, A. Bombyxin, an insulin-related peptide of insects, reduces the major storage carbohydrates in the silkworm Bombyx mori. Comp. Biochem. Physiol. Part B: Biochem. Mol. Biol. 1997, 118, 349–357. [Google Scholar] [CrossRef]
- Edgar, B.A.; Orr-Weaver, T.L. Endoreplication cell cycles: More for less. Cell 2001, 105, 297–306. [Google Scholar] [CrossRef] [PubMed]
- Lilly, M.A.; Spradling, A.C. The Drosophila endocycle is controlled by Cyclin E and lacks a checkpoint ensuring S-phase completion. Genes Dev. 1996, 10, 2514–2526. [Google Scholar] [CrossRef] [PubMed]
- Gratzner, H.G. Monoclonal antibody to 5-bromo- and 5-iododeoxyuridine: A new reagent for detection of DNA replication. Science 1982, 218, 474–475. [Google Scholar] [CrossRef] [PubMed]
- Nagata, S.; Hakuno, F.; Takahashi, S.I.; Nagasawa, H. Identification of Bombyx mori Akt and its phosphorylation by bombyxin stimulation. Comp. Biochem. Physiol. Part B: Biochem. Mol. Biol. 2008, 151, 355–360. [Google Scholar] [CrossRef]
- Gu, S.H.; Young, S.C.; Tsai, W.H.; Lin, J.L.; Lin, P.L. Involvement of 4E-BP phosphorylation in embryonic development of the silkworm, Bombyx mori. J. Insect Physiol. 2011, 57, 978–985. [Google Scholar] [CrossRef] [PubMed]
- Gu, S.H.; Yeh, W.L.; Young, S.C.; Lin, P.L.; Li, S. TOR signaling is involved in PTTH-stimulated ecdysteroidogenesis by prothoracic glands in the silkworm, Bombyx mori. Insect Biochem. Mol. Biol. 2012, 42, 296–303. [Google Scholar] [CrossRef] [PubMed]
- Fujiwara, Y.; Shindome, C.; Takeda, M.; Shiomi, K. The roles of ERK and P38 MAPK signaling cascades on embryonic diapause initiation and termination of the silkworm, Bombyx mori. Insect Biochem. Mol. Biol. 2006, 36, 47–53. [Google Scholar] [CrossRef] [PubMed]
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, Y.; Chen, X.; Tang, X.; Zhang, C.; Wang, L.; Chen, P.; Pan, M.; Lu, C. DNA Synthesis during Endomitosis Is Stimulated by Insulin via the PI3K/Akt and TOR Signaling Pathways in the Silk Gland Cells of Bombyx mori. Int. J. Mol. Sci. 2015, 16, 6266-6280. https://doi.org/10.3390/ijms16036266
Li Y, Chen X, Tang X, Zhang C, Wang L, Chen P, Pan M, Lu C. DNA Synthesis during Endomitosis Is Stimulated by Insulin via the PI3K/Akt and TOR Signaling Pathways in the Silk Gland Cells of Bombyx mori. International Journal of Molecular Sciences. 2015; 16(3):6266-6280. https://doi.org/10.3390/ijms16036266
Chicago/Turabian StyleLi, Yaofeng, Xiangyun Chen, Xiaofang Tang, Chundong Zhang, La Wang, Peng Chen, Minhui Pan, and Cheng Lu. 2015. "DNA Synthesis during Endomitosis Is Stimulated by Insulin via the PI3K/Akt and TOR Signaling Pathways in the Silk Gland Cells of Bombyx mori" International Journal of Molecular Sciences 16, no. 3: 6266-6280. https://doi.org/10.3390/ijms16036266
APA StyleLi, Y., Chen, X., Tang, X., Zhang, C., Wang, L., Chen, P., Pan, M., & Lu, C. (2015). DNA Synthesis during Endomitosis Is Stimulated by Insulin via the PI3K/Akt and TOR Signaling Pathways in the Silk Gland Cells of Bombyx mori. International Journal of Molecular Sciences, 16(3), 6266-6280. https://doi.org/10.3390/ijms16036266