Tetraspanin 8-Rictor-Integrin α3 Complex Is Required for Glioma Cell Migration
Abstract
:1. Introduction
2. Results
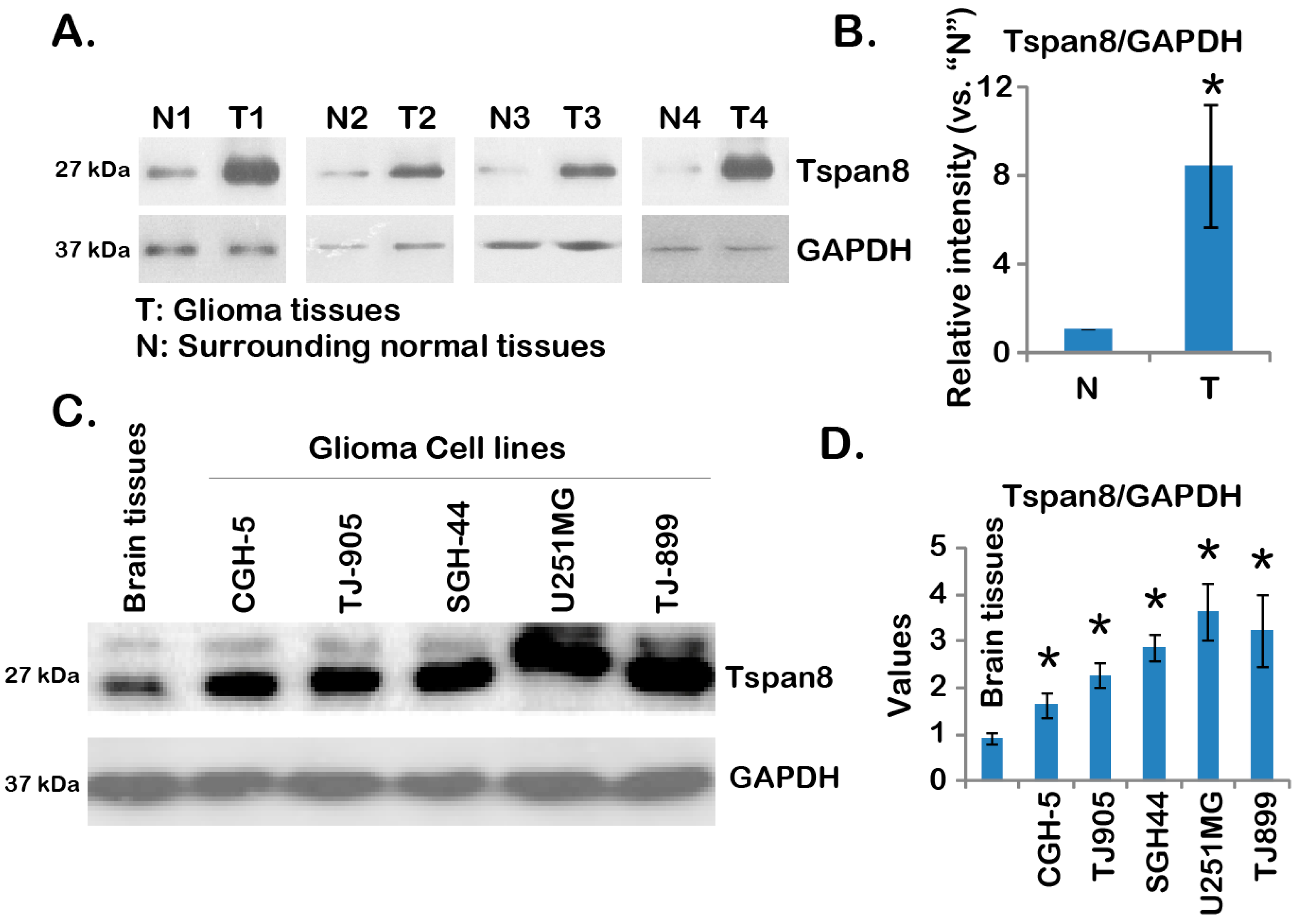
2.1. Over-Expression of Tspan8 in Human Malignant Glioma Tissues and Cell Lines
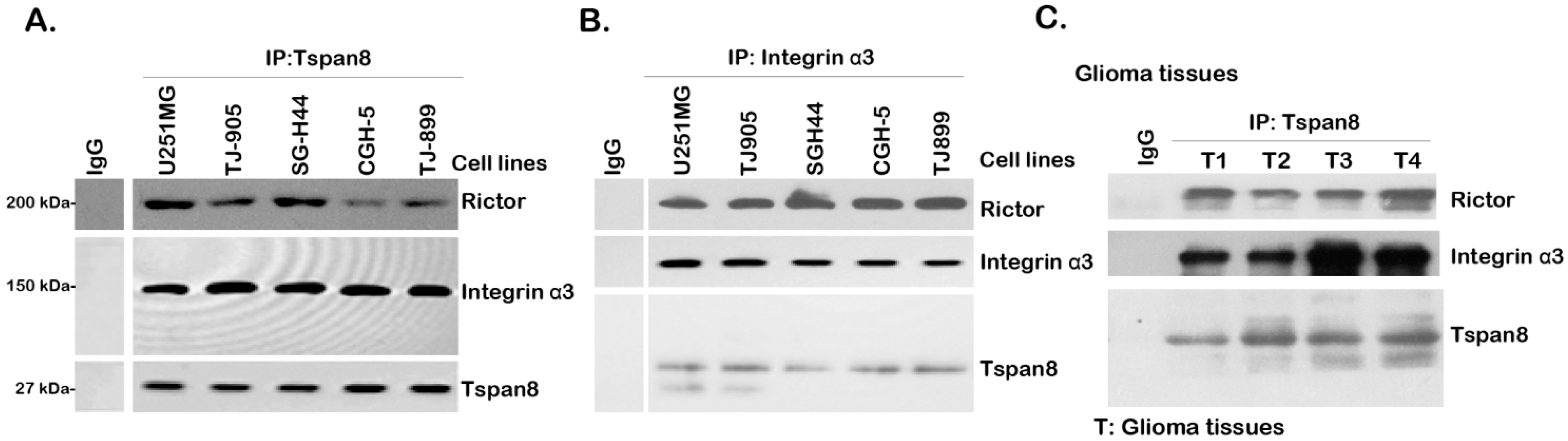
2.2. Tspan8 Associates with Rictor and Integrin α3 in both Human Glioma Tissues and Cell Lines
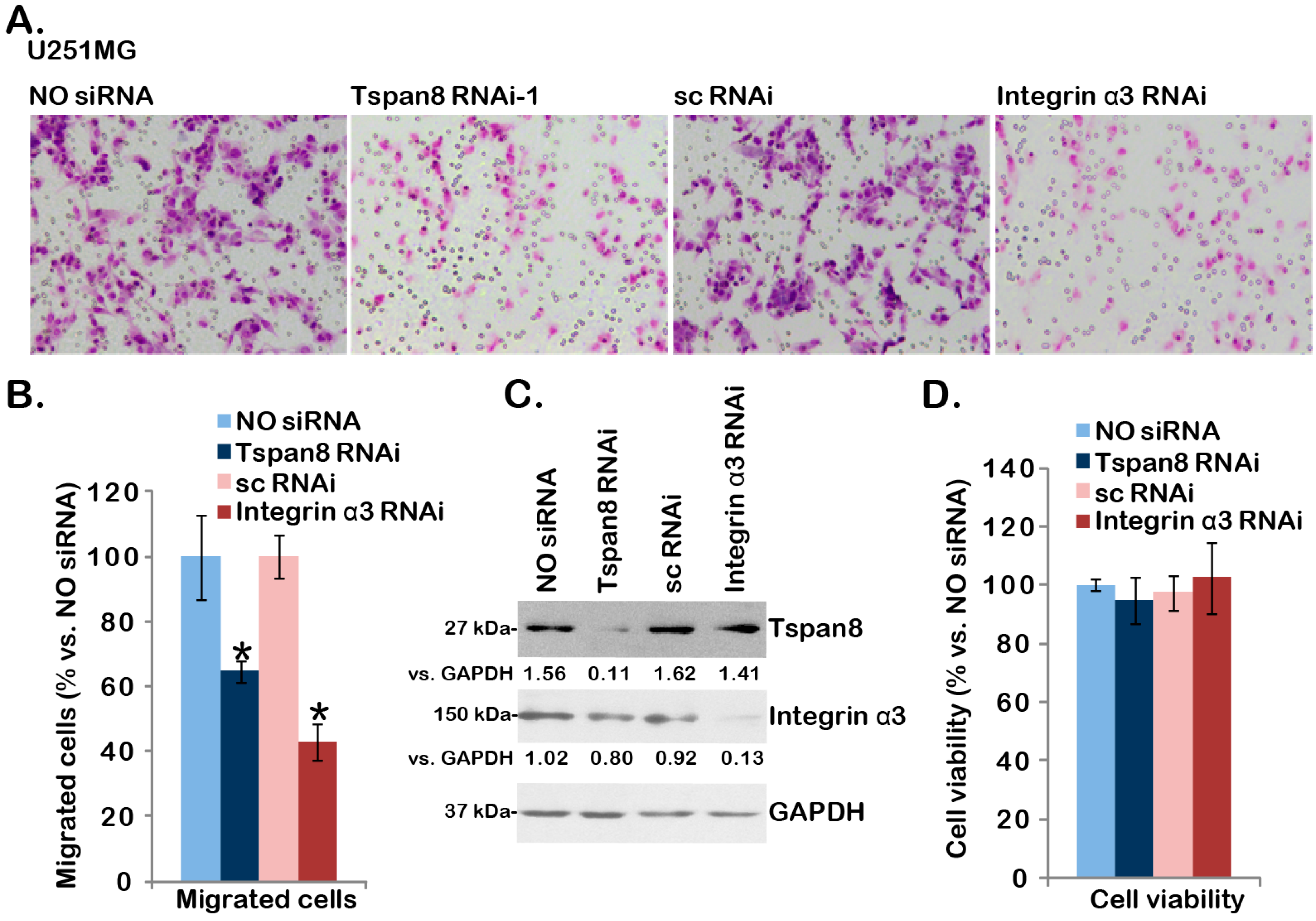
2.3. SiRNA-Mediated Knockdown of Tspan8 or Integrin α3 Inhibits U251MG Cell Migration
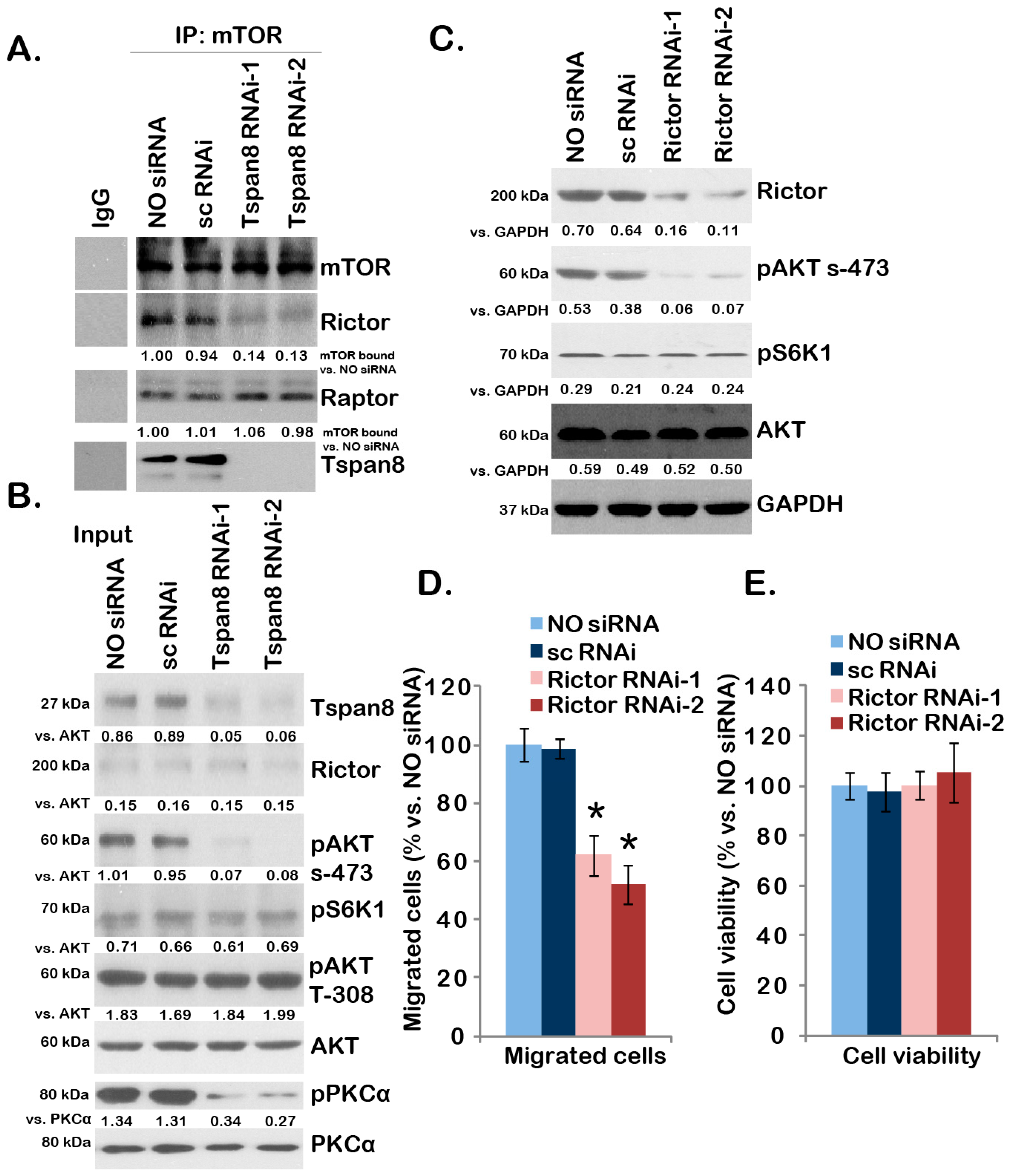
2.4. Tspan8-Rictor Association Is Required for mTORC2 Activation, AKT Ser-473 Phosphorylation and U251MG Cell Migration
3. Discussion
4. Experimental Section
4.1. Cell Culture and Reagents
4.2. Western Blot Analysis
4.3. Co-Immunoprecipitation (IP)
4.4. Transwell Assay
4.5. MTT Cell Viability Assay
4.6. siRNA
4.7. Patient’s Glioma Tissues Isolation and Preparation
4.8. Statistical Analysis
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Siegel, R.; Ma, J.; Zou, Z.; Jemal, A. Cancer statistics, 2014. CA Cancer J. Clin. 2014, 64, 9–29. [Google Scholar] [CrossRef] [PubMed]
- Lefranc, F.; Rynkowski, M.; DeWitte, O.; Kiss, R. Present and potential future adjuvant issues in high-grade astrocytic glioma treatment. Adv. Tech. Stand. Neurosurg. 2009, 34, 3–35. [Google Scholar] [PubMed]
- Wang, Y.; Jiang, T. Understanding high grade glioma: Molecular mechanism, therapy and comprehensive management. Cancer Lett. 2013, 331, 139–146. [Google Scholar] [CrossRef] [PubMed]
- Westphal, M.; Lamszus, K. The neurobiology of gliomas: From cell biology to the development of therapeutic approaches. Nat. Rev. Neurosci. 2011, 12, 495–508. [Google Scholar] [CrossRef] [PubMed]
- Charrin, S.; le Naour, F.; Silvie, O.; Milhiet, P.E.; Boucheix, C.; Rubinstein, E. Lateral organization of membrane proteins: Tetraspanins spin their web. Biochem. J. 2009, 420, 133–154. [Google Scholar] [CrossRef] [PubMed]
- Levy, S.; Shoham, T. The tetraspanin web modulates immune-signalling complexes. Nat. Rev. Immunol. 2005, 5, 136–148. [Google Scholar] [CrossRef] [PubMed]
- Yue, S.; Mu, W.; Zoller, M. Tspan8 and CD151 promote metastasis by distinct mechanisms. Eur. J. Cancer 2013, 49, 2934–2948. [Google Scholar] [CrossRef] [PubMed]
- Berditchevski, F. Complexes of tetraspanins with integrins: More than meets the eye. J. Cell Sci. 2001, 114, 4143–4151. [Google Scholar] [PubMed]
- Winterwood, N.E.; Varzavand, A.; Meland, M.N.; Ashman, L.K.; Stipp, C.S. A critical role for tetraspanin CD151 in alpha3beta1 and alpha6beta4 integrin-dependent tumor cell functions on laminin-5. Mol. Biol. Cell 2006, 17, 2707–2721. [Google Scholar] [CrossRef] [PubMed]
- Hasegawa, M.; Furuya, M.; Kasuya, Y.; Nishiyama, M.; Sugiura, T.; Nikaido, T.; Momota, Y.; Ichinose, M.; Kimura, S. CD151 dynamics in carcinoma-stroma interaction: Integrin expression, adhesion strength and proteolytic activity. Lab. Investig. 2007, 87, 882–892. [Google Scholar] [CrossRef] [PubMed]
- Sala-Valdes, M.; Ailane, N.; Greco, C.; Rubinstein, E.; Boucheix, C. Targeting tetraspanins in cancer. Expert Opin. Ther. Targets 2012, 16, 985–997. [Google Scholar] [CrossRef] [PubMed]
- Veenbergen, S.; van Spriel, A.B. Tetraspanins in the immune response against cancer. Immunol. Lett. 2 2011, 138, 129–136. [Google Scholar] [CrossRef]
- Zoller, M. Tetraspanins: Push and pull in suppressing and promoting metastasis. Nat. Rev. Cancer 2009, 9, 40–55. [Google Scholar] [CrossRef] [PubMed]
- Ailane, N.; Greco, C.; Zhu, Y.; Sala-Valdes, M.; Billard, M.; Casal, I.; Bawa, O.; Opolon, P.; Rubinstein, E.; Boucheix, C.; et al. Effect of an anti-human Co-029/tspan8 mouse monoclonal antibody on tumor growth in a nude mouse model. Front. Physiol. 2014, 5. [Google Scholar] [CrossRef] [PubMed]
- Greco, C.; Bralet, M.P.; Ailane, N.; Dubart-Kupperschmitt, A.; Rubinstein, E.; le Naour, F.; Boucheix, C. E-cadherin/p120-catenin and tetraspanin Co-029 cooperate for cell motility control in human colon carcinoma. Cancer Res. 2010, 70, 7674–7683. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Z.; Ran, Y.L.; Hu, H.; Pan, J.; Li, Z.F.; Chen, L.Z.; Sun, L.C.; Peng, L.; Zhao, X.L.; Yu, L.; et al. TM4SF3 promotes esophageal carcinoma metastasis via upregulating ADAM12m expression. Clin. Exp. Metastasis 2008, 25, 537–548. [Google Scholar] [CrossRef] [PubMed]
- Kanetaka, K.; Sakamoto, M.; Yamamoto, Y.; Yamasaki, S.; Lanza, F.; Kanematsu, T.; Hirohashi, S. Overexpression of tetraspanin CO-029 in hepatocellular carcinoma. J. Hepatol. 2001, 35, 637–642. [Google Scholar] [CrossRef] [PubMed]
- Berthier-Vergnes, O.; Kharbili, M.E.; de la Fouchardiere, A.; Pointecouteau, T.; Verrando, P.; Wierinckx, A.; Lachuer, J.; le Naour, F.; Lamartine, J. Gene expression profiles of human melanoma cells with different invasive potential reveal TSPAN8 as a novel mediator of invasion. Br. J. Cancer 2011, 104, 155–165. [Google Scholar] [CrossRef] [PubMed]
- Guertin, D.A.; Stevens, D.M.; Thoreen, C.C.; Burds, A.A.; Kalaany, N.Y.; Moffat, J.; Brown, M.; Fitzgerald, K.J.; Sabatini, D.M. Ablation in mice of the mTORC components raptor, rictor, or mLST8 reveals that mTORC2 is required for signaling to Akt-FOXO and PKCalpha, but not S6K1. Dev. Cell 2006, 11, 859–871. [Google Scholar] [CrossRef] [PubMed]
- Sarbassov, D.D.; Guertin, D.A.; Ali, S.M.; Sabatini, D.M. Phosphorylation and regulation of Akt/PKB by the rictor-mTOR complex. Science 2005, 307, 1098–1101. [Google Scholar] [CrossRef] [PubMed]
- Masri, J.; Bernath, A.; Martin, J.; Jo, O.D.; Vartanian, R.; Funk, A.; Gera, J. mTORC2 activity is elevated in gliomas and promotes growth and cell motility via overexpression of rictor. Cancer Res. 2007, 67, 11712–11720. [Google Scholar] [CrossRef] [PubMed]
- Guertin, D.A.; Sabatini, D.M. Defining the role of mTOR in cancer. Cancer Cell 2007, 12, 9–22. [Google Scholar] [CrossRef] [PubMed]
- Sabatini, D.M. mTOR and cancer: Insights into a complex relationship. Nat. Rev. Cancer 2006, 6, 729–734. [Google Scholar] [CrossRef] [PubMed]
- Gesierich, S.; Paret, C.; Hildebrand, D.; Weitz, J.; Zgraggen, K.; Schmitz-Winnenthal, F.H.; Horejsi, V.; Yoshie, O.; Herlyn, D.; Ashman, L.K.; et al. Colocalization of the tetraspanins, CO-029 and CD151, with integrins in human pancreatic adenocarcinoma: Impact on cell motility. Clin. Cancer Res. 2005, 11, 2840–2852. [Google Scholar] [CrossRef] [PubMed]
- Herlevsen, M.; Schmidt, D.S.; Miyazaki, K.; Zoller, M. The association of the tetraspanin D6.1A with the α6β4 integrin supports cell motility and liver metastasis formation. J. Cell Sci. 2003, 116, 4373–4390. [Google Scholar] [CrossRef] [PubMed]
- Claas, C.; Seiter, S.; Claas, A.; Savelyeva, L.; Schwab, M.; Zoller, M. Association between the rat homologue of CO-029, a metastasis-associated tetraspanin molecule and consumption coagulopathy. J. Cell Biol. 1998, 141, 267–280. [Google Scholar] [CrossRef] [PubMed]
- Kanetaka, K.; Sakamoto, M.; Yamamoto, Y.; Takamura, M.; Kanematsu, T.; Hirohashi, S. Possible involvement of tetraspanin CO-029 in hematogenous intrahepatic metastasis of liver cancer cells. J. Gastroenterol. Hepatol. 2003, 18, 1309–1314. [Google Scholar] [CrossRef] [PubMed]
- Fukushima, Y.; Ohnishi, T.; Arita, N.; Hayakawa, T.; Sekiguchi, K. Integrin α3β1-mediated interaction with laminin-5 stimulates adhesion, migration and invasion of malignant glioma cells. Int. J. Cancer 1998, 76, 63–72. [Google Scholar] [CrossRef] [PubMed]
- Tysnes, B.B.; Larsen, L.F.; Ness, G.O.; Mahesparan, R.; Edvardsen, K.; Garcia-Cabrera, I.; Bjerkvig, R. Stimulation of glioma-cell migration by laminin and inhibition by anti-α3 and anti-β1 integrin antibodies. Int. J. Cancer 1996, 67, 777–784. [Google Scholar] [CrossRef] [PubMed]
- Doherty, L.; Gigas, D.C.; Kesari, S.; Drappatz, J.; Kim, R.; Zimmerman, J.; Ostrowsky, L.; Wen, P.Y. Pilot study of the combination of EGFR and mTOR inhibitors in recurrent malignant gliomas. Neurology 2006, 67, 156–158. [Google Scholar] [CrossRef] [PubMed]
- Akhavan, D.; Cloughesy, T.F.; Mischel, P.S. mTOR signaling in glioblastoma: Lessons learned from bench to bedside. Neuro Oncol. 2010, 12, 882–889. [Google Scholar] [CrossRef] [PubMed]
- Gan, X.; Wang, J.; Wang, C.; Sommer, E.; Kozasa, T.; Srinivasula, S.; Alessi, D.; Offermanns, S.; Simon, M.I.; Wu, D.; et al. PRR5L degradation promotes mTORC2-mediated PKC-δ phosphorylation and cell migration downstream of Galpha12. Nat. Cell Biol. 2012, 14, 686–696. [Google Scholar] [CrossRef] [PubMed]
- Kim, E.K.; Yun, S.J.; Ha, J.M.; Kim, Y.W.; Jin, I.H.; Yun, J.; Shin, H.K.; Song, S.H.; Kim, J.H.; Lee, J.S.; et al. Selective activation of Akt1 by mammalian target of rapamycin complex 2 regulates cancer cell migration, invasion, and metastasis. Oncogene 2011, 30, 2954–2963. [Google Scholar] [CrossRef] [PubMed]
- Laplante, M.; Sabatini, D.M. mTOR signaling in growth control and disease. Cell 2012, 149, 274–293. [Google Scholar] [CrossRef] [PubMed]
- Chen, M.B.; Zhang, Y.; Wei, M.X.; Shen, W.; Wu, X.Y.; Yao, C.; Lu, P.H. Activation of AMP-activated protein kinase (AMPK) mediates plumbagin-induced apoptosis and growth inhibition in cultured human colon cancer cells. Cell Signal. 2013, 25, 1993–2002. [Google Scholar] [CrossRef] [PubMed]
- Chen, M.B.; Wu, X.Y.; Gu, J.H.; Guo, Q.T.; Shen, W.X.; Lu, P.H. Activation of AMP-activated protein kinase contributes to doxorubicin-induced cell death and apoptosis in cultured myocardial H9c2 cells. Cell Biochem. Biophys. 2011, 60, 311–322. [Google Scholar] [CrossRef] [PubMed]
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pan, S.-J.; Zhan, S.-K.; Pan, Y.-X.; Liu, W.; Bian, L.-G.; Sun, B.; Sun, Q.-F. Tetraspanin 8-Rictor-Integrin α3 Complex Is Required for Glioma Cell Migration. Int. J. Mol. Sci. 2015, 16, 5363-5374. https://doi.org/10.3390/ijms16035363
Pan S-J, Zhan S-K, Pan Y-X, Liu W, Bian L-G, Sun B, Sun Q-F. Tetraspanin 8-Rictor-Integrin α3 Complex Is Required for Glioma Cell Migration. International Journal of Molecular Sciences. 2015; 16(3):5363-5374. https://doi.org/10.3390/ijms16035363
Chicago/Turabian StylePan, Si-Jian, Shi-Kun Zhan, Yi-Xin Pan, Wei Liu, Liu-Guan Bian, Bomin Sun, and Qing-Fang Sun. 2015. "Tetraspanin 8-Rictor-Integrin α3 Complex Is Required for Glioma Cell Migration" International Journal of Molecular Sciences 16, no. 3: 5363-5374. https://doi.org/10.3390/ijms16035363
APA StylePan, S.-J., Zhan, S.-K., Pan, Y.-X., Liu, W., Bian, L.-G., Sun, B., & Sun, Q.-F. (2015). Tetraspanin 8-Rictor-Integrin α3 Complex Is Required for Glioma Cell Migration. International Journal of Molecular Sciences, 16(3), 5363-5374. https://doi.org/10.3390/ijms16035363




