Identification and Pharmacokinetics of Multiple Potential Bioactive Constituents after Oral Administration of Radix Astragali on Cyclophosphamide-Induced Immunosuppression in Balb/c Mice
Abstract
:1. Introduction
2. Results and Discussion
2.1. Identification of Compounds in RA Extract
| No. | TR (min) | Formula | [M + H]+ (Error, ppm) | [M − H]− (Error, ppm) | Fragment Ions in Positive (+) Ion Mode | Fragment Ions in Negative (−) Ion Mode | Identification | Source | ||
|---|---|---|---|---|---|---|---|---|---|---|
| RA | Control Plasma | Dosed Plasma | ||||||||
| 1 | 0.96 | C6H12O6 | 179.0563 (+0.79) | 203.0521 [M + Na]+ (−2.4), 143.0327 [M + Na − C2H4O2]+ | - | Hexose | + | + | 0.25–36 h | |
| 2 | 0.97 | C4H8N2O3 | 133.0607 (−0.7) | 131.0472 (+7.6) | - | 114.0194 [M − H − NH3]−, 113.0354 [M − H − H2O]−, 95.0257 [M − H − CH2O2]− | Asparagine a | + | + | 1–36 h |
| 3 | 1.00 | C12H22O11 | - | 341.1089 (−0.08) | 365.1053 [M + Na]+ (−0.36), 203.0532 [M + Na − glc]+, 185.0425 [M + Na − C6H12O6]+ | 179.0553 [M − H − glc]− | Disaccharide | + | - | 0.25–4 h |
| 4 | 1.02 | C5H13NO | 104.1071 (1.0) | - | 60.0833 [M + H − C2H5O]+ | - | Choline a | + | + | 0.25–36 h |
| 5 | 1.05 | C4H9NO2 | 104.0706 (−0.1) | 102.0615 (+1.0) | - | - | γ-Aminobutyric acid a | + | + | 0.25–36 h |
| 6 | 1.12 | C18H32O16 | - | 503.1621 (+1.7) | 527.1583 [M + Na]+ (+0.1) | 161.0453 [M + H − 2glc]− | Raffinose a | + | - | 0.25–3 h |
| 7 | 1.21 | C5H5N5 | 136.0617 (−0.6) | 134.0473 (+1.0) | 119.0351 [M + H − NH3]+, 92.0248 [M + H − NH3 − HCN]+ | 107.0366 [M + H − HCN]+, 72.0186 | Adenine a | + | - | - |
| 8 | 1.37 | C6H5NO2 | 124.0393 (−0) | 106.0285 [M + H − H2O]+, 80.0500 [M + H − CO2]+, 78.0343, 53.0417 | - | Nicotinic acid a | + | 0.25–4 h | ||
| 9 | 1.63 | C6H13NO2 | 132.1018 (−1.0) | 130.0874 (+1.0) | 86.0975 [M + H − CH2O2]+, 69.0714 [M + H−NH3 − CH2O2]+ | - | Leucine a | + | + | 0.25–36 h |
| 10 | 1.86 | C10H13N5O4 | 268.1040 (−0.3) | 266.0899 (+0.7) | 136.0613 [M + H − ribose]+, 119.0347 [M + H − ribose − NH3]+ | 135.0315 [M − H − ribose]−, 107.0377 [M − H − C6 H12O5 − HCN]− | Adenine nucleoside a | + | + | 0.25–36 h |
| 11 | 2.60 | C9H11NO2 | 166.0864 (+1.0) | 164.0717 (−0.12) | 149.0588 [M + H − NH3]+, 131.0507 [M + H − NH3 − H2O]+, 120.0814 [M + H − CH2O2]+, 103.0550 [M + H − NH3 − CH2O2]+ | 147.0460 [M − H − NH3]−, 103.0571 [M − H − NH3 − CO2]−, | Phenylalanine a | + | + | 0.25–36 h |
| 12 | 3.70 | C7H6O4 | - | 153.0196 (+2.0) | - | 109.0293 [M − H − CO2]−, 81.0349 | Protocatechuic acid a | + | + | 0.25–36 h |
| 13 | 4.01 | C18H26O12 | - | 433.1355 (+0.9) | 457.1319 [M + Na]+ (+0.25) | 301.0939 [M − H − C5H8O4]−, 139.0399 [M − H − C5H8O4 − glc]−, 161.0454, 124.0168 | Markhamioside F | + | - | 0.25–8 h |
| 14 | 4.87 | C11H12N2O2 | 205.0970 (−0.25) | 203.0830 (+2) | 188.0704 [M + H − NH3]+, 170.0596 [M + H − NH3 − H2O]+, 159.0915 [M + H − CH2O2]+, 132.0806 [M + H − NH3 − CH2O2]+, 146.0600, 118.0652, 91.0553 | 185.0443 [M − H−NH3]−, 159.0916 [M − H−CO2]−, 142.0659 [M − H − NH3 − CO2]−, 116.0507, 74.0276 | Tryptophan a | + | + | 0.25–36 h |
| 15 | 5.34 | C7H6O3 | 139.0388 (−0.8) | 137.0249 (+3.6) | 121.0278 [M + H − H2O]+, 111.0072 [M + H − CO]+, 95.0482 [M + H − CO2]+, 77.0400 [M + H − H2O − CO2]+ | 93.0369 [M − H − CO2]−, 75.0272 [M − H − H2O − CO2]− | Hydroxybenzoic acid a | + | - | - |
| 16 | 6.04 | C9H11NO3 | 182.0810 (−0.6) | 180.0666 (+0) | 165.0550 [M + NH3]+ | - | Tyrosine a | + | + | 0.25–36 h |
| 17 | 8.17 | C16H18O9 | 355.1024 (+0.3) | 353.0878 (−0.11) | - | - | Chlorogenic acid a | + | - | - |
| 18 | 8.27 | C28H32O16 | 625.1763 (0) | 623.1624 (+1.1) | 463.1225 [M + H − glc]+, 301.0707 [M + H − 2glc]+, | 461.1109 [M − H − glc]−, 299.0574 [M − H − 2glc]− | Rhamnocitrin 3,4'-di-O-glucoside | + | - | 0.25-6 h |
| 19 | 8.97 | C9H8O4 | 181.0490 (−2.8) | 179.0357 (3.9) | 153.0544 [M + H − CO]+, 137.0314 [M + H − CO2]+, 123.0051, 93.0364 | 135.0467 [M − H − CO2]− | Caffeic acid a | + | - | 0.25−0.5 h |
| 20 | 9.49 | C9H10O5 | 199.0600 (−0.4) | 197.0455 (−0.14) | 181.0478 [M + H − H2O]+, 155.0694 [M + H − CO2]+, 125.0228 [M + H − CO2 − CH2O]+, 97.0298, 77.0388 | 182.0231 [M − H − CH3]−, 166.9992 [M − H − CH3O]−, 153.0572 [M − H − CO2]−, 123.0098 [M − H − CO2 − CH2O]−, 95.0153 | Syringic acid a | + | + | 0.25–36 h |
| 21 | 9.95 | C29H38O16 | - | 641.2085 (−0.28) | 665.2056 [M + Na] + (+0.7) | 479.1604 [M − H − glc]−, 317.1053 [M − H − 2glc]−, 195.0653 [M − H − 2glc − RDA]−, 121.0292 | Isomucronulatol-2',5'-di-O-glucoside | + | - | 0.25–3 h |
| 22 | 10.19 | C27H30O15 | 595.1652 (−0.8) | 593.1512 (−1.1) | 433.1137 [M + H − glc]+, 271.0596 [M + H − 2glc]+ | - | Emodin-di-O-glucoside | + | - | 0.25–4 h |
| 23 | 10.40 | C9H10O4 | 183.0654 (−0.8) | 181.0511 (+2.6) | 155.0696 [M + H − CO]+, 140.0466 [M + H − CO − CH3]+, 125.0220 [M + H − CO − CH2O]+, 123.0418 [M + H − 2CH2O]+, 95.0511, 77.0400 | - | Syringaldehyde a | + | - | 0.25–6 h |
| 24 | 10.73 | C17H24O9 | - | 371.1352 (+1.3) | 395.1314 [M + Na]+ (+0.4) | 209.0824 [M − H − glc]−, 193.0506, 178.0269 | Syringin a | + | + | 0.25–36 h |
| 25 | 11.20 | C10H10O4 | 195.0652 (+0.25) | 193.0510 (+1.9) | 177.0547 [M + H − H2O]+, 149.0590 [M + H − CH2O2]+, 163.0388 [M + H − CH3OH]+, 117.0333 [M + H − CH2O2 − CH3OH]+, 109.0271, 89.0380, 77.0387 | 178.0257 [M − H − CH3]−, 149.0611 [M − H − CO2]−, 134.0380 [M − H − CO2 − CH3]− | Ferulic acid a | + | + | 0.25–36 h |
| 26 | 11.31 | C22H22O11 | 463.1232 (−0.25) | 461.1099 (+2.1) | 301.0700 [M + H − glc]+, 269.0430 [M + H − glc − CH3OH]+, 241.0500, 213.0561, 197.0560 | 299.0568 [M − H − glc]−, 284.0336 [M − H − glc − CH3]+, 255.0308, 227.0334, 135.0092 | Pratensein-7-O-glucoside | + | - | 0.25–8 h |
| 27 | 11.90 | C10H10O4 | 195.0648 (−1.6) | 193.0509 (+1.6) | 177.0553 [M + H − H2O]+, 149.0631 [M + H − CH2O2]+, 117.0319 [M + H − CH2O2 − CH3OH]+, 89.0377 | 178.0259 [M − H − CH3]−, 149.069 [M − H − CO2]−, 134.0374 [M − H − CO2 − CH3]− | Isoferulic acid | + | + | 0.25–36 h |
| 28 | 12.03 | C11H12O5 | 225.0759 (+0.8) | 223.0617 (+2.3) | 247.0578 [M + Na]+, 207.0648 [M + H − H2O]+, 179.0671 [M + H − CH2O2]+, 175.0409 [M + H−H2O − CH3OH]+, 147.0418 [M + H − CH2O2 − CH3OH]+ | 205.0514 [M − H − H2O]−, 179.0710 [M − H − CO2]−, 163.0401 [M − H − 2CH2O]−, 161.0608 [M − H − H2O − CO2]−, 133.0661, 117.0720, 91.0569 | Sinapic acid | + | - | 0.25–3 h |
| 29 | 12.85 | C22H22O10 | 447.1290 (+1.1) | 445.1142 (+0.6) | 469.1106 [M + Na]+ (−0.3), 285.0767 [M + H − glc]+, 270.0523 [M + H − glc − CH3]+, 253.0501, 225.0553, 137.0238 | 283.0620 [M − H − glc]−, 268.0366 [M − H − glc − CH3]−, 233.0890, 203.0819, 159.0361 | Calycosin-7-O-glucoside a | + | - | 0.25–24 h |
| 30 | 13.25 | C21H20O10 | 433.1132 (+0.7) | 431.0985 (+0.25) | 271.0602 [M + H − glc]+, 215.0702 | 269.0390 [M − H − glc]−, 195.0509 | Cosmosiin a | + | + | 0.25–36 h |
| 31 | 15.08 | C29H38O15 | - | 625.2156 (+2.9) | 649.2115 [M + Na]+ (2.0), 487.1555 [M + Na − glc]+, 325.1390 [M + Na − 2glc]+, 177.0552 | 463.1635 [M − H − glc], 301.1097 [M − H − 2glc]−, 286.0850, 121.0300 | Isomucronulatol-7,2'-di-O-glucoside | + | - | 0.25–12 h |
| 32 | 15.27 | C9H16O4 | 189.1120 (−0.7) | 187.0985 (+4.7) | 211.0939 [M + Na]+ (+0.4), 165.1231 | 169.0875 [M − H − H2O]−, 143.1091 [M − H − CO2]−, 125.0980 [M − H − H2O − CO2]−, 97.0680, 95.0521 | Azelaic acid | + | + | 0.25–36 h |
| 33 | 15.75 | C22H22O9 | 431.1344 (+1.7) | 429.1193 (+0.6) | 453.1159 [M + Na]+ (+0.8), 269.0823 [M + H − glc]+, 254.0588, 213.0912 | 267.0681 [M − H − glc]− | Ononin a | + | - | 0.25–24 h |
| 34 | 15.94 | C23H26O10 | 463.1603 (+1.0) | 461.1449 (−0.9) | 301.1083 [M + H − glc]+, | 167.0706 | 9,10-dimethoxypterocarpan-3-O-glucoside | + | - | 0.25–12 h |
| 35 | 16.83 | C9H14O2 | 155.1065 (−1.1) | 153.0923 (+1.4) | 137.0969 [M + H − H2O]+, 109.1014 [M + H − CH2O2]+, 95.0859, 67.0569 | - | 2,4-Nonadienic acid | + | + | 0.25–36 h |
| 36 | 17.18 | C23 h28O10 | 465.1754 (−0.3) | 463.1611 (+0.21) | 487.1574 [M + Na]+ (+0), 303.1225 [M + H − glc]+, 167.0704, 123.0439 | 301.1092 [M − H − glc]−, 271.0629 [M − H − glc − CH3O]−, 165.0489, 121.0307, | Isomucronulatol-7-O-glucoside | + | - | 0.25–12 h |
| 37 | 17.42 | C16H12O5 | 285.0759 (+0.8) | 283.0622 (+3.8) | 270.0525 [M + H − CH3]+, 253.0490, 225.0534, 197.0596, 157.0641, 137.0223 | 239.0350, 211.0398, 195.0452, 167.0506, 148.0168, 135.0089 | Calycosin a | + | - | 0.25–24 h |
| 38 | 19.01 | C24H24O10 | 473.1442 (−0.1) | - | 455.3400 [M + H − H2O]+, 269.0795 [M + H−glc − C2H4O2]+ | - | 6'-O-acetyl ononin | + | - | 0.25–8 h |
| 39 | 20.09 | C16H12O4 | 269.0809 (+0.25) | 267.0669 (+2.4) | 253.0503, 237.0552, 225.0551, 213.0918, 197.0599, 181.0647 | 251.0358, 223.0401, 195.0453, 167.0505, 132.0223 | Formononetin a | + | - | 0.25–24 h |
| 40 | 22.25 | C47H78O19 | 947.5211 (+0.1) | 945.5112 (+5.0) | 969.5027 [M + Na]+ (−0.2) | 783.4625 [M − H − glc]−, 654.4278, 541.1990 | Astragaloside V | + | - | 0.25–24 h |
| 41 | 22.72 | C47H78O19 | 947.5223 (+1.3) | 945.5108 (+4.6) | 749.4493,617.4075, 587.3937, 969.5037 [M + Na]+ (+0.8), 473.3647,455.3527, 437.3421, 305.1585, 143.1067, 125.0987 | 783.4651 [M − H − glc]−, 489.3570, 161.0466 | Astragaloside VI | + | - | 0.25–24 h |
| 42 | 22.94 | C41H68O14 | 785.4676 (−0.7) | 783.4563 (3.5) | 807.4505 [M + Na]+ (+0.25) 605.4145, 455.3490, 437.1977, 419.3328 | 621.4056, 489.3691, 161.0458 | Astragaloside III | + | - | 0.25–12 h |
| 43 | 23.06 | C41H70O14 | - | 785.4704 (+1.5) | 809.4659 [M + Na]+ (+0.2) | 623.4223 [M − H − glc], 491.3752 [M − H − glc − xyl]−, 415.3217, 616.0448 | Cyclocanthoside E | + | - | 3 h |
| 44 | 24.03 | C47H78O19 | 947.5216 (+0.6) | 945.5116 (+5.5) | 965.5039 [M + Na]+ (+1.0) | 783.4630 [M − H − glc],765.4541, 651.4267, 489.3623, 179.0557, 161.0444 | Astragaloside VII | + | - | 0.25–12 h |
| 45 | 24.41 | C41H68O14 | 785.4682 (+0) | 783.4569 (+4.2) | 807.4089 [M + Na]+ (+0.9) | 621.4019, 489.3605, 383.2991, 161.0453 | Astragaloside IV a | + | - | 0.25–24 h |
| 46 | 24.63 | C43H70O15 | 827.4795 (+1.0) | 825.4669 (3.3) | 849.4615 [M + Na]+ (+1.0), 665.4299,647.4172, 473.3602, 455.3547, 437.3413, 175.0601 | - | Astragaloside II | + | - | 0.25−0.5 h, 6 h |
| 47 | 24.89 | C48H78O18 | 943.5254 (−0.7) | 941.5163 (+5.1) | 965.5072 [M + Na]+ (−0.8), 797.4635, 635.4105, 617.4020, 599.3916, 441.3704, 423.3577, 405.3532, 309.1134, 203.1766 | 923.5120, 733.4538, 615.4003, 457.3725, 247.0823, 205.7828 | Soyasaponin I | + | - | 0.25–24 h |
| 48 | 24.97 | C47H76O17 | - | 911.5032 (+2.5) | 935.4967 [M + Na]+ (−0.8), 477.115 | 703.4545, 615.3994, 571.4053, 457.3800, 247.0808, 205.0718 | Astragaloside VIII | + | - | 0.25–24 h |
| 49 | 25.71 | C45H72O16 | 869.4898 (+0.6) | 867.4785 (+4.4) | 831.4571, 711.4038, 651.3955, 591.3675, 447.3377 | 765.4573 | Astragaloside I | + | - | 0.25−0.5 h |
| 50 | 26.01 | C45H72O16 | 869.4887 (−0.6) | 867.4756 (+1.0) | 711.4037, 477.3364 | 807.4652, 765.4568, 179.0567 | Isoastragaloside I | + | - | 3 h |
| 51 | 27.35 | C47H74O17 | 911.4998 (+0) | 909.4888 (+3.8) | 875.4590, 753.4225, 731.4360, 477.3428, 437.3368, 259.0854, | 867.4787, 807.4666, 747.4616 | Acetyl astragaloside I | + | - | 8 h |
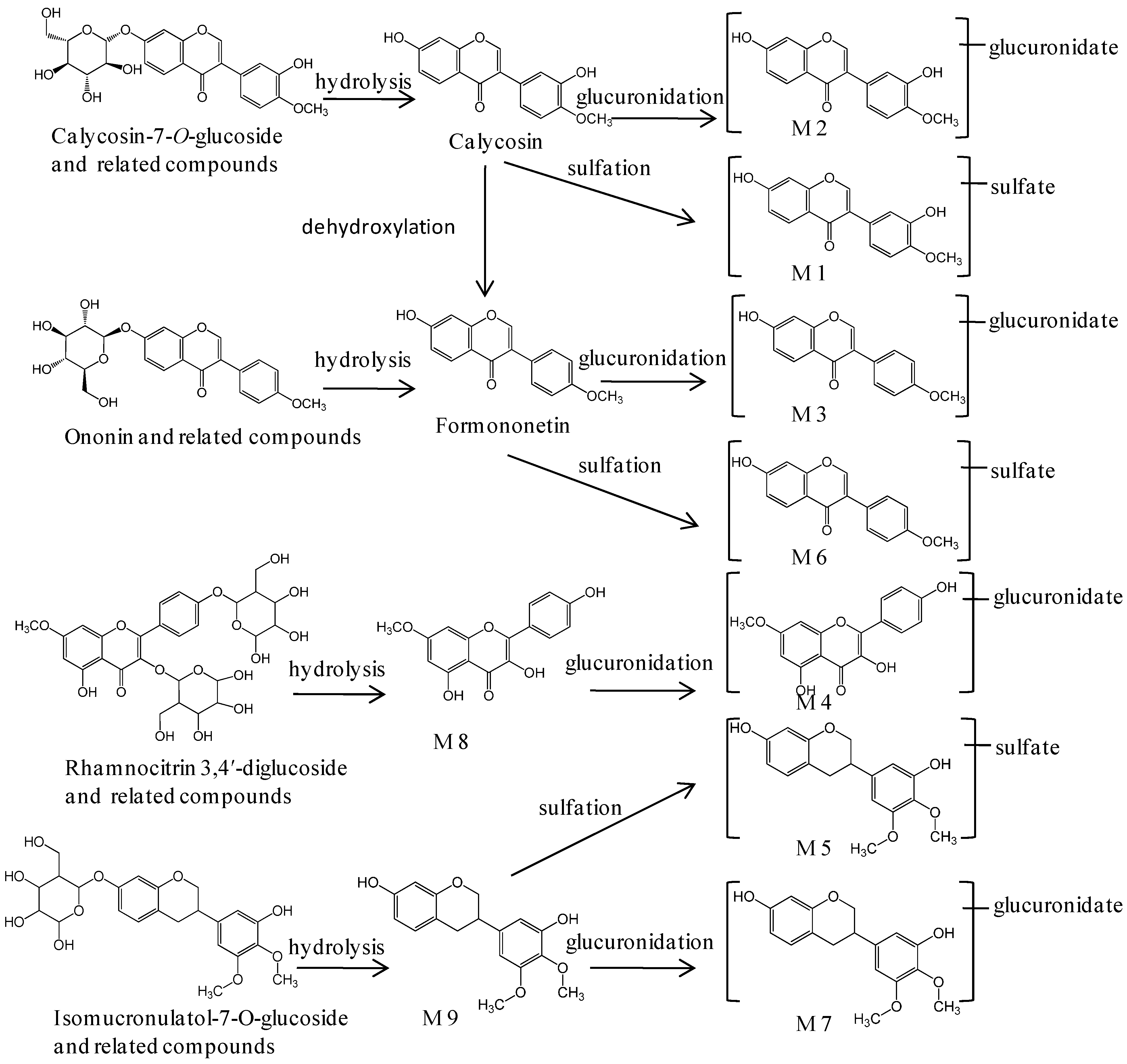
| M1 | 14.34 | C16 H12O8S | 365.0323 (−0.5) | 363.0177 (−0.8) | 285.0782, 270.0522, 225.0537, 137.0254 | 283.0609, 268.0375, 211.0407, 148.0174 | Sulfated calycosin | - | - | 0.25–8 h |
| M2 | 14.82 | C22H20O11 | 461.1081 (+0.7) | 459.0928 (−0.9) | 285.0766, 270.0534, 225.0541, 137.0230 | 283.0617, 268.0385, 239.0346, 211.0408, 175.0255, 117.0191, 113.0254, 85.0320 | Glucuronidated calycosin | - | - | 0.25–12 h |
| M3 | 15.41 | C22H20O10 | 445.1133 (+1.0) | 443.0979 (−0.9) | 269.0817, 253.0486, 237.0516, 213.0904, 181.0650 | 267.0665, 252.0441, 175.0243, 113.0249 | Glucuronidated formononetin | - | - | 0.25–8 h |
| M4 | 16.09 | C22H20O12 | 477.1027 (−0.1) | 475.0883 (+0.3) | 301.0761, 286.0475, 167.0661 | 299.0583, 284.0328, 255.0291, 227.0344, 175.0242, 151.0391, 113.0261 | Glucuronidated rhamnocitrin | - | - | 0.25–8 h |
| M5 | 16.28 | C17H18O8S | 383.0798 (+0.9) | 381.0649 (+0.1) | - | 301.1082, 286.0827, 271.0624, 256.0363, 135.0450, 109.0312 | Sulfated isomucronulatol | - | - | 0.25–8 h |
| M6 | 16.85 | C16H12O7S | 349.0378 (+0.6) | 347.0228 (−0.6) | 269.0792, 253.0507, 213.0941, 181.0629 | 267.0667, 252.0429, 223.0390 | Sulfated formononetin | - | - | 0.25–8 h |
| M7 | 17.27 | C23H26O11 | 479.1544 (−0.7) | 477.1396 (−1.2) | 303.1219, 167.0707 | 301.1085, 286.0855, 271.0615, 256.0370, 175.0252, 135.0449, 113.0254 | Glucuronidated isomucronulatol | - | - | 0.25–12 h |
| M8 | 18.45 | C16H12O6 | 301.0709 (+0.8) | 299.0561 (+0.3) | - | 284.0305, 255.0312, 227.0353, 183.0432, 135.0085 | Rhamnocitrin | - | - | 0.25–8 h |
| M9 | 19.63 | C17H18O5 | 303.1229 (+0.7) | 301.1084 (+1.0) | 181.0841, 167.0688, 135.0339, 123.0440 | 286.0836, 271.0599, 256.0377, 135.0445, 121.0245, 109.0340 | Isomucronulatol | - | - | 0.25–8 h |
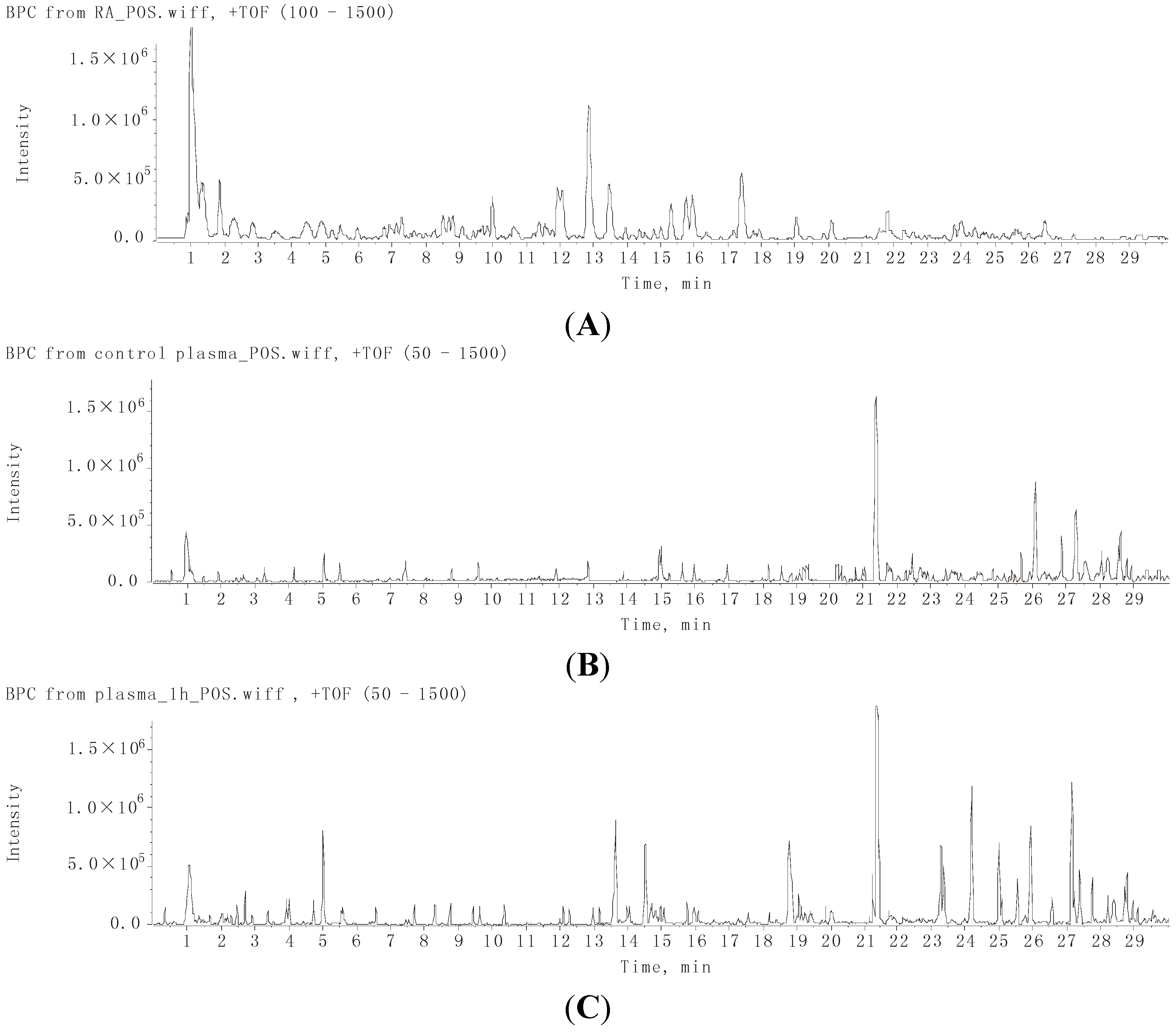

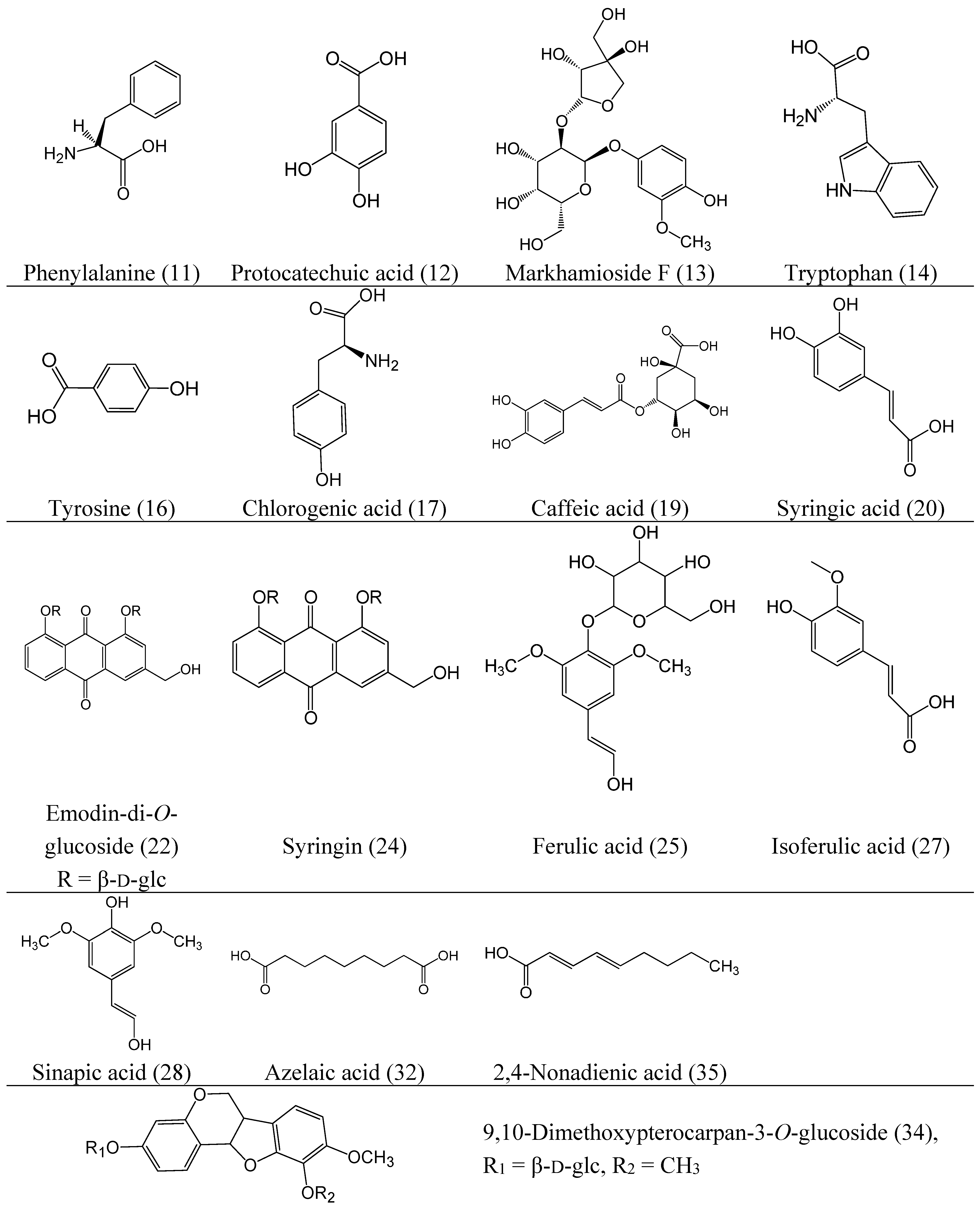
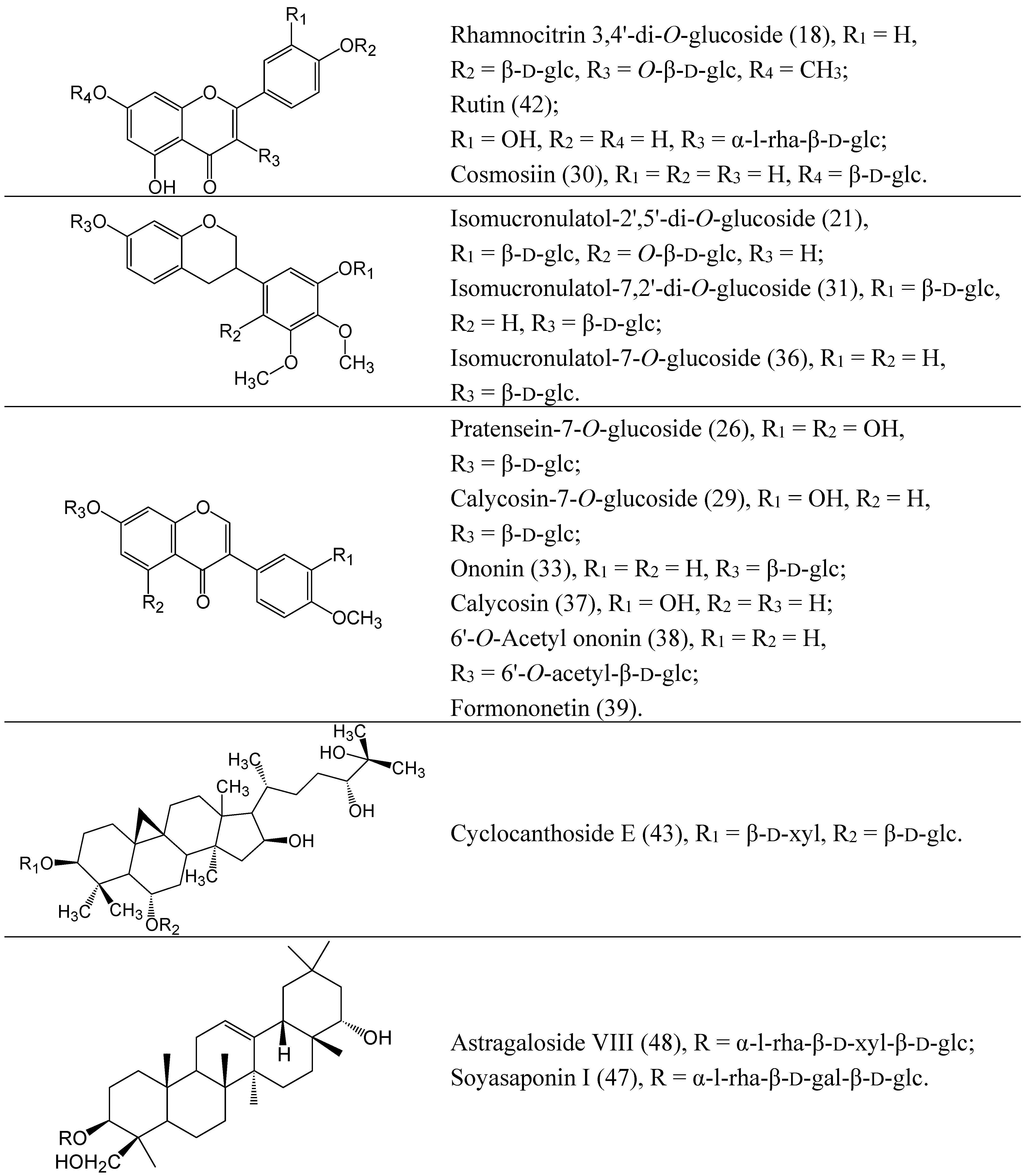
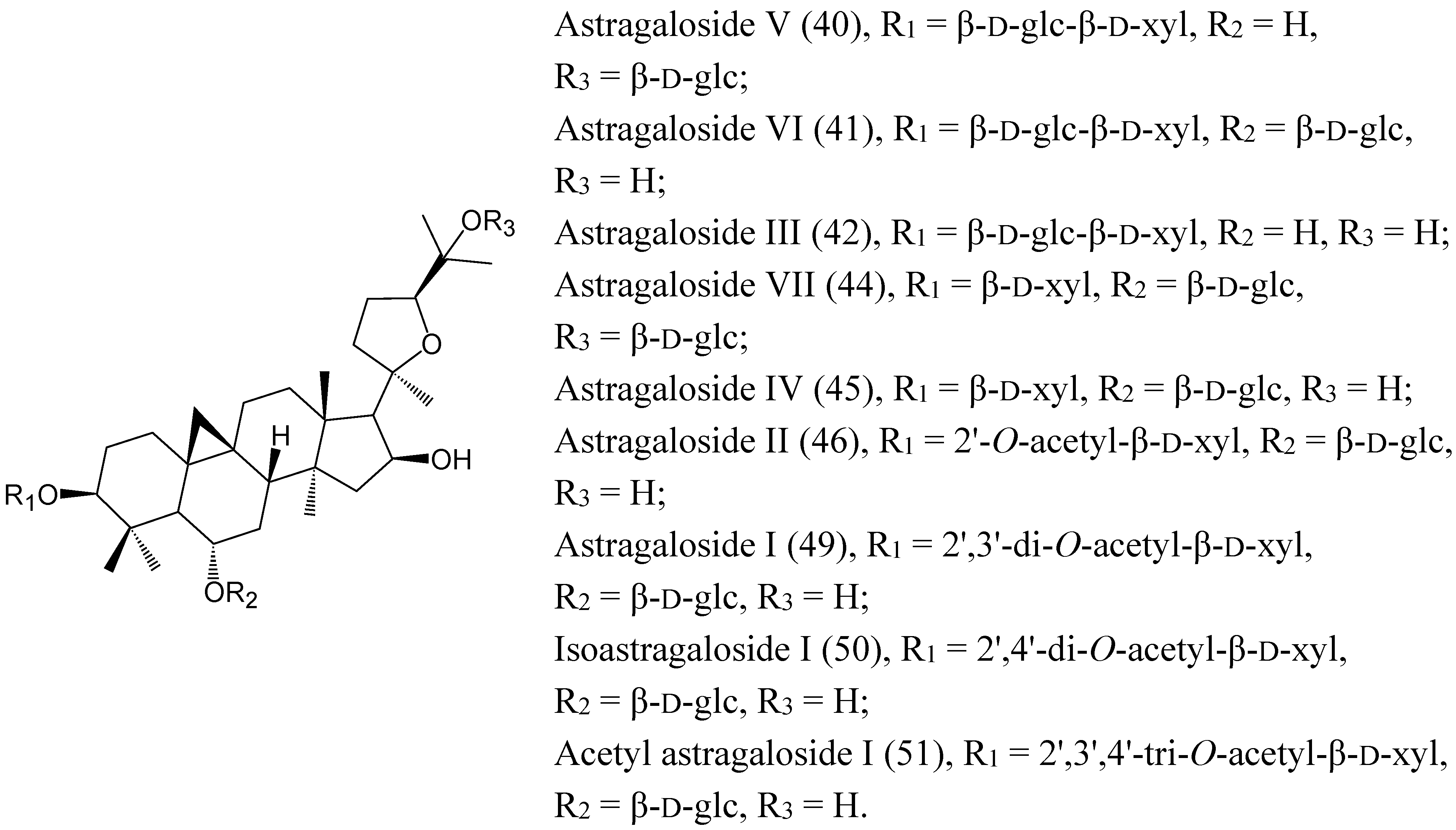
2.2. Characterization of the Absorbed Constituents in Mice Plasma
2.3. Identification of Metabolites in Mice Plasma
2.4. Pharmacokinetics of Calycosin-7-O-glucoside, Ononin, Calycosin, Formononetin and Astragaloside IV in Mice Plasma
2.4.1. Assay Validation
| Compounds | Linear Range (ng/mL) | Linearity | r | LOD (ng/mL) | LOQ (ng/mL) | Intra-Day RSD (n = 6) | Inter-Day RSD (n = 3) | Accuracy | |
|---|---|---|---|---|---|---|---|---|---|
| Mean (%) | RSD (%) | ||||||||
| Calycosin-7-O-glucoside | 4.48–448.00 | y = 0.7300x − 0.0017 | 0.9982 | 0.64 | 2.28 | 1.28 | 3.20 | 95.21 | 1.63 |
| Ononin | 3.40–340.00 | y = 0.2847x + 0.0009 | 0.9994 | 0.52 | 1.70 | 2.16 | 3.11 | 97.03 | 2.18 |
| Calycosin | 4.47–447.00 | y = 0.7951x − 0.0047 | 0.9987 | 0.84 | 2.24 | 1.22 | 2.95 | 104.58 | 2.56 |
| Formononetin | 5.52–552.00 | y = 0.3547x + 0.0021 | 0.9949 | 0.68 | 2.76 | 2.77 | 3.64 | 102.21 | 1.40 |
| Astragaloside IV | 8.98–898.00 | y = 0.8558x + 0.0031 | 0.9923 | 1.28 | 4.49 | 3.84 | 4.37 | 98.48 | 2.87 |
2.4.2. Pharmacokinetics Study
| Compounds | Parameter | ||||
|---|---|---|---|---|---|
| AUC(0–t) (ng/mL∙h) | AUC(0–∞) (ng/mL∙h) | t1/2z (h) | tmax (h) | Cmax (ng/mL) | |
| Calycosin-7-O-glucoside | 108.16 ± 37.14 | 127.17 ± 40.31 | 2.61 ± 0.67 | 1.5 | 33.41 |
| Ononin | 227.84 ± 59.69 | 239.24 ± 47.38 | 1.96 ± 0.34 | 1.5 | 51.38 |
| Calycosin | 135.88 ± 41.41 | 154.87 ± 37.69 | 2.21 ± 0.92 | 1.5 | 32.98 |
| Formononetin | 233.38 ± 47.83 | 256.42 ± 45.68 | 3.99 ± 1.03 | 2.0 | 47.93 |
| Astragaloside IV | 695.37 ± 178.57 | 722.49 ± 196.52 | 3.48 ± 1.15 | 1.5 | 128.95 |
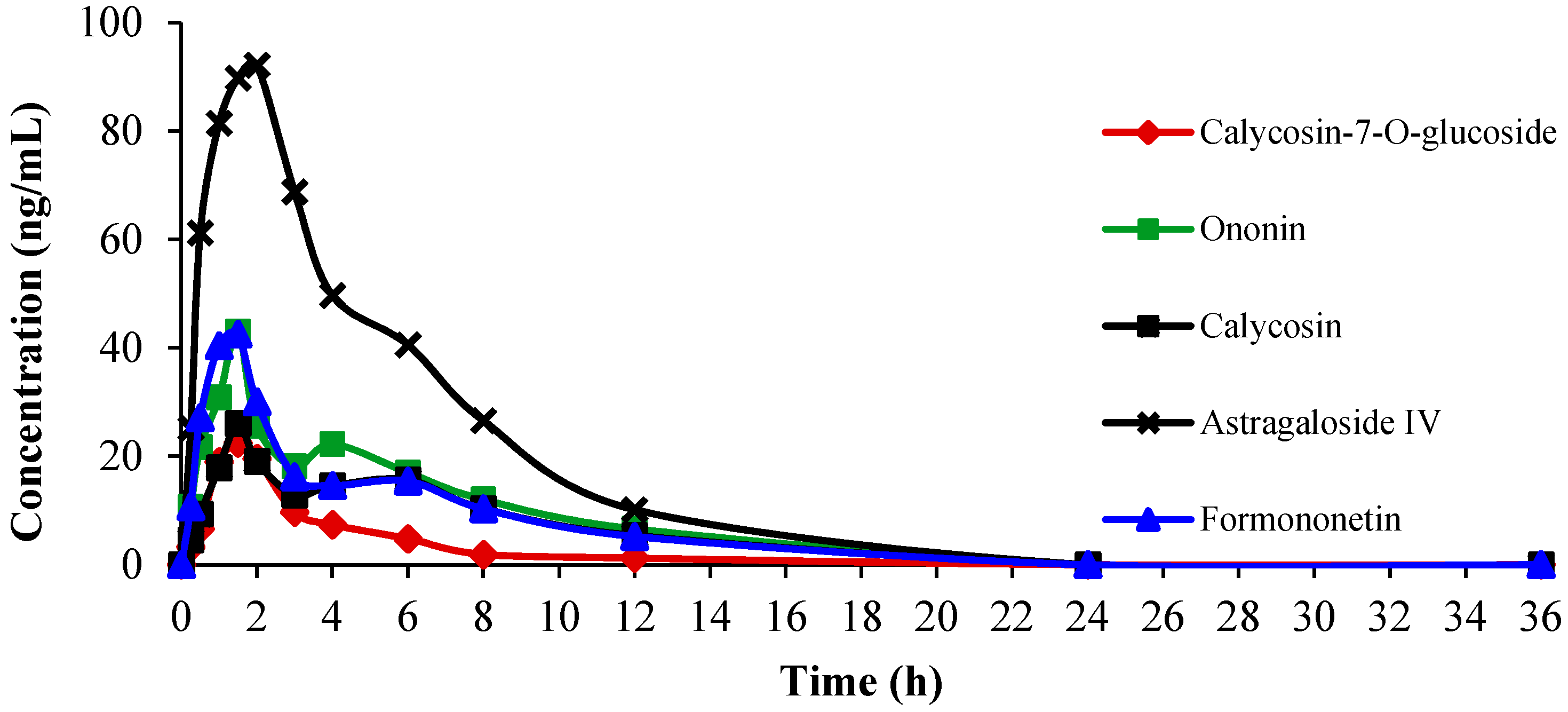
2.5. Discussion
3. Experimental Section
3.1. Chemicals and Reagents
3.2. Plant Material and Sample Preparation
3.3. Animals and Experiment Design
3.4. Preparation of Plasma Samples
3.5. Bioanalytical Methods
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Rasmussen, L.; Arvin, A. Chemotherapy-induced immunosuppression. Environ. Health Perspect. 1982, 43, 21–25. [Google Scholar] [CrossRef] [PubMed]
- Harris, J.; Sengar, D.; Stewart, T.; Hyslop, D. The effect of immunosuppressive chemotherapy on immune function in patients with malignant disease. Cancer 1976, 37, 1058–1069. [Google Scholar] [CrossRef] [PubMed]
- Chinese Pharmacopoeia Commission of PR China. Pharmacopoeia of PR China (2010 Edition); People’s Health Publishing House: Beijing, China, 2010; Volume I, p. 283. [Google Scholar]
- The Committee on Japanese Pharmacopoeia. The Japanese Pharmacopoeia, 16th ed.; The Minister of Health, Labour and Welfare: Tokyo, Japan, 2011; p. 1604. [Google Scholar]
- Korea Food and Drug Administration. The Korean Herbal Pharmacopoeia, 10th ed.; Ministry of Food and Drug Safety: Seoul, Korea, 2002; p. 1013. [Google Scholar]
- Wang, X.; Li, Y.; Yang, X.; Yao, J. Astragalus polysaccharide reduces inflammatory response by decreasing permeability of LPS-infected Caco2 cells. Int. J Biol. Macromol. 2013, 61, 347–352. [Google Scholar] [CrossRef] [PubMed]
- Huang, X.; Tang, L.; Wang, F.; Song, G. Astragaloside IV attenuates allergic inflammation by regulation Th1/Th2 cytokine and enhancement CD4+CD25+Foxp3 T cells in ovalbumin-induced asthma. Immunobiology 2014, 219, 565–571. [Google Scholar] [CrossRef] [PubMed]
- Wang, F.; Zhao, S.C.; Li, F.; Zhang, B.; Yi, Q.; Sun, T.L.; Luo, T.; Li, D.P. Investigation of antioxidant interactions between Radix Astragali and Cimicifuga foetida and identification of synergistic antioxidant compounds. PLoS One 2014, 9, e87221. [Google Scholar] [CrossRef] [PubMed]
- Su, Z.Y.; Hwang, S.L.; Chiang, B.H.; Sheen, L.Y. Antihepatoma and liver protective potentials of ganoderma lucidum (ling zhi) fermented in a medium containing black soybean (hei dou) and astragalus membranaceus (sheng huang qi). J. Tradit. Complement. Med. 2013, 3, 110–118. [Google Scholar] [CrossRef] [PubMed]
- Zhu, C.; Huang, S.; Ding, G.; Yuan, Y.; Chen, Q.; Pan, X.; Chen, R.; Zhang, A. Protective effects of Huang Qi Huai granules on adriamycin nephrosis in rats. Pediatr. Nephrol. 2011, 26, 905–913. [Google Scholar] [CrossRef] [PubMed]
- Guo, C.; Tong, L.; Xi, M.; Yang, H.; Dong, H.; Wen, A. Neuroprotective effect of calycosin on cerebral ischemia and reperfusion injury in rats. J. Ethnopharmacol. 2012, 144, 768–774. [Google Scholar] [CrossRef] [PubMed]
- Huang, G.C.; Wu, L.S.; Chen, L.G.; Yang, L.L.; Wang, C.C. Immuno-enhancement effects of Huang Qi Liu Yi Tang in a murine model of cyclophosphamide-induced leucopenia. J. Ethnopharmacol. 2007, 109, 229–235. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.X.; Tong, X.; Li, P.B.; Cao, H.; Su, W.W. Immuno-enhancement effects of Shenqi Fuzheng Injection on cyclophosphamide-induced immunosuppression in Balb/c mice. J. Ethnopharmacol. 2012, 139, 88–795. [Google Scholar]
- Li, J.; Bao, Y.; Lam, W.; Li, W.; Lu, F.; Zhu, X.; Liu, J.; Wang, H. Immunoregulatory and anti-tumor effects of polysaccharopeptide and Astragalus polysaccharides on tumor-bearing mice. Immunopharmacol. Immunotoxicol. 2008, 30, 771–782. [Google Scholar] [CrossRef] [PubMed]
- Zhang, R.P.; Zhang, X.P.; Ruan, Y.F.; Ye, S.Y.; Zhao, H.C.; Cheng, Q.H.; Wu, D.J. Protective effect of Radix Astragali injection on immune organs of rats with obstructive jaundice and its mechanism. World J. Gastroenterol. 2009, 15, 2862–2869. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.F.; He, H.G.; Zhang, J.P.; Shi, L.Y. Effects of Astragalus on the Cyclophosphamide-induced immune compromised mice. J. Zhejiang Univ. Tradit. Chin. Med. 2012, 6, 749–751. [Google Scholar]
- Fu, J.; Wang, Z.; Huang, L.; Zheng, S.; Wang, D.; Chen, S.; Zhang, H.; Yang, S. Review of the botanical characteristics, phytochemistry, and pharmacology of Astragalus membranaceus (Huangqi). Phytother. Res. 2014, 28, 1275–1283. [Google Scholar] [CrossRef] [PubMed]
- Liu, M.H.; Tong, X.; Wang, J.X.; Zou, W.; Cao, H.; Su, W.W. Rapid separation and identification of multiple constituents in traditional Chinese medicine formula Shenqi Fuzheng injection by ultra-fast liquid chromatography combined with quadrupole-time-of-flight mass spectrometry. J. Pharm. Biomed. Anal. 2013, 74, 141–155. [Google Scholar] [CrossRef] [PubMed]
- <em>Liu, X.H.; Zhao, L.G.; Liang, J.; Guo, L.; Yang, Y.L.; Hu, F.; Zhu, R.J.; Feng, S.L. Component analysis and structure identification of active substances for anti-gastric ulcer effects in Radix Astragali by liquid chromatography and tandem mass spectrometry. J. Chromatogr. B Anal. Technol. Biomed. Life Sci. 2014, 43, 43–51. [Google Scholar] [CrossRef]
- Wang, J.X.; Liu, M.H.; Tong, X.; Peng, W.; Cao, H.; Su, W.W. Chromatographic fingerprint analysis and characterization of constituents in Shenqi Fuzheng injection by HPLC-DAD-ELSD and UFLC-DAD-Q-TOF tandem mass spectrometry techniques. Acta Chromatogr. 2014. [Google Scholar] [CrossRef]
- Yang, L.P.; Shen, J.G.; Xu, W.C.; Li, J.; Jiang, J.Q. Secondary metabolites of the genus Astragalus: Structure and biological-activity update. Chem. Biodivers. 2013, 10, 1004–1054. [Google Scholar] [CrossRef] [PubMed]
- Liang, J.; Xu, F.; Zhang, Y.Z.; Huang, S.; Zang, X.Y.; Zhao, X.; Zhang, L.; Shang, M.Y.; Yang, D.H.; Wang, X.; et al. The profiling and identification of the absorbed constituents and metabolites of Paeoniae Radix Rubra decoction in rat plasma and urine by the HPLC-DAD-ESI-IT-TOF-MS(n) technique: A novel strategy for the systematic screening and identification of absorbed constituents and metabolites from traditional Chinese medicines. J. Pharm. Biomed. Anal. 2013, 83, 108–121. [Google Scholar] [CrossRef] [PubMed]
- Silveman, R.B. Drug Metabolism. In The Organic Chemistry of Drug Design and Drug Action, 3rd ed.; Silveman, R.B., Holladay, M.W., Eds.; Academic Press: Salt Lake, UT, USA, 2014; pp. 357–422. [Google Scholar]
- Crozier, A.; del Rio, D.; Clifford, M.N. Bioavailability of dietary flavonoids and phenolic compounds. Mol. Aspects. Med. 2010, 31, 446–467. [Google Scholar] [CrossRef] [PubMed]
- Serra, A.; Macià, A.; Romero, M.P.; Reguant, J.; Ortega, N.; Motilva, M.J. Metabolic pathways of the colonic metabolism of flavonoids (flavonols, flavones and flavanones) and phenolic acids. Food Chem. 2012, 383–393. [Google Scholar] [PubMed]
- Tang, D.Q.; Li, Z.; Jiang, X.L.; Li, Y.J.; Du, Q.; Yang, D.Z. Fingerprint analysis and multi-ingredient quantitative analysis for quality evaluation of Xiaoyanlidan tablets by ultra high performance liquid chromatography with diode array detection. J. Sep. Sci. 2014, 37, 2131–2137. [Google Scholar] [CrossRef]
- Chen, X.Z.; Cao, Z.Y.; Liao, L.M.; Liu, Z.Z.; Du, J. Application of serum pharmacology in evaluating the antitumor effect of Fuzheng Yiliu decoction from Chinese medicine. Chin. J. Integr. Med. 2014, 20, 450–455. [Google Scholar] [CrossRef]
- He, X.Y.; Liu, Q.C.; Peng, W.; Huang, Y.L.; Wu, C.J. Bioactivities and serum pharmacochemistry of Qi-Wei-Xiao-Yan-Tang. Pharm. Biol. 2013, 51, 629–634. [Google Scholar] [CrossRef]
- Zheng, K.Y.; Choi, R.C.; Cheung, A.W.; Guo, A.J.; Bi, C.W.; Zhu, K.Y.; Fu, Q.; Du, Y.; Zhang, W.L.; Zhan, J.Y.; et al. Flavonoids from Radix Astragali induce the expression of erythropoietin in cultured cells: A signaling mediated via the accumulation of hypoxia-inducible factor-1α. J. Agric. Food Chem. 2011, 59, 1697–1704. [Google Scholar]
- Tang, D.; He, B.; Zheng, Z.G.; Wang, R.S.; Gu, F.; Duan, T.T.; Cheng, H.Q.; Zhu, Q. Inhibitory effects of two major isoflavonoids in Radix Astragali on high glucose-induced mesangial cells proliferation and AGEs-induced endothelial cells apoptosis. Planta Med. 2011, 77, 729–732. [Google Scholar] [CrossRef]
- Tian, J.; Duan, Y.X.; Bei, C.Y.; Chen, J. Calycosin induces apoptosis by upregulation of RASD1 in human breast cancer cells MCF-7. Horm. Metab. Res. 2013, 45, 593–598. [Google Scholar]
- Wang, L.; Chi, Y.F.; Yuan, Z.T.; Zhou, W.C.; Yin, P.H.; Zhang, X.M.; Peng, W.; Cai, H. Astragaloside IV inhibits renal tubulointerstitial fibrosis by blocking TGF-β/Smad signaling pathway in vivo and in vitro. Exp. Biol. Med. 2014, 239, 1310–1324. [Google Scholar] [CrossRef]
- Sun, L.; Li, W.; Li, W.; Xiong, L.; Li, G.; Ma, R. Astragaloside IV prevents damage to human mesangial cells through the inhibition of the NADPH oxidase/ROS/Akt/NF-κB pathway under high glucose conditions. Int. J. Mol. Med. 2014, 34, 167–176. [Google Scholar]
- Yuan, X.; Sun, S.; Wang, S.; Sun, Y. Effects of astragaloside IV on IFN-γ level and prolonged airway dysfunction in a murine model of chronic asthma. Planta Med. 2011, 77, 328–333. [Google Scholar] [CrossRef]
- Wang, X.; Zhao, X.; Gu, L.; Lv, C.; He, B.; Liu, Z.; Hou, P.; Bi, K.; Chen, X. Simultaneous determination of five free and total flavonoids in rat plasma by ultra HPLC-MS/MS and its application to a comparative pharmacokinetic study in normal and hyperlipidemic rats. J. Chromatogr. B Anal. Technol. Biomed. Life Sci. 2014, 953, 1–10. [Google Scholar] [CrossRef]
- Liu, X.H.; Zhao, J.B.; Guo, L.; Yang, Y.L.; Hu, F.; Zhu, R.J.; Feng, S.L. Simultaneous determination of calycosin-7-O-β-d-glucoside, ononin, calycosin, formononetin, astragaloside IV, and astragaloside II in rat plasma after oral administration of Radix Astragali extraction for their pharmacokinetic studies by ultra-pressure liquid chromatography with tandem mass spectrometry. Cell Biochem. Biophys. 2014, 70, 677–686. [Google Scholar] [CrossRef] [PubMed]
- Shaw, L.H.; Lin, L.C.; Tsai, T.H. HPLC-MS/MS analysis of a traditional Chinese medical formulation of Bu-Yang-Huan-Wu-Tang and its pharmacokinetics after oral administration to rats. PLoS One 2012, 7, e43848. [Google Scholar] [CrossRef] [PubMed]
- Wu, B.; Basu, S.; Meng, S.; Wang, X.; Hu, M. Regioselective sulfation and glucuronidation of phenolics: Insights into the structural basis. Curr. Drug Metable 2011, 12, 900–916. [Google Scholar] [CrossRef]
- Tang, L.; Zhou, J.; Yang, C.H.; Xia, B.J.; Hu, M.; Liu, Z.Q. Systematic studies of sulfation and glucuronidation of 12 flavonoids in the mouse liver S9 fraction reveal both unique and shared positional preferences. J. Agric. Food Chem. 2012, 60, 3223–3233. [Google Scholar] [CrossRef]
- ICH. Q2(R1): Validation of analytical procedures: Text and methodology. 1994. Available online: http://www.ich.org/products/guidelines/quality/article/quality-guidelines.html (accessed on 2 March 2015).
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, M.; Li, P.; Zeng, X.; Wu, H.; Su, W.; He, J. Identification and Pharmacokinetics of Multiple Potential Bioactive Constituents after Oral Administration of Radix Astragali on Cyclophosphamide-Induced Immunosuppression in Balb/c Mice. Int. J. Mol. Sci. 2015, 16, 5047-5071. https://doi.org/10.3390/ijms16035047
Liu M, Li P, Zeng X, Wu H, Su W, He J. Identification and Pharmacokinetics of Multiple Potential Bioactive Constituents after Oral Administration of Radix Astragali on Cyclophosphamide-Induced Immunosuppression in Balb/c Mice. International Journal of Molecular Sciences. 2015; 16(3):5047-5071. https://doi.org/10.3390/ijms16035047
Chicago/Turabian StyleLiu, Menghua, Panlin Li, Xuan Zeng, Huanxian Wu, Weiwei Su, and Jingyu He. 2015. "Identification and Pharmacokinetics of Multiple Potential Bioactive Constituents after Oral Administration of Radix Astragali on Cyclophosphamide-Induced Immunosuppression in Balb/c Mice" International Journal of Molecular Sciences 16, no. 3: 5047-5071. https://doi.org/10.3390/ijms16035047
APA StyleLiu, M., Li, P., Zeng, X., Wu, H., Su, W., & He, J. (2015). Identification and Pharmacokinetics of Multiple Potential Bioactive Constituents after Oral Administration of Radix Astragali on Cyclophosphamide-Induced Immunosuppression in Balb/c Mice. International Journal of Molecular Sciences, 16(3), 5047-5071. https://doi.org/10.3390/ijms16035047






