Transcriptome MicroRNA Profiling of Bovine Mammary Glands Infected with Staphylococcus aureus
Abstract
:1. Introduction
2. Results
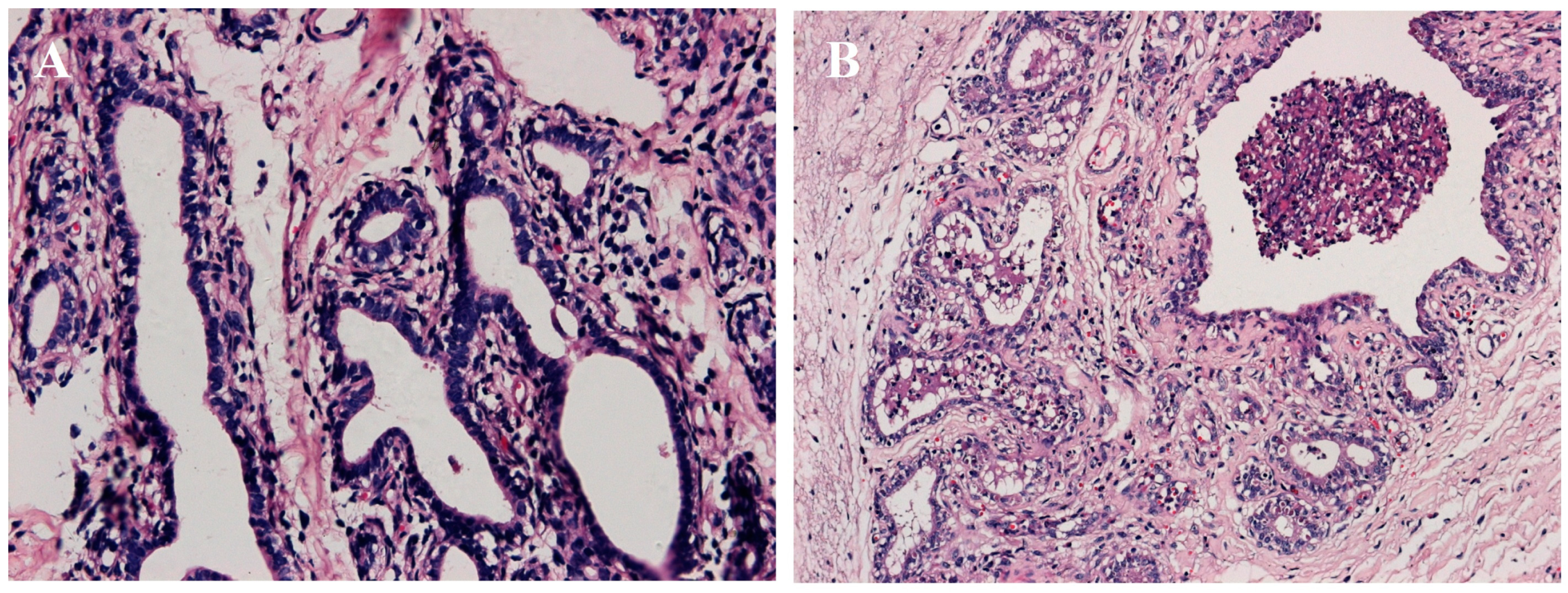
2.1. Establishment of Mastitis Model
2.2. Standard Bioinformatics Analyses
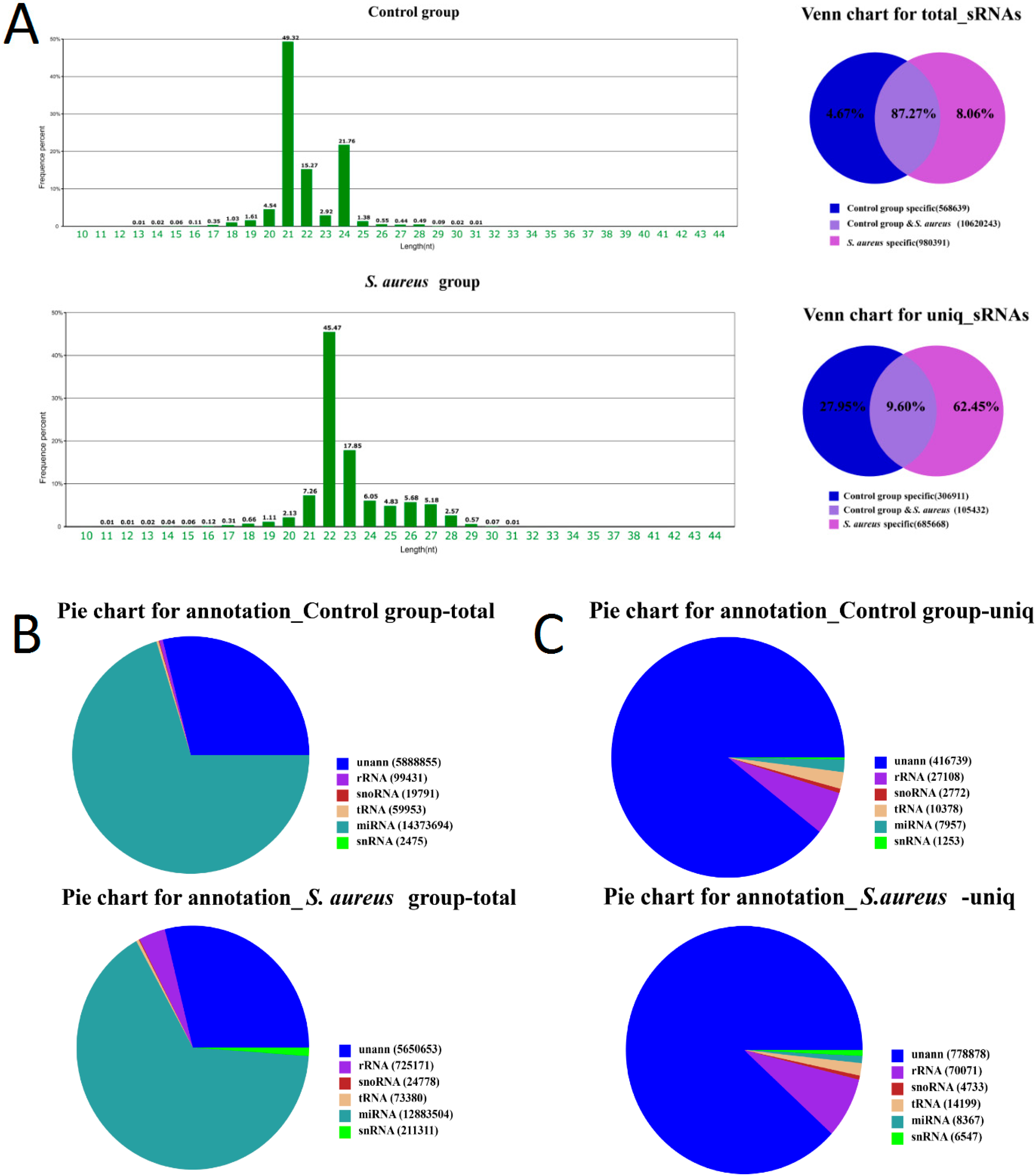
2.3. Characteristics of Known miRNAs
2.4. Novel miRNA Predictions
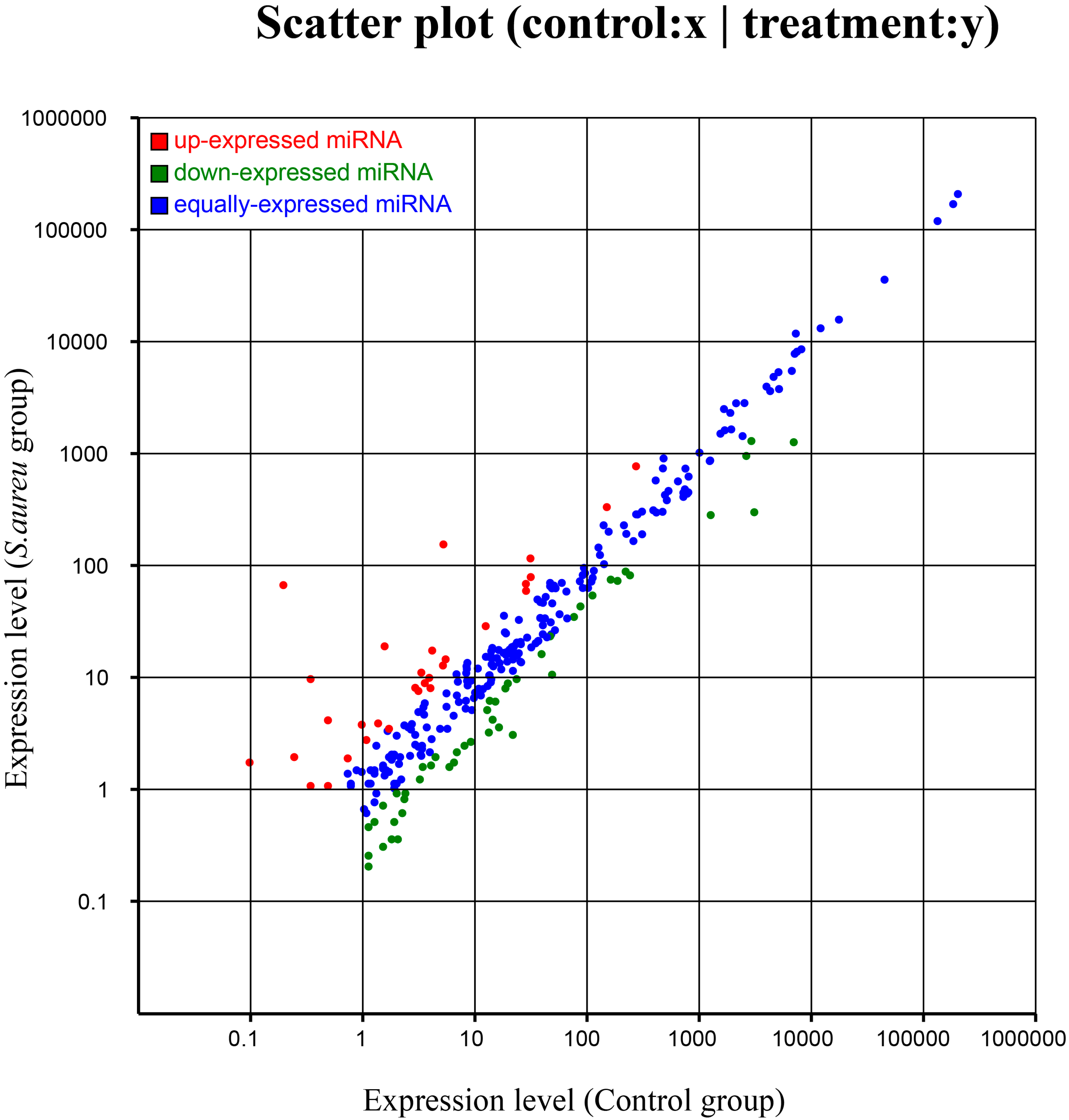
2.5. Differential Expression of miRNAs
| miR-Name | A1B1C1-std | A2B3C4-std | Fold-Change (log2 A2B3C4/A1B1C1) | p-Value | Expression Level | Significance Level |
|---|---|---|---|---|---|---|
| bta-miR-1246 | 0.1957 | 66.79 | 8.4148 | 0.00 | up | ** |
| bta-miR-223 | 5.2338 | 154.28 | 4.8815 | 0.00 | up | ** |
| bta-miR-184 | 0.3424 | 9.6582 | 4.818 | 3.68 × 10−49 | up | ** |
| bta-miR-2313-3p | 0.0978 | 1.7375 | 4.151 | 4.92 × 10−9 | up | ** |
| bta-miR-1298 | 1.5652 | 18.959 | 3.5984 | 7.68 × 10−78 | up | ** |
| bta-miR-2887 | 0.4891 | 4.1392 | 3.0812 | 6.65 × 10−16 | up | ** |
| bta-miR-1940 | 0.2446 | 1.9419 | 2.989 | 6.57 × 10−8 | up | ** |
| bta-miR-877 | 4.1577 | 17.375 | 2.0631 | 7.00 × 10−40 | up | ** |
| bta-miR-2484 | 0.9783 | 3.7815 | 1.9506 | 3.37 × 10−9 | up | ** |
| bta-miR-451 | 31.256 | 115.64 | 1.8875 | 3.51 × 10−227 | up | ** |
| bta-miR-21-3p | 3.3261 | 11.038 | 1.7306 | 9.25 × 10−21 | up | ** |
| bta-miR-2422 | 0.3424 | 1.0731 | 1.648 | 5.72 × 10−3 | up | ** |
| bta-miR-3141 | 1.3696 | 3.8837 | 1.5037 | 6.05 × 10−7 | up | ** |
| bta-miR-142-5p | 273.33 | 769.54 | 1.4934 | 0.00 | up | ** |
| bta-miR-142-3p | 2.9348 | 8.0741 | 1.46 | 1.69 × 10−12 | up | ** |
| bta-miR-339b | 5.4783 | 14.513 | 1.4055 | 3.42 × 10−20 | up | ** |
| bta-miR-2468 | 0.7337 | 1.8908 | 1.3657 | 1.28 × 10−3 | up | ** |
| bta-miR-138 | 1.0761 | 2.7595 | 1.3586 | 1.01 × 10−4 | up | ** |
| bta-miR-2904 | 3.9131 | 9.9137 | 1.3411 | 2.21 × 10−13 | up | ** |
| bta-miR-486 | 31.452 | 78.799 | 1.325 | 1.29 × 10−93 | up | ** |
| bta-miR-130b | 3.5707 | 8.8917 | 1.3163 | 7.60 × 10−12 | up | ** |
| bta-miR-2284w | 5.1848 | 12.775 | 1.301 | 4.04 × 10−16 | up | ** |
| bta-miR-425-3p | 3.1305 | 7.5631 | 1.2726 | 7.85 × 10−10 | up | ** |
| bta-miR-2284ab | 28.468 | 68.476 | 1.2663 | 3.65 × 10−76 | up | ** |
| bta-miR-2284z | 12.473 | 28.719 | 1.2032 | 1.58 × 10−30 | up | ** |
| bta-miR-132 | 149.92 | 332.26 | 1.1481 | 3.10 × 10−310 | up | ** |
| bta-miR-2332 | 0.4891 | 1.0731 | 1.1336 | 3.73 × 10−2 | up | * |
| bta-miR-6529 | 28.566 | 59.329 | 1.0545 | 7.46 × 10−50 | up | ** |
| bta-miR-935 | 1.7120 | 3.4749 | 1.0213 | 4.95 × 10−4 | up | ** |
| bta-miR-2284aa | 4.0109 | 8.023 | 1.0002 | 1.82 × 10−7 | up | ** |
| bta-miR-99a-3p | 46.859 | 23.302 | −1.0079 | 7.89 × 10−37 | down | ** |
| bta-miR-365-3p | 87.262 | 43.079 | −1.0184 | 3.29 × 10−68 | down | ** |
| bta-miR-200b | 111.72 | 53.964 | −1.0498 | 3.77 × 10−91 | down | ** |
| bta-miR-455-5p | 1.5163 | 0.7154 | −1.0837 | 1.71 × 10−2 | down | * |
| bta-miR-1249 | 3.424 | 1.5842 | −1.1119 | 2.25 × 10−4 | down | ** |
| bta-miR-139 | 162.54 | 74.762 | −1.1204 | 3.41 × 10−146 | down | ** |
| bta-miR-2431-3p | 2.0055 | 0.9198 | −1.1246 | 4.58 × 10−3 | down | ** |
| bta-miR-133a | 13.549 | 6.1833 | −1.1317 | 7.30 × 10−14 | down | ** |
| bta-miR-196a | 76.452 | 34.698 | −1.1397 | 1.19 × 10−71 | down | ** |
| bta-miR-664b | 19.663 | 8.8406 | −1.1533 | 5.39 × 10−20 | down | ** |
| bta-miR-1 | 2917.8 | 1294.4 | −1.1726 | 0.00 | down | ** |
| bta-miR-874 | 4.4511 | 1.9419 | −1.1967 | 7.91 × 10−6 | down | ** |
| bta-miR-503-5p | 18.685 | 7.9719 | −1.2289 | 6.26 × 10−21 | down | ** |
| bta-miR-424-5p | 39.278 | 16.148 | −1.2823 | 5.21 × 10−45 | down | ** |
| bta-miR-361 | 23.528 | 9.6582 | −1.2845 | 1.09 × 10−27 | down | ** |
| bta-miR-491 | 1.1250 | 0.4599 | −1.2905 | 1.90 × 10−2 | down | * |
| bta-miR-32 | 4.0598 | 1.6353 | −1.3119 | 4.61 × 10−6 | down | ** |
| bta-miR-450b | 1.2718 | 0.511 | −1.3155 | 1.12 × 10−2 | down | * |
| bta-miR-20a | 15.212 | 6.0811 | −1.3228 | 3.09 × 10−19 | down | ** |
| bta-miR-195 | 220.8 | 88.253 | −1.323 | 1.65 × 10−256 | down | ** |
| bta-miR-19b | 12.864 | 5.1102 | −1.3319 | 1.17 × 10−16 | down | ** |
| bta-miR-205 | 186.85 | 72.871 | −1.3585 | 2.62 × 10−226 | down | ** |
| bta-miR-450a | 2.3968 | 0.9198 | −1.3817 | 2.65 × 10−4 | down | ** |
| bta-miR-2285o | 3.2283 | 1.2264 | −1.3963 | 1.88 × 10−5 | down | ** |
| bta-miR-23a | 2628.1 | 952.28 | −1.4645 | 0.00 | down | ** |
| bta-miR-328 | 2.3479 | 0.8176 | −1.5219 | 1.05 × 10−4 | down | ** |
| bta-miR-31 | 241.39 | 81.712 | −1.5627 | 0.00 | down | ** |
| bta-miR-1388-3p | 6.8968 | 2.1463 | −1.6841 | 5.88 × 10−13 | down | ** |
| bta-miR-299 | 8.0707 | 2.4529 | −1.7182 | 2.62 × 10−15 | down | ** |
| bta-miR-150 | 14.381 | 4.1903 | −1.779 | 2.47 × 10−27 | down | ** |
| bta-miR-96 | 9.1958 | 2.6573 | −1.791 | 3.56 × 10−18 | down | ** |
| bta-miR-331-5p | 2.25 | 0.6132 | −1.8755 | 1.10 × 10−5 | down | ** |
| bta-miR-136 | 1.9076 | 0.511 | −1.9004 | 4.61 × 10−5 | down | ** |
| bta-miR-335 | 5.9185 | 1.5842 | −1.9015 | 4.13 × 10−13 | down | ** |
| bta-miR-1247-5p | 6.5055 | 1.7375 | −1.9046 | 2.66 × 10−14 | down | ** |
| bta-miR-196b | 13.305 | 3.2194 | −2.0471 | 2.51 × 10−30 | down | ** |
| bta-miR-24 | 1.125 | 0.2555 | −2.1385 | 8.30 × 10−4 | down | ** |
| bta-miR-23b-3p | 1265.7 | 282.13 | −2.1655 | 0.00 | down | ** |
| bta-miR-487b | 16.386 | 3.5771 | −2.1956 | 2.80 × 10−40 | down | ** |
| bta-miR-204 | 48.767 | 10.578 | −2.2048 | 3.91 × 10−117 | down | ** |
| bta-miR-135a | 1.5163 | 0.3066 | −2.3061 | 4.30 × 10−5 | down | ** |
| bta-miR-655 | 1.8098 | 0.3577 | −2.339 | 6.14 × 10−6 | down | ** |
| bta-miR-410 | 1.125 | 0.2044 | −2.4605 | 2.79 × 10−4 | down | ** |
| bta-miR-26a | 6964.3 | 1262.7 | −2.4634 | 0.00 | down | ** |
| bta-miR-378b | 2.0544 | 0.3577 | −2.5219 | 4.60 × 10−7 | down | ** |
| bta-miR-380-3p | 21.767 | 3.0661 | −2.8276 | 2.55 × 10−70 | down | ** |
| bta-miR-145 | 3096.8 | 299.05 | −3.3723 | 0.00 | down | ** |
2.6. Verification of miRNA Expression by Real Time PCR

2.7. Target Gene Prediction
| miRNA | Target Genes |
|---|---|
| miR-1246 | ATP2B4, MAP3K1, ADCK3, PSD2, SLC5A1 |
| miR-130b | EXOC3L1, TIE1, BAZ2B, C3, GRAMD1C |
| miR-145 | HSD3B7, SLCO4A1, PDIA4, ACADL, PTPN11, KRT9, RASSF6, RNF43, LAMC2 |
| miR-196a | ADAP1, GPR97, POMT1 |
| miR-200b | ARID3A, MLXIP, GPR110 |
| miR-205 | IL13RA2, COL5A2, ADM, CXCR2, XPO6, SPSB1, FMO5, PSMF1 |
| miR-31 | MEX3D, PFKFB3, ST3GAL3, IL2RB, ANKRD32, MGST1 |
| miR-184 | HSPA1L, SLC25A15, HEG1, MAPRE2, ACP6, SYNE2 |
| miR-223 | TMEM165 |
| miR-132 | IQCA1 |

| Pathway | Target Genes with Pathway Annotation (15970) | All Genes with This Type of Pathway Annotation (16078) | p-Value | Q-Value | Pathway ID |
|---|---|---|---|---|---|
| Olfactory transduction | 1051 (6.58%) | 1051 (6.54%) | 0.0006579 | 0.2033013 | Ko04740 |
| Pathways in cancer | 528 (3.31%) | 528 (3.28%) | 0.0268226 | 1.0000000 | Ko05200 |
| Focal adhesion | 425 (2.66%) | 425 (2.64%) | 0.0548559 | 1.0000000 | Ko04510 |
3. Discussion
4. Experimental Section
4.1. Animals
4.2. Construction of a Mastitis Model in Dairy Cows
4.3. Collection of Tissue Samples
4.4. Histologic Examination
4.5. RNA Sequencing
4.6. Data Analysis
4.7. Verification of miRNA Expression by qRT-PCR
| miRNA | Primer Sequences(5'–3') |
|---|---|
| bta-miR-223 | CCTGTCAGTTTGTCAAATACCCCA |
| bta-miR-31 | GGAAGGCAAGATGCTGGCA |
| bta-miR-205 | TCCTTCATTCCACCGGAGTCTG |
| bta-miR-145 | GTCCAGTTTTCCCAGGAATCCCT |
| bta-miR-132 | TAACAGTCTACAGCCATGGTCGAAA |
| bta-miR-130b | AGCAGGCAGTGCAATGATGA |
| bta-miR-200b | GCTGACGGTGCTAATACTGCCT |
| bta-miR-1246 | GAATGGATTTTTGGAGCAGGAA |
| bta-miR-196a | GCTGCGACCGTAGGTAGTTTCAT |
| bta-miR-184 | TGGACGGAGAACTGATAAGGGTAAA |
| bta-S18(F) | CACCGAGGATGAGGTGGA |
| bta-S18(R) | TATTGGCGTGGATTCTGC |
5. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Ethics Statement
Abbreviations
| RB | right back |
| LF | left front |
| LB | left back |
Conflicts of Interest
References
- Hogeveen, H.; Huijps, K.; Lam, T.J. Economioytc aspects of mastitis: New developments. N. Z. Vet. J. 2011, 59, 16–23. [Google Scholar] [CrossRef] [PubMed]
- Günther, J.; Esch, K.; Poschadel, N.; Petzl, W.; Zerbe, H.; Mitterhuemer, S.; Blum, H.; Seyfert, H.M. Comparative kinetics of Escherichia coli- and S. aureus-specific activation of key immune pathways in mammary epithelial cells demonstrates that S. aureus elicits a delayed response dominated by interleukin-6 (IL-6) but not by IL-1A or tumor necrosis factor α. Infect. Immun. 2011, 79, 695–707. [Google Scholar]
- Buitenhuis, B.; Røntved, C.M.; Edwards, S.M.; Ingvartsen, K.L.; Sørensen, P. In depth analysis of genes and pathways of the mammary gland involved in the pathogenesis of bovine Escherichia coli-mastitis. BMC Genomics 2011, 12. [Google Scholar] [CrossRef] [PubMed]
- Gilbert, F.B.; Cunha, P.; Jensen, K.; Glass, E.J.; Foucras, G.; Robert-Granié, C.; Rainard, P. Differential response of bovine mammary epithelial cells to Staphylococcus aureus or Escherichia coli agonists of the innate immune system. Vet. Res. 2013, 44, 1–23. [Google Scholar] [CrossRef] [PubMed]
- Jin, W.; Ibeagha-Awemu, E.M.; Liang, G.; Beaudoin, F.; Zhao, X. Transcriptome microRNA profiling of bovine mammary epithelial cells challenged with Escherichia coli or Staphylococcus aureus bacteria reveals pathogen directed microRNA expression profiles. BMC Genomics 2014, 15. [Google Scholar] [CrossRef] [PubMed]
- Rinaldi, M.; Li, R.W.; Capuco, A.V. Mastitis associated transcriptomic disruptions in cattle. Vet. Immunol. Immunopathol. 2010, 138, 267–279. [Google Scholar] [CrossRef] [PubMed]
- Condorelli, G.; Latronico, M.V.; Dorn, G.W. microRNAs in heart disease: Putative novel therapeutic targets? Eur. Heart J. 2010, 31, 649–658. [Google Scholar] [CrossRef] [PubMed]
- Dorn, G.W. MicroRNAs in cardiac disease. Transl. Res. 2011, 157, 226–235. [Google Scholar] [CrossRef] [PubMed]
- Lindsay, M.A. microRNAs and the immune response. Trends Immunol. 2008, 29, 343–351. [Google Scholar] [CrossRef] [PubMed]
- Xiao, C.; Rajewsky, K. MicroRNA control in the immune system: Basic principles. Cell 2009, 136, 26–36. [Google Scholar] [CrossRef]
- Zhou, R.; O’Hara, S.P.; Chen, X.M. MicroRNA regulation of innate immune responses in epithelial cells. Cell. Mol. Immunol. 2011, 8, 371–379. [Google Scholar] [CrossRef] [PubMed]
- Eulalio, A.; Schulte, L.; Vogel, J. The mammalian microRNA response to bacterial infections. RNA Biol. 2012, 9, 742–750. [Google Scholar] [CrossRef] [PubMed]
- Tili, E.; Michaille, J.J.; Cimino, A.; Costinean, S.; Dumitru, C.D.; Adair, B.; Croce, C.M. Modulation of miR-155 and miR-125b levels following lipopolysaccharide/TNF-α stimulation and their possible roles in regulating theresponse to endotoxin shock. J. Immunol. 2007, 179, 5082–5089. [Google Scholar] [CrossRef] [PubMed]
- Dilda, F.; Gioia, G.; Pisani, L.; Restelli, L.; Lecchi, C.; Albonico, F.; Ceciliani, F. Escherichia coli lipopolysaccharides and Staphylococcus aureus enterotoxin B differentially modulate inflammatory microRNAs in bovine monocytes. Vet. J. 2012, 192, 514–516. [Google Scholar] [CrossRef] [PubMed]
- Naeem, A.; Zhong, K.; Moisa, S.J.; Drackley, J.K.; Moyes, K.M.; Loor, J.J. Bioinformatics analysis of microRNA and putative target genes in bovine mammary tissue infected with Streptococcus uberis. J. Dairy Sci. 2012, 95, 6397–6408. [Google Scholar] [CrossRef] [PubMed]
- Lawless, N.; Foroushani, A.B.; Mccabe, M.S.; O’Farrelly, C.; Lynn, D.J. Next generation sequencing reveals the expression of a unique miRNA profile in response to a Gram-positive bacterial infection. PLoS One 2013, 8, e57543. [Google Scholar] [CrossRef] [PubMed]
- O’Connell, R.M.; Rao, D.S.; Chaudhuri, A.A.; Baltimore, D. Physiological and pathological roles for microRNAs in the immune system. Nat. Rev. Immunol. 2010, 10, 111–122. [Google Scholar] [CrossRef] [PubMed]
- Williams, A.E.; Perry, M.M.; Moschos, S.A.; Larner-Svensson, H.M.; Lindsay, M.A. Role of miRNA-146a in the regulation of the innate immune response and cancer. Biochem. Soc. Trans. 2008, 36, 1211–1215. [Google Scholar] [CrossRef] [PubMed]
- Hou, Q.; Huang, J.; Ju, Z.; Li, Q.; Li, L.; Wang, C.; Zhong, J. Identification of splice variants, targeted microRNAs and functional single nucleotide polymorphisms of the BOLA-DQA2 gene in dairy cattle. DNA Cell Biol. 2012, 31, 739–744. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Liu, X.; Li, Z.; Wang, H.; Liu, Y.; He, H.; Guo, J. Expression differences of miRNAs and genes on NF-κB pathway between the healthy and the mastitis Chinese Holstein cows. Gene 2014, 545, 117–125. [Google Scholar] [CrossRef] [PubMed]
- Fatima, A.; Lynn, D.J.; Padraic, O.; Seoighe, C.; Morris, D. The miRNAome of the postpartum dairy cow liver in negative energy balance. BMC Genomics 2014, 15. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Bian, Y.; Wang, Z.; Li, D.; Wang, C.; Li, Q.; Gao, X. MicroRNA-152 regulates DNA Methyltransferase 1 and is involved in the development and lactation of mammary glands in dairy cows. PLoS One 2014, 9, e101358. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Zhang, H.; Song, N.; Wang, H.; Chen, L.; Zhai, M.; Liu, X. Molecular cloning, characterization and expression of miR-15a-3pand miR-15b-3p in dairy cattle. Mol. Cell. Probes 2014, 28, 255–258. [Google Scholar] [CrossRef] [PubMed]
- Gao, Q.; Ju, Z.; Zhang, Y.; Huang, J.; Zhang, X.; Qi, C.; Wang, C. Association of TNP2 Gene polymorphisms of the bta-miR-154 target Site with the semen quality traits of Chinese Holstein bulls. PLoS One 2014, 9, e84355. [Google Scholar] [CrossRef] [PubMed]
- Vimalraj, S.; Miranda, P.J.; Ramyakrishna, B.; Selvamurugan, N. Regulation of breast cancer and bone metastasis by microRNAs. Dis. Markers 2013, 35, 369–387. [Google Scholar] [CrossRef] [PubMed]
- Creighton, C.J.; Fountain, M.D.; Yu, Z.; Nagaraja, A.K.; Zhu, H.; Khan, M.; Anderson, M.L. Molecular profiling uncovers a p53-associated role for microRNA-31 in inhibiting the proliferation of serous ovarian carcinomas and other cancers. Cancer Res. 2010, 70, 1906–1915. [Google Scholar] [CrossRef] [PubMed]
- Johnnidis, J.B.; Harris, M.H.; Wheeler, R.T.; Stehling-Sun, S.; Lam, M.H.; Kirak, O.; Camargo, F.D. Regulation of progenitor cell proliferation and granulocyte function by microRNA-223. Nature 2008, 451, 1125–1129. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.F.; Yu, M.L.; Yu, G.; Bian, J.J.; Deng, X.M.; Wan, X.J.; Zhu, K.M. Serum miR-146a and miR-223 as potential new biomarkers for sepsis. Biochem. Biophys. Res. Commun. 2010, 394, 184–188. [Google Scholar] [CrossRef] [PubMed]
- Tognini, P.; Pizzorusso, T. MicroRNA212/132 family: Molecular transducer of neuronal function and plasticity. Int. J. Biochem. Cell Biol. 2012, 44, 6–10. [Google Scholar] [CrossRef] [PubMed]
- Lagos, D.; Pollara, G.; Henderson, S.; Gratrix, F.; Fabani, M.; Milne, R.S.; Boshoff, C. MiR-132 regulates antiviral innate immunity through suppression of the p300 transcriptional co-activator. Nat. Cell Biol. 2010, 12, 513–519. [Google Scholar] [CrossRef] [PubMed]
- Fen, L.; Rong, J.; Zhenguo, Z.; Ning, Z.; Liang, X.; Cheng, N.; Kejian, Q. The expression changes in microRNA-132 in the lipopolysaccharide-induced inflammation of rat alveolar macrophages. Zhonghua Wei Zhong Bing Ji Jiu Yi Xue 2014, 26, 80–83. [Google Scholar] [PubMed]
- Schmittgen, T.D.; Livak, K.J. Analyzing real-time PCR data by the comparative Ct method. Nat. Protoc. 2008, 3, 1101–1108. [Google Scholar] [CrossRef] [PubMed]
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, R.; Zhang, C.-L.; Liao, X.-X.; Chen, D.; Wang, W.-Q.; Zhu, Y.-H.; Geng, X.-H.; Ji, D.-J.; Mao, Y.-J.; Gong, Y.-C.; et al. Transcriptome MicroRNA Profiling of Bovine Mammary Glands Infected with Staphylococcus aureus. Int. J. Mol. Sci. 2015, 16, 4997-5013. https://doi.org/10.3390/ijms16034997
Li R, Zhang C-L, Liao X-X, Chen D, Wang W-Q, Zhu Y-H, Geng X-H, Ji D-J, Mao Y-J, Gong Y-C, et al. Transcriptome MicroRNA Profiling of Bovine Mammary Glands Infected with Staphylococcus aureus. International Journal of Molecular Sciences. 2015; 16(3):4997-5013. https://doi.org/10.3390/ijms16034997
Chicago/Turabian StyleLi, Rui, Cheng-Long Zhang, Xiang-Xiang Liao, Dan Chen, Wen-Qiang Wang, Yi-Hui Zhu, Xiao-Han Geng, De-Jun Ji, Yong-Jiang Mao, Yun-Chen Gong, and et al. 2015. "Transcriptome MicroRNA Profiling of Bovine Mammary Glands Infected with Staphylococcus aureus" International Journal of Molecular Sciences 16, no. 3: 4997-5013. https://doi.org/10.3390/ijms16034997
APA StyleLi, R., Zhang, C.-L., Liao, X.-X., Chen, D., Wang, W.-Q., Zhu, Y.-H., Geng, X.-H., Ji, D.-J., Mao, Y.-J., Gong, Y.-C., & Yang, Z.-P. (2015). Transcriptome MicroRNA Profiling of Bovine Mammary Glands Infected with Staphylococcus aureus. International Journal of Molecular Sciences, 16(3), 4997-5013. https://doi.org/10.3390/ijms16034997





