Sinomenine Hydrochloride Protects against Polymicrobial Sepsis via Autophagy
Abstract
:1. Introduction
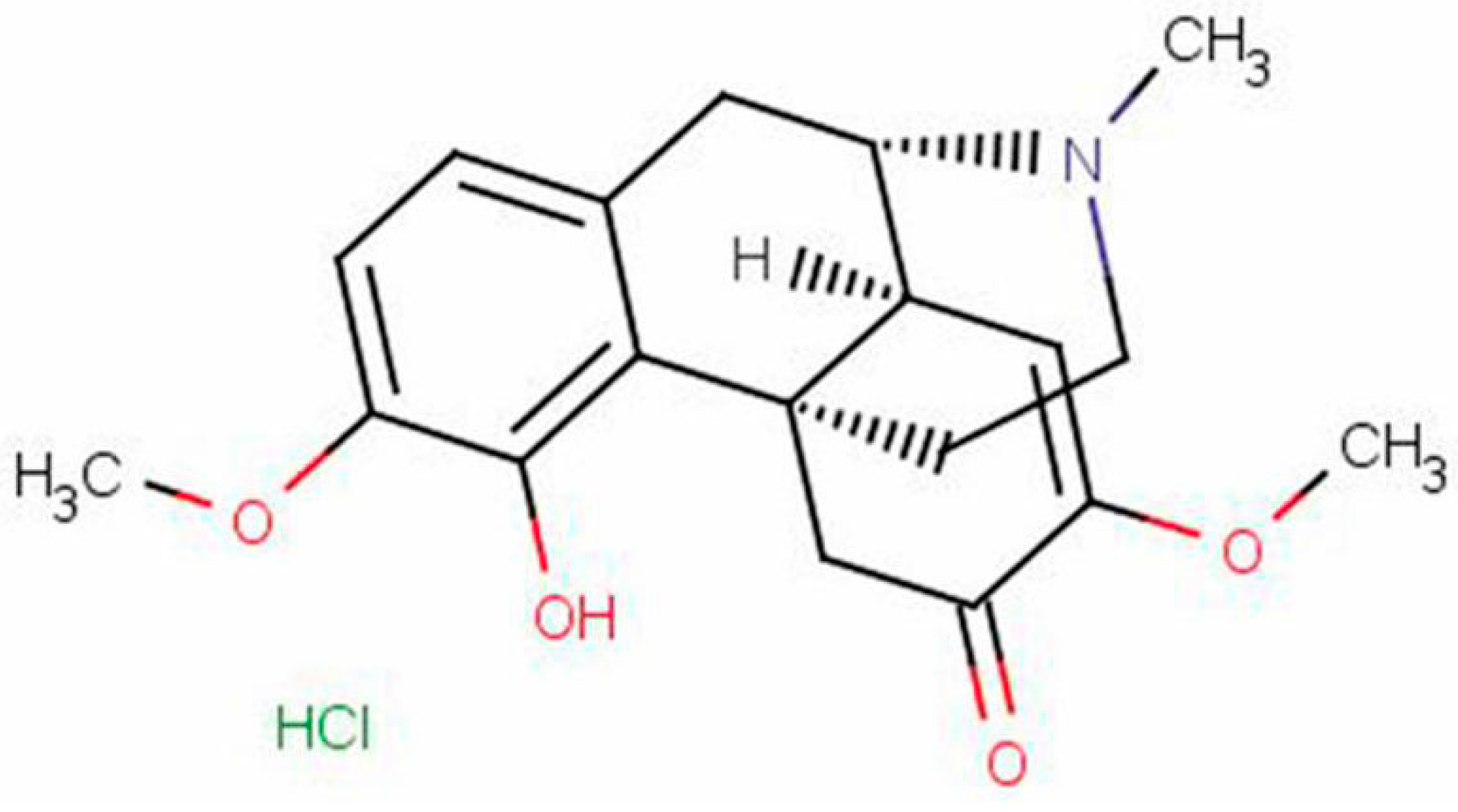
2. Results
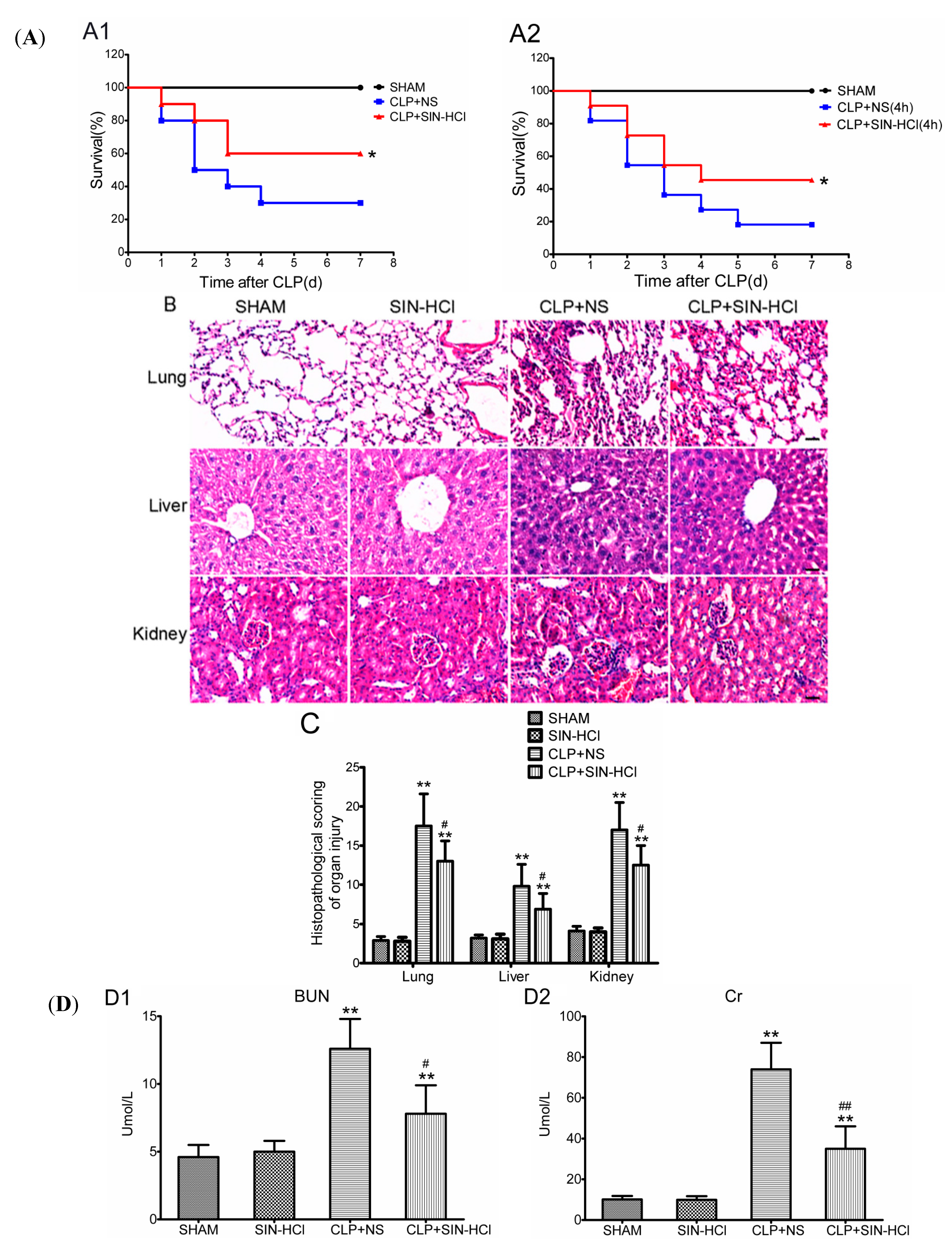
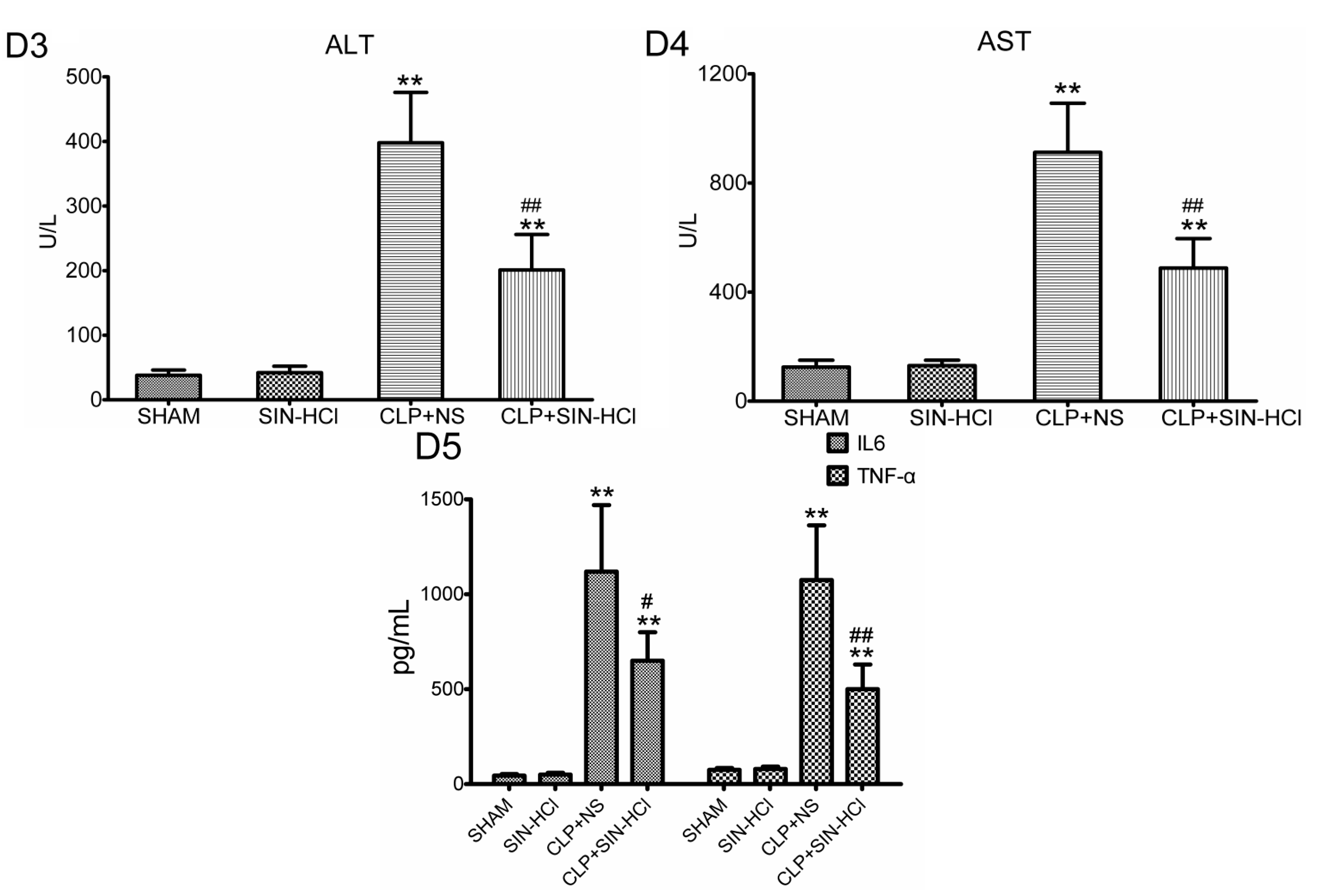
2.1. SIN-HCl Protected against Polymicrobial Sepsis in Model Mice and Attenuated Multiple Organ Dysfunction and Systemic Inflammatory Response
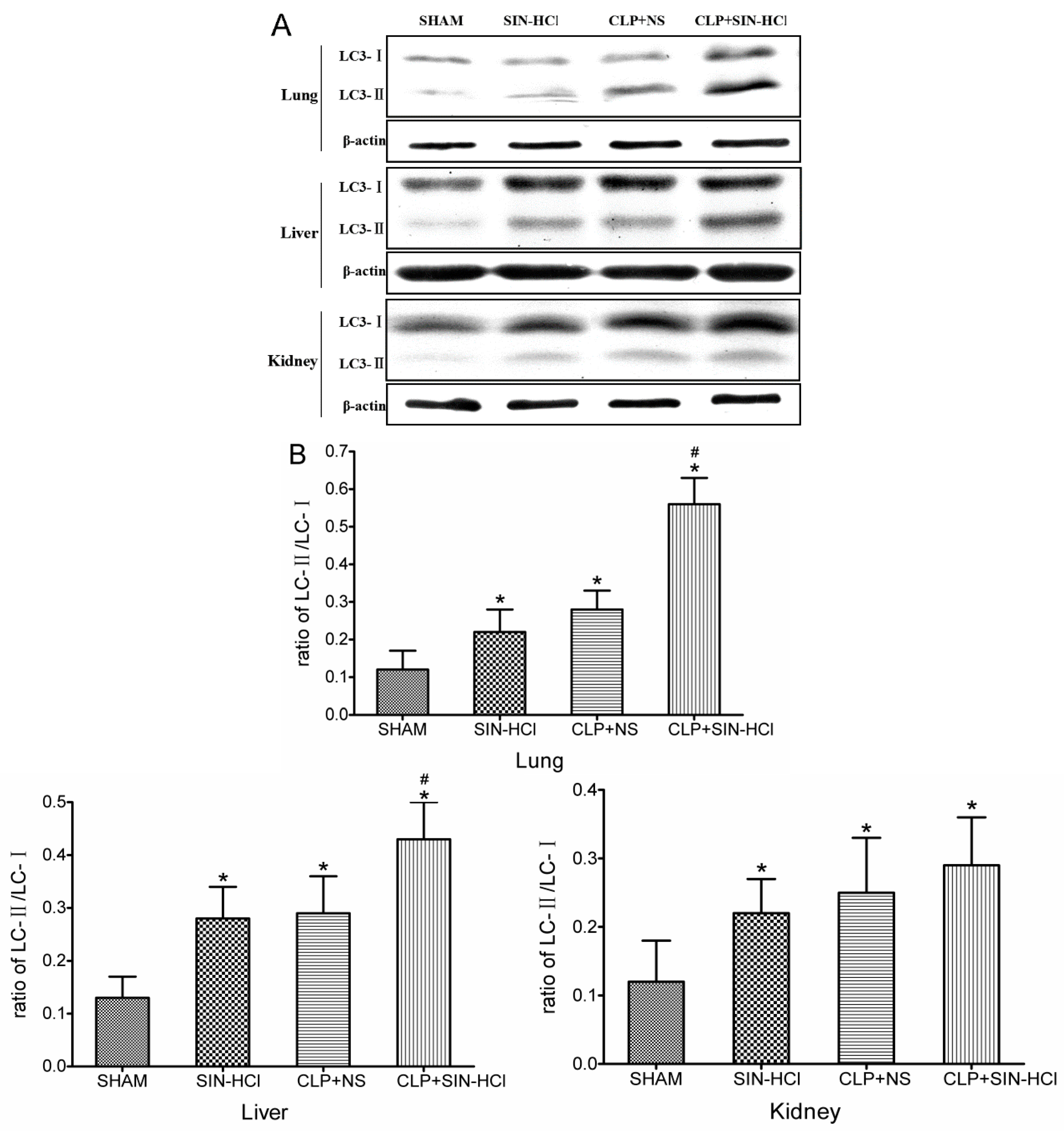
2.2. CLP-Induced Autophagy in Multiple Organs Was Strengthened in SIN-HCl Treatment in Mice
2.3. SIN-HCl Blocked Lps-Induced Inflammatory Cytokine Release Induced by LPS and Increased Autophagy in Peritoneal Macrophages (PM)

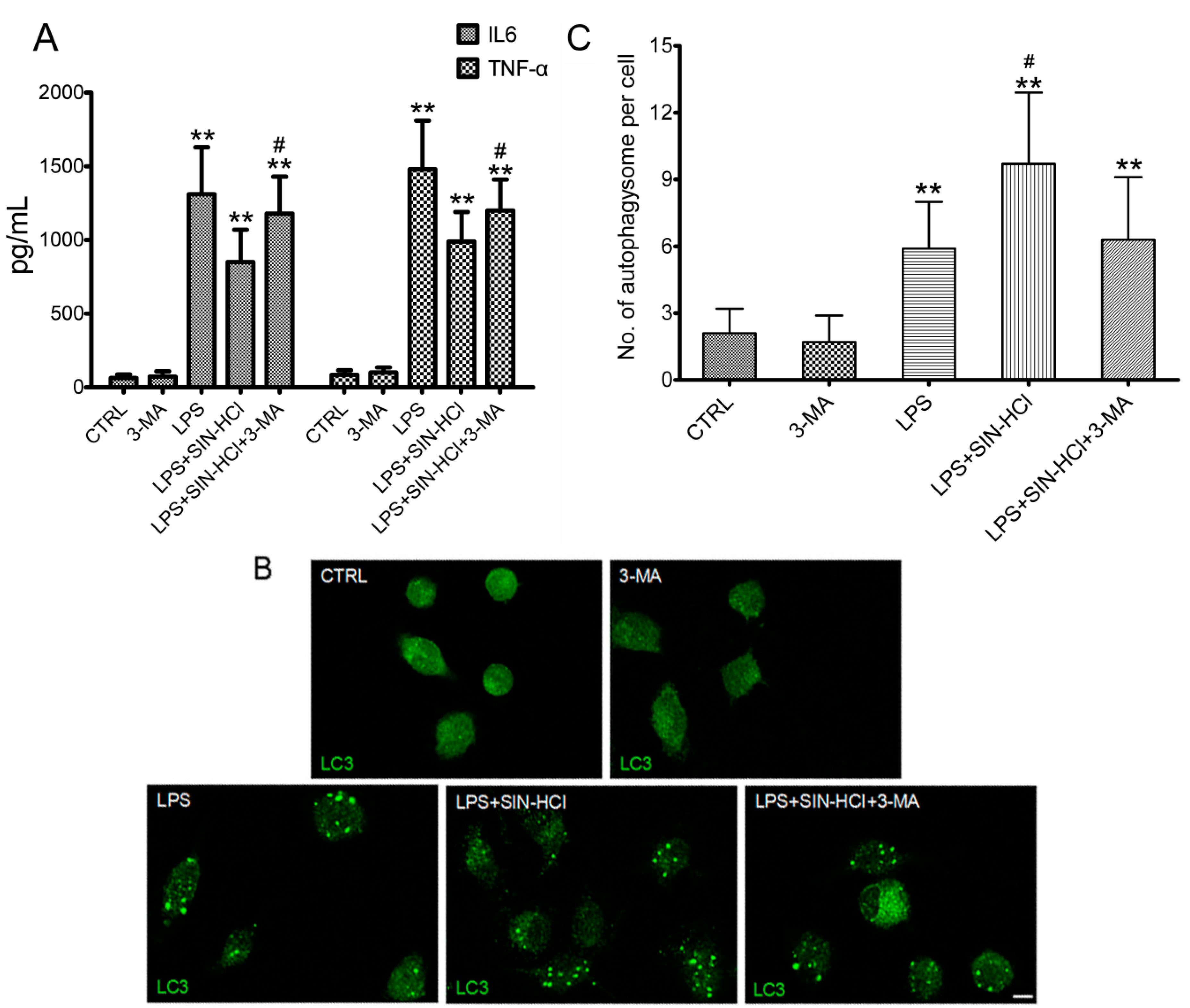
2.4. The Effects of 3-MA on SIN-HCl-Induced Autophagy and Inflammatory Responses in PM
3. Discussion
4. Experimental Section
4.1. Materials
4.2. Animals
4.3. CLP Model and SIN-HCl Treatment
4.4. Serum Biochemical Parameters and Cytokine Determination
4.5. Morphological Analysis
4.6. Western Blotting Analyses
4.7. Isolation of Peritoneal Macrophages and Cell Treatments
4.8. Immunofluorescence Staining
4.9. Statistical Analyses
5. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Abbreviation
| SIN | Sinomenine |
| SIN-HCl | Sinomenine hydrochloride |
| CLP | Cecal ligation and puncture |
| PM | Peritoneal macrophages |
| LPS | Lipopolysaccharide |
| LC3-II | Microtubule-associated protein light chain 3-II |
| LC3-I | Microtubule-associated protein 1 light chain-3-I |
| TNF-α | Tumor necrosis factor α |
| IL-6 | Interleukin 6 |
| 3-MA | 3-Methyladenine |
| ICU | Intensive care unit |
| ELISA | Enzyme-linked immunosorbent assay |
Conflicts of Interest
References
- Hotchkiss, R.S.; Karl, I.E. The pathophysiology and treatment of sepsis. N. Engl. J. Med. 2003, 348, 138–150. [Google Scholar]
- Riedemann, N.C.; Guo, R.F.; Ward, P.A. Novel strategies for the treatment of sepsis. Nat. Med. 2003, 9, 517–524. [Google Scholar]
- Levine, B.; Klionsky, D.J. Development by self-digestion: Molecular mechanisms and biological functions of autophagy. Dev. Cell 2004, 6, 463–477. [Google Scholar]
- Mizushima, N.; Levine, B. Autophagy in mammalian development and differentiation. Nat. Cell Biol. 2010, 12, 823–830. [Google Scholar]
- Kuma, A.; Hatano, M.; Matsui, M.; Yamamoto, A.; Nakaya, H.; Yoshimori, T.; Ohsumi, Y.; Tokuhisa, T.; Mizushima, N. The role of autophagy during the early neonatal starvation period. Nature 2004, 432, 1032–1036. [Google Scholar]
- Watanabe, E.; Muenzer, J.T.; Hawkins, W.G.; Davis, C.G.; Dixon, D.J.; McDunn, J.E.; Brackett, D.J.; Lerner, M.R.; Swanson, P.E.; Hotchkiss, R.S. Sepsis induces extensive autophagic vacuolization in hepatocytes: A clinical and laboratory-based study. Lab. Investig. 2009, 89, 549–561. [Google Scholar]
- Takahashi, W.; Watanabe, E.; Fujimura, L.; Watanabe-Takano, H; Yoshidome, H; Swanson, P.E.; Tokuhisa, T.; Oda, S.; Hatano, M. Kinetics and protective role of autophagy in a mouse cecal ligation and puncture-induced sepsis. Crit. Care 2013, 17. [Google Scholar] [CrossRef]
- Lo, S.; Yuan, S.S.; Hsu, C.; Cheng., Y.J.; Chang, Y.F.; Hsueh, H.W.; Lee, P.H.; Hsieh, Y.C. Lc3 over-expression improves survival and attenuates lung injury through increasing autophagosomal clearance in septic mice. Ann. Surg. 2013, 257, 352–363. [Google Scholar]
- Hsieh, C.H; Pai, P.Y.; Hsueh, H.W.; Yuan, S.S; Hsieh, Y.C. Complete induction of autophagy is essential for cardioprotection in sepsis. Ann. Surg. 2011, 253, 1190–1200. [Google Scholar]
- Chien, W.S.; Chen, Y.H.; Chiang, P.C.; Hsiao, H.W.; Chuang, S.M.; Lue, S.I.; Hsu, C. Suppression of autophagy in rat liver at late stage of polymicrobial sepsis. Shock 2011, 35, 506–511. [Google Scholar]
- Lin, C.W.; Lo, S; Perng, D.S.; Wu, D.B.; Lee, P.H.; Chang, Y.F.; Kuo, P.L.; Yu, M.L.; Yuan, S.S.; Hsieh, Y.C. Complete activation of autophagic process attenuates liver injury and improves survival in septic mice. Shock 2014, 41, 241–249. [Google Scholar]
- Hsiao, H.W.; Tsai, K.L.; Wang, L.F.; Chen, Y.H.; Chiang, P.C.; Chuang, S.M.; Hsu, C. The decline of autophagy contributes to proximal tubular dysfunction during sepsis. Shock 2012, 37, 289–296. [Google Scholar]
- Xu, M.; Liu, L.; Qi, C.; Deng, B.; Cai, X. Sinomenine versus NSAIDs for the treatment of rheumatoid arthritis: A systematic review and meta-analysis. Planta Med. 2008, 74, 1423–1429. [Google Scholar]
- Yamasaki, H. Pharmacology of sinomenine, an anti-rheumatic alkaloid from Sinomenium acutum. Acta Med. Okayama 1976, 30, 1–20. [Google Scholar]
- Wang, Y.; Fang, Y.; Huang, W.; Zhou, X.; Wang, M.; Zhong, B.; Peng, D. Effect of sinomenine on cytokine expression of macrophages and synoviocytes in adjuvant arthritis rats. J. Ethnopharmacol. 2005, 98, 37–43. [Google Scholar]
- He, X.; Wang, J.; Guo, Z.; Liu, Q.; Chen, T.; Wang, X.; Cao, X. Requirement for ERK activation in sinomenine-i, nduced apoptosis of macrophages. Immunol. Lett. 2005, 98, 91–96. [Google Scholar]
- Kok, T.W.; Yue, P.Y.; Mak, N.K.; Fan, T.P.; Liu, L.; Wong, R.N. The anti-angiogenic effect of sinomenine. Angiogenesis 2005, 8, 3–12. [Google Scholar]
- Hubbard, W.J.; Choudhry, M.; Schwacha, M.G.; Kerby, J.D.; Rue, L.R.; Bland, K.I.; Chaudry, I.H. Cecal ligation and puncture. Shock 2005, 24, S152–S157. [Google Scholar]
- Cavaillon, J.M.; Adib-Conquy, M.; Fitting, C.; Adrie, C.; Payen, D. Cytokine cascade in sepsis. Scand. J. Infect. Dis. 2003, 35, 535–544. [Google Scholar]
- Teng, P; Liu, H.L.; Zhang, L.; Feng, L.L.; Huai, Y.; Deng, Z.S.; Sun, Y.; Xu, Q.; Li, J.X. Synthesis and biological evaluation of novel sinomenine derivatives as anti-inflammatory agents. Eur. J. Med. Chem. 2012, 5063–5074. [Google Scholar]
- Yen, Y.T.; Yang, H.R.; Lo, H.C.; Hsieh, Y.C.; Tsai, S.C.; Hong, C.W.; Hsieh, C.H. Enhancing autophagy with activated protein C and rapamycin protects against sepsis-induced acute lung injury. Surgery 2013, 153, 689–698. [Google Scholar]
- Saitoh, T.; Fujita, N.; Jang, M.H.; Uematsu, S.; Yang, B.G.; Satoh, T.; Omori, H.; Noda, T.; Yamamoto, N.; Komatsu, M.; et al. Loss of the autophagy protein Atg16L1 enhances endotoxin-induced IL-1β production. Nature 2008, 456, 264–268. [Google Scholar]
- Savva, A.; Plantinga, T.S.; Kotanidou, A.; Farcas, M.; Baziaka, F.; Raftogiannis, M.; Orfanos, S.E.; Dimopoulos, G.; Netea, M.G.; Giamarellos-Bourboulis, E.J. Association of autophagy-related 16-like 1 (ATG16L1) gene polymorphism with sepsis severity in patients with sepsis and ventilator-associated pneumonia. Eur. J. Clin. Microbiol. Infect. Dis. 2014, 33, 1609–1614. [Google Scholar]
- Kimura, T.; Watanabe, E.; Sakamoto, T.; Takasu, O.; Ikeda, T.; Ikeda, K.; Kotani, J.; Kitamura, N.; Sadahiro, T.; Tateishi, Y.; et al. Autophagy-related IRGM polymorphism is associated with mortality of patients with severe sepsis. PLoS One 2014, 9, e91522. [Google Scholar]
- Seglen, P.O.; Gordon, P.B. 3-Methyladenine: Specific inhibitor of autophagic/lysosomal protein degradation in isolated rat hepatocytes. Proc. Natl. Acad. Sci. USA 1982, 79, 1889–1892. [Google Scholar]
- Puleston, D.J.; Simon, A.K. Autophagy in the immune system. Immunology 2014, 141, 1–8. [Google Scholar]
- Ma, Y.; Galluzzi, L.; Zitvogel, L.; Kroemer, G. Autophagy and cellular immune responses. Immunity 2013, 39, 211–227. [Google Scholar]
- Rittirsch, D.; Huber-Lang, M.S.; Flierl, M.A.; Ward, P.A. Immunodesign of experimental sepsis by cecal ligation and puncture. Nat. Protoc. 2009, 4, 31–36. [Google Scholar]
- Gao, M.; Zhang, L.; Liu, Y.; Yang, M.; Wang, N.; Wang, K.; Ou, D.; Liu, M.; Chen, G.; Liu, K.; Xiao, X. Use of blood urea nitrogen, creatinine, interleukin-6, granulocyte-macrophage colony stimulating factor in combination to predict the severity and outcome of abdominal sepsis in rats. Inflamm. Res. 2012, 61, 889–897. [Google Scholar]
- Smith, K.M.; Mrozek, J.D.; Simonton, S.C.; Bing, D.R.; Meyers, P.A.; Connett, J.E.; Mammel, M.C. Prolonged partial liquid ventilation using conventional and high-frequency ventilatory techniques: Gas exchange and lung pathology in an animal model of respiratory distress syndrome. Crit. Care Med. 1997, 25, 1888–1897. [Google Scholar]
- Coimbra, R.; Porcides, R.D.; Melbostad, H.; Loomis, W.; Tobar, M.; Hoyt, D.B; Wolf, P. Nonspecific phosphodiesterase inhibition attenuates liver injury in acute endotoxemia. Surg. Infect. 2005, 6, 73–85. [Google Scholar]
- Yasuda, H.; Yuen, P.S.; Hu, X.; Zhou, H.; Star, R.A. Simvastatin improves sepsis-induced mortality and acute kidney injury via renal vascular effects. Kidney Int. 2006, 69, 1535–1542. [Google Scholar]
- Tong, Z.; Jiang, B.; Zhang, L.; Liu, Y.; Gao, M.; Jiang, Y; Li, Y.; Lu, Q.; Yao, Y.; Xiao, X. HSF-1 is involved in attenuating the release of inflammatory cytokines induced by LPS through regulating autophagy. Shock 2014, 41, 449–453. [Google Scholar]
- Gomes, R.N.; Teixeira-Cunha, M.G.; Figueiredo, R.T.; Almeida, P.E.; Alves, S.C.; Bozza, P.T.; Bozza, F.A.; Bozza, M.T.; Zimmerman, G.A.; Castro-Faria-Neto, H.C. Bacterial clearance in septic mice is modulated by MCP-1/CCL2 and nitric oxide. Shock 2013, 39, 63–69. [Google Scholar]
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jiang, Y.; Gao, M.; Wang, W.; Lang, Y.; Tong, Z.; Wang, K.; Zhang, H.; Chen, G.; Liu, M.; Yao, Y.; et al. Sinomenine Hydrochloride Protects against Polymicrobial Sepsis via Autophagy. Int. J. Mol. Sci. 2015, 16, 2559-2573. https://doi.org/10.3390/ijms16022559
Jiang Y, Gao M, Wang W, Lang Y, Tong Z, Wang K, Zhang H, Chen G, Liu M, Yao Y, et al. Sinomenine Hydrochloride Protects against Polymicrobial Sepsis via Autophagy. International Journal of Molecular Sciences. 2015; 16(2):2559-2573. https://doi.org/10.3390/ijms16022559
Chicago/Turabian StyleJiang, Yu, Min Gao, Wenmei Wang, Yuejiao Lang, Zhongyi Tong, Kangkai Wang, Huali Zhang, Guangwen Chen, Meidong Liu, Yongming Yao, and et al. 2015. "Sinomenine Hydrochloride Protects against Polymicrobial Sepsis via Autophagy" International Journal of Molecular Sciences 16, no. 2: 2559-2573. https://doi.org/10.3390/ijms16022559
APA StyleJiang, Y., Gao, M., Wang, W., Lang, Y., Tong, Z., Wang, K., Zhang, H., Chen, G., Liu, M., Yao, Y., & Xiao, X. (2015). Sinomenine Hydrochloride Protects against Polymicrobial Sepsis via Autophagy. International Journal of Molecular Sciences, 16(2), 2559-2573. https://doi.org/10.3390/ijms16022559




