The Influence of AHI1 Variants on the Diagnosis and Treatment Outcome in Schizophrenia
Abstract
:1. Introduction
2. Results and Discussion
| Variable | Schizophrenia | Controls (n = 345) | ||
|---|---|---|---|---|
| Total Sample (n = 426) | Sample with Follow-up (n = 238) | |||
| Gender | Males | 198 (46.5%) | 136 (57.1%) | 138 (40%) |
| Females | 181 (42.5%) | 102 (42.9%) | 207 (60%) | |
| Missing | 47 (11.0%) | |||
| Age (years) | 36.2 ± 11.73 | 37 ± 12.16 | 43.39 ± 14.05 | |
| PANSS total score | Baseline | 93.91 ± 13.55 | 94.02 ± 13.95 | |
| Discharge | NA | 76.63 ± 8.96 | ||
| Age at onset (years) | 23.65 ± 6.6 | 23.28 ± 6.5 | ||
| Family history of psychiatric disorders | Yes | 65 (15.2%) | 38 (16.0%) | |
| No | 317 (74.4%) | 200 (84.0%) | ||
| Missing | 44 (10.3%) | |||
| Suicide attempts | Yes | 73 (17.1%) | 46 (19.3%) | |
| No | 309 (72.5%) | 192 (80.7%) | ||
| Missing | 44 (10.3%) | |||
| SNPs | Position a | HWE’s p-Value | Location | Schizophrenia (n = 426) | Controls (n = 345) | χ2 | p-Value | |
|---|---|---|---|---|---|---|---|---|
| Alleles | ||||||||
| rs11154801 | 135739355 | 1.0 | Intron | C | 671 (78.8) | 542 (78.5) | 0.01 | 0.92 |
| A | 181 (21.2) | 148 (21.4) | ||||||
| rs7750586 | 135827673 | 0.6839 | Promoter | T | 680 (79.8) | 551 (79.8) | 0.001 | 0.98 |
| C | 172 (20.2) | 139 (20.1) | ||||||
| rs9647635 | 135841056 | 0.7021 | Intron | A | 718 (84.3) | 551 (79.9) | 5.10 | 0.02 |
| C | 134 (15.7) | 139 (20.1) | ||||||
| rs9321501 | 135641417 | 1.0 | Intron | A | 634 (74.4) | 527 (76.4) | 0.79 | 0.37 |
| C | 218 (25.6) | 163 (23.6) | ||||||
| Genotypes | ||||||||
| rs11154801 | 135739355 | 1.0 | Intron | C/C | 266 (62.4) | 213 (61.7) | 0.10 | 0.94 |
| A/C | 139 (32.6) | 116 (33.6) | ||||||
| A/A | 21 (4.9) | 16 (4.6) | ||||||
| rs7750586 | 135827673 | 0.6839 | Promoter | T/T | 273 (64.1) | 220 (63.8) | 0.10 | 0.95 |
| C/T | 134 (31.5) | 111 (32.2) | ||||||
| C/C | 19 (4.5) | 14 (4.0) | ||||||
| rs9647635 | 135841056 | 0.7021 | Intron | A/A | 302 (70.9) | 221 (64.1) | 5.20 | 0.07 |
| A/C | 114 (26.8) | 109 (31.6) | ||||||
| C/C | 10 (2.3) | 15 (4.3) | ||||||
| rs9321501 | 135641417 | 1.0 | Intron | A/A | 236 (55.4) | 204 (59.1) | 1.13 | 0.57 |
| A/C | 162 (38.0) | 119 (34.5) | ||||||
| C/C | 28 (6.6) | 22 (6.4) | ||||||
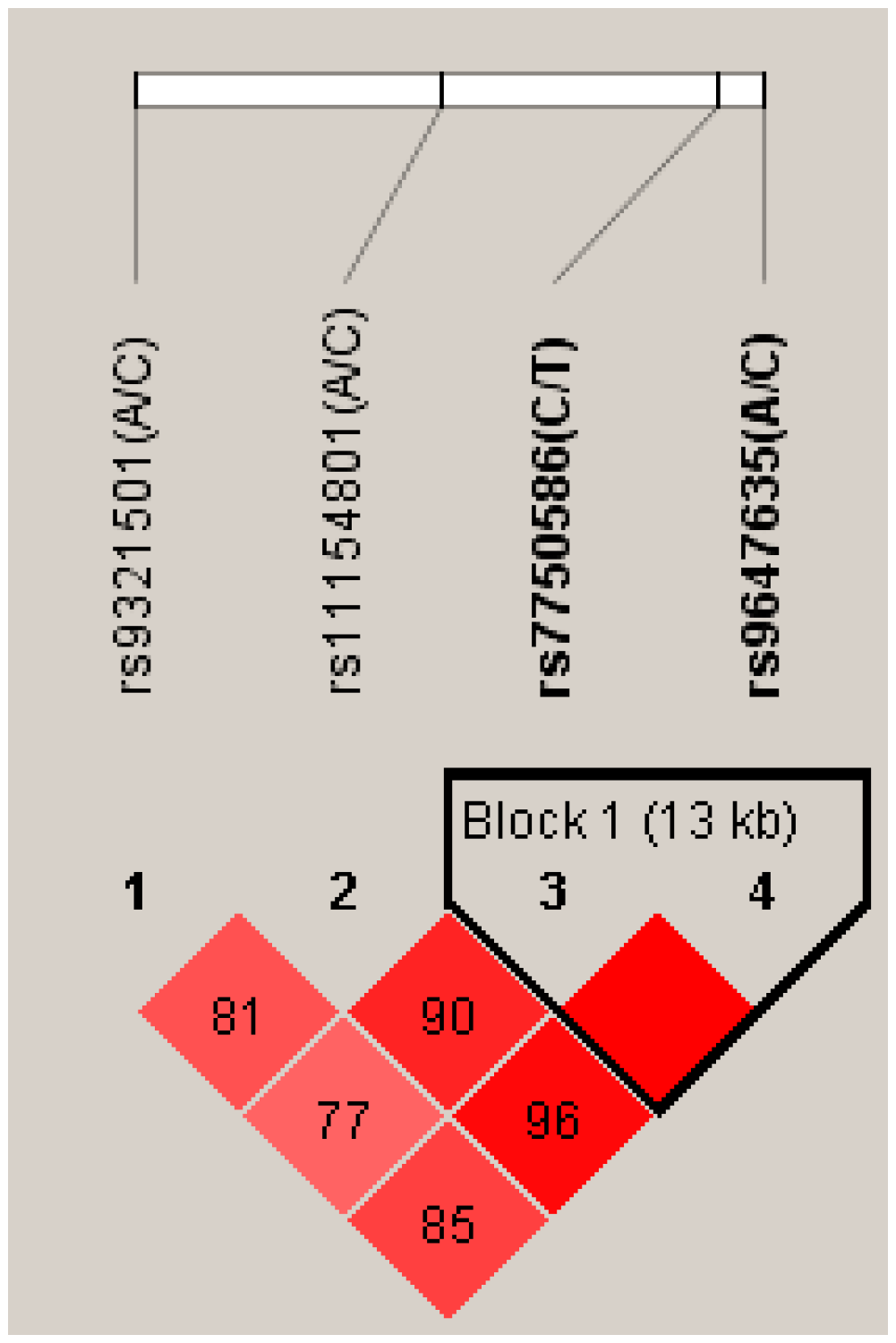
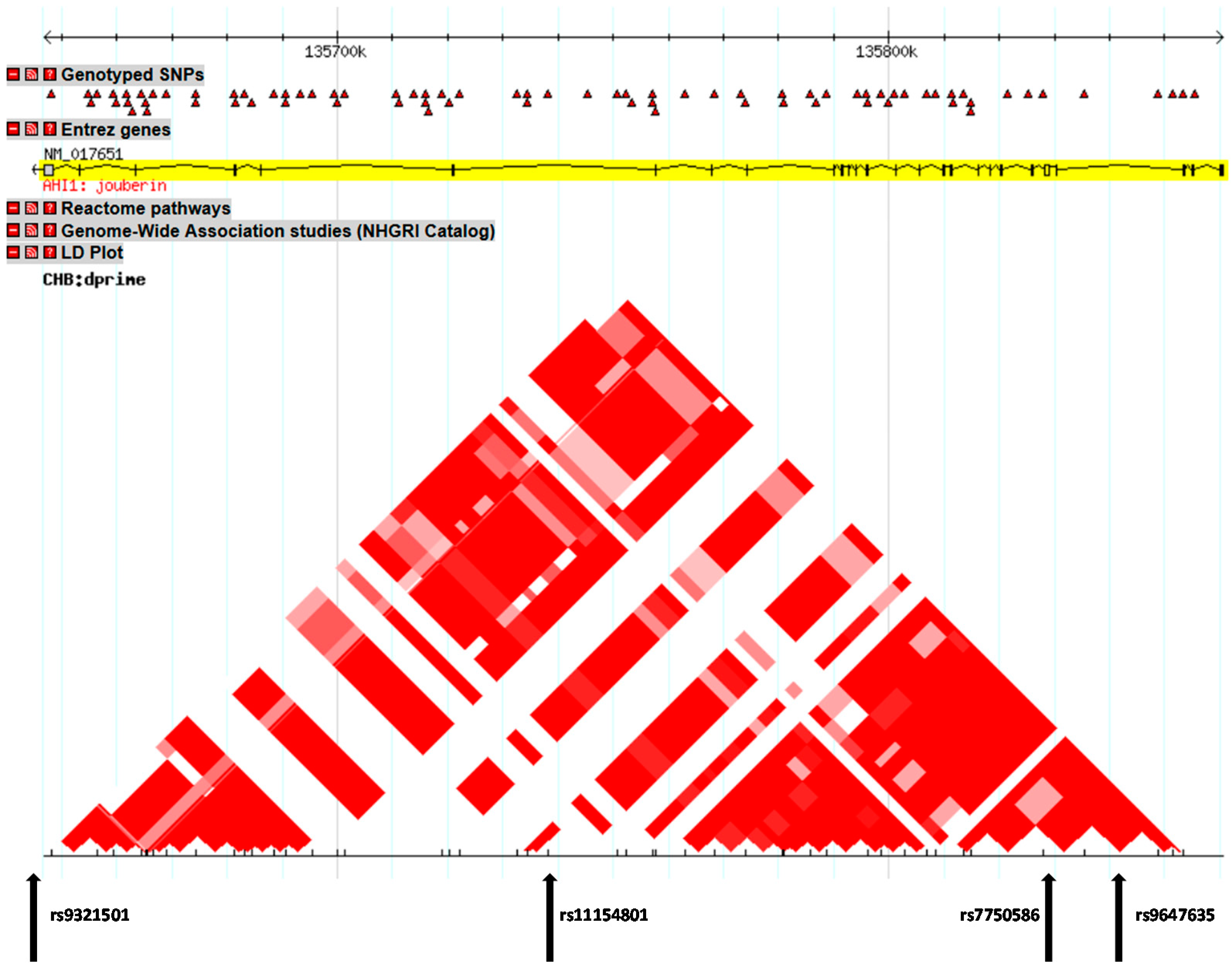
2.1. Differences between Genotype and Allele Frequencies in SCZ Patients and Healthy Controls
| rs11154801 | rs7750586 | rs9647635 | rs9321501 | Cases Hap-Freq a | Controls Hap-Freq a | p-Value | Sim b p-Value | Odds Ratio |
|---|---|---|---|---|---|---|---|---|
| A | C | C | C | 0.14 | 0.18 | 0.04 | 0.04 | 0.82 (0.62–1.09) |
| C | T | A | A | 0.70 | 0.74 | 0.19 | 0.18 | 1 (NA) |
| A | C | C | A | 0.01 | 0.02 | 0.40 | 0.40 | 0.77 (0.35–1.69) |
| C | T | A | C | 0.07 | 0.05 | 0.10 | 0.10 | 1.46 (0.92–2.30) |
| A | T | A | C | 0.02 | 0.01 | 0.04 | 0.03 | 2.45 (0.86–6.99) |
| A | C | A | C | 0.03 | 0.001 | <0.001 | <0.001 | 18.85 (1.89–187.55) |
2.2. AHI1 Variants and Clinical Improvement in SCZ Subjects
| rs11154801 | rs7750586 | rs9647635 | rs9321501 | Hap-Freq | p-Value | Sim p-Value |
|---|---|---|---|---|---|---|
| A | C | C | C | 0.14 | 0.17 | 0.17 |
| A | C | C | A | 0.02 | 0.48 | 0.42 |
| C | T | A | A | 0.72 | 0.68 | 0.68 |
| A | T | A | C | 0.02 | 0.52 | 0.48 |
| A | C | A | C | 0.02 | 0.45 | 0.41 |
| C | T | A | C | 0.07 | 0.41 | 0.41 |
3. Methods
3.1. Subjects
3.2. Statistical Analyses
3.3. Genotyping
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Tandon, R.; Keshavan, M.S.; Nasrallah, H.A. Schizophrenia, “just the facts” what we know in 2008. 2. Epidemiology and etiology. Schizophr. Res. 2008, 102, 1–18. [Google Scholar] [CrossRef] [PubMed]
- Lichtenstein, P.; Yip, B.H.; Bjork, C.; Pawitan, Y.; Cannon, T.D.; Sullivan, P.F.; Hultman, C.M. Common genetic determinants of schizophrenia and bipolar disorder in Swedish families: A population-based study. Lancet 2009, 373, 234–239. [Google Scholar] [CrossRef] [PubMed]
- Crisafulli, C.; Chiesa, A.; Han, C.; Lee, S.J.; Park, M.H.; Balzarro, B.; Andrisano, C.; Patkar, A.A.; Pae, C.U.; Serretti, A. Case-control association study for 10 genes in patients with schizophrenia: Influence of 5HTR1A variation rs10042486 on schizophrenia and response to antipsychotics. Eur. Arch. Psychiatry Clin. Neurosci. 2009, 262, 199–205. [Google Scholar] [CrossRef]
- Alda, M. Genetic factors and treatment of mood disorders. Bipolar. Disord. 2001, 3, 318–324. [Google Scholar] [CrossRef] [PubMed]
- Pae, C.U.; Chiesa, A.; Porcelli, S.; Han, C.; Patkar, A.A.; Lee, S.J.; Park, M.H.; Serretti, A.; de Ronchi, D. Influence of BDNF variants on diagnosis and response to treatment in patients with major depression, bipolar disorder and schizophrenia. Neuropsychobiology 2012, 65, 1–11. [Google Scholar] [CrossRef] [PubMed]
- National Library of Medicine (US). Available online: http://www.ncbi.nlm.nih.gov/pubmed/ (accessed on 1 January 2011).
- Ferland, R.J.; Eyaid, W.; Collura, R.V.; Tully, L.D.; Hill, R.S.; Al-Nouri, D.; Al-Rumayyan, A.; Topcu, M.; Gascon, G.; Bodell, A.; et al. Abnormal cerebellar development and axonal decussation due to mutations in AHI1 in Joubert syndrome. Nat. Genet. 2004, 36, 1008–1013. [Google Scholar] [CrossRef] [PubMed]
- Sheng, G.; Xu, X.; Lin, Y.F.; Wang, C.E.; Rong, J.; Cheng, D.; Peng, J.; Jiang, X.; Li, S.H.; Li, X.J. Huntingtin-associated protein 1 interacts with Ahi1 to regulate cerebellar and brainstem development in mice. J. Clin. Investig. 2008, 118, 2785–2795. [Google Scholar] [CrossRef] [PubMed]
- Weng, L.; Lin, Y.F.; Li, A.L.; Wang, C.E.; Yan, S.; Sun, M.; Gaertig, M.A.; Mitha, N.; Kosaka, J.; Wakabayashi, T.; et al. Loss of Ahi1 affects early development by impairing BM88/Cend1-mediated neuronal differentiation. J. Neurosci. Off. J. Soc. Neurosci. 2013, 33, 8172–8184. [Google Scholar] [CrossRef]
- Lisman, J.E.; Coyle, J.T.; Green, R.W.; Javitt, D.C.; Benes, F.M.; Heckers, S.; Grace, A.A. Circuit-based framework for understanding neurotransmitter and risk gene interactions in schizophrenia. Trends Neurosci. 2008, 31, 234–242. [Google Scholar] [CrossRef] [PubMed]
- Lotan, A.; Lifschytz, T.; Slonimsky, A.; Broner, E.C.; Greenbaum, L.; Abedat, S.; Fellig, Y.; Cohen, H.; Lory, O.; Goelman, G.; et al. Neural mechanisms underlying stress resilience in Ahi1 knockout mice: relevance to neuropsychiatric disorders. Mol. Psychiatry 2014, 19, 243–252. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.; Yang, H.; Lin, Y.F.; Li, X.; Cape, A.; Ressler, K.J.; Li, S.; Li, X.J. Neuronal Abelson helper integration site-1 (Ahi1) deficiency in mice alters TrkB signaling with a depressive phenotype. Proc. Natl. Acad. Sci. USA 2010, 107, 19126–19131. [Google Scholar] [CrossRef] [PubMed]
- Torri, F.; Akelai, A.; Lupoli, S.; Sironi, M.; Amann-Zalcenstein, D.; Fumagalli, M.; dal Fiume, C.; Ben-Asher, E.; Kanyas, K.; Cagliani, R.; et al. Fine mapping of AHI1 as a schizophrenia susceptibility gene: from association to evolutionary evidence. FASEB J. 2010, 24, 3066–3082. [Google Scholar] [CrossRef] [PubMed]
- Amann-Zalcenstein, D.; Avidan, N.; Kanyas, K.; Ebstein, R.P.; Kohn, Y.; Hamdan, A.; Ben-Asher, E.; Karni, O.; Mujaheed, M.; Segman, R.H.; et al. AHI1, a pivotal neurodevelopmental gene, and C6orf217 are associated with susceptibility to schizophrenia. Eur. J. Hum. Genet. 2006, 14, 1111–1119. [Google Scholar] [CrossRef] [PubMed]
- Ingason, A.; Giegling, I.; Cichon, S.; Hansen, T.; Rasmussen, H.B.; Nielsen, J.; Jurgens, G.; Muglia, P.; Hartmann, A.M.; Strengman, E.; et al. A large replication study and meta-analysis in European samples provides further support for association of AHI1 markers with schizophrenia. Hum. Mol. Genet. 2010, 19, 1379–1386. [Google Scholar] [CrossRef] [PubMed]
- Ingason, A.; Sigmundsson, T.; Steinberg, S.; Sigurdsson, E.; Haraldsson, M.; Magnusdottir, B.B.; Frigge, M.L.; Kong, A.; Gulcher, J.; Thorsteinsdottir, U.; et al. Support for involvement of the AHI1 locus in schizophrenia. Eur. J. Hum. Genet. 2007, 15, 988–991. [Google Scholar] [CrossRef] [PubMed]
- Rivero, O.; Reif, A.; Sanjuan, J.; Molto, M.D.; Kittel-Schneider, S.; Najera, C.; Topner, T.; Lesch, K.P. Impact of the AHI1 gene on the vulnerability to schizophrenia: A case-control association study. PLoS One 2010, 5, e12254. [Google Scholar] [CrossRef] [PubMed]
- Ren, L.; Qian, X.; Zhai, L.; Sun, M.; Miao, Z.; Li, J.; Xu, X. Loss of Ahi1 impairs neurotransmitter release and causes depressive behaviors in mice. PLoS One 2014, 9, e93640. [Google Scholar] [CrossRef] [PubMed]
- Niu, S.; Wang, H.; Huang, Z.; Rao, X.; Cai, X.; Liang, T.; Xu, J.; Xu, X.; Sheng, G. Expression changes of hypothalamic Ahi1 in mice brain: implication in sensing insulin signaling. Mol. Biol. Rep. 2012, 39, 9697–9705. [Google Scholar] [CrossRef] [PubMed]
- Huang, L.; Teng, D.; Wang, H.; Sheng, G.; Liu, T. Association of copy number variation in the AHI1 gene with risk of obesity in the Chinese population. Eur. J. Endocrinol./Eur. Fed. Endocr. Soc. 2012, 166, 727–734. [Google Scholar] [CrossRef]
- Wang, H.; Huang, Z.; Huang, L.; Niu, S.; Rao, X.; Xu, J.; Kong, H.; Yang, J.; Yang, C.; Wu, D.; et al. Hypothalamic Ahi1 mediates feeding behavior through interaction with 5-HT2C receptor. J. Biol. Chem. 2012, 287, 2237–2246. [Google Scholar] [CrossRef]
- Han, S.B.; Choi, B.I.; Lee, D.; Kee, S.H.; Kim, H.S.; Sun, W.; Kim, H. Regulation of AHI1 expression in adult rat brain: Implication in hypothalamic feeding control. Biochem. Biophys. Res. Commun. 2009, 390, 535–540. [Google Scholar] [CrossRef] [PubMed]
- Lerer, B.; Segman, R.H.; Hamdan, A.; Kanyas, K.; Karni, O.; Kohn, Y.; Korner, M.; Lanktree, M.; Kaadan, M.; Turetsky, N.; et al. Genome scan of Arab Israeli families maps a schizophrenia susceptibility gene to chromosome 6q23 and supports a locus at chromosome 10q24. Mol. Psychiatry 2003, 8, 488–498. [Google Scholar] [CrossRef] [PubMed]
- Levi, A.; Kohn, Y.; Kanyas, K.; Amann, D.; Pae, C.U.; Hamdan, A.; Segman, R.H.; Avidan, N.; Karni, O.; Korner, M.; et al. Fine mapping of a schizophrenia susceptibility locus at chromosome 6q23: Increased evidence for linkage and reduced linkage interval. Eur. J. Hum. Genet. 2005, 13, 763–771. [Google Scholar] [CrossRef] [PubMed]
- Slonimsky, A.; Levy, I.; Kohn, Y.; Rigbi, A.; Ben-Asher, E.; Lancet, D.; Agam, G.; Lerer, B. Lymphoblast and brain expression of AHI1 and the novel primate-specific gene, C6orf217, in schizophrenia and bipolar disorder. Schizophr. Res. 2010, 120, 159–166. [Google Scholar] [CrossRef] [PubMed]
- Cao, Q.; Martinez, M.; Zhang, J.; Sanders, A.R.; Badner, J.A.; Cravchik, A.; Markey, C.J.; Beshah, E.; Guroff, J.J.; Maxwell, M.E.; et al. Suggestive evidence for a schizophrenia susceptibility locus on chromosome 6q and a confirmation in an independent series of pedigrees. Genomics 1997, 43, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Martinez, M.; Goldin, L.R.; Cao, Q.; Zhang, J.; Sanders, A.R.; Nancarrow, D.J.; Taylor, J.M.; Levinson, D.F.; Kirby, A.; Crowe, R.R.; et al. Follow-up study on a susceptibility locus for schizophrenia on chromosome 6q. Am. J. Med. Genet. 1999, 88, 337–343. [Google Scholar] [CrossRef] [PubMed]
- Levinson, D.F.; Holmans, P.; Straub, R.E.; Owen, M.J.; Wildenauer, D.B.; Gejman, P.V.; Pulver, A.E.; Laurent, C.; Kendler, K.S.; Walsh, D.; et al. Multicenter linkage study of schizophrenia candidate regions on chromosomes 5q, 6q, 10p, and 13q: Schizophrenia linkage collaborative group III. Am. J. Hum. Genet. 2000, 67, 652–663. [Google Scholar] [CrossRef] [PubMed]
- Alvarez Retuerto, A.I.; Cantor, R.M.; Gleeson, J.G.; Ustaszewska, A.; Schackwitz, W.S.; Pennacchio, L.A.; Geschwind, D.H. Association of common variants in the Joubert syndrome gene (AHI1) with autism. Hum. Mol. Genet. 2008, 17, 3887–3896. [Google Scholar] [CrossRef] [PubMed]
- Holroyd, S.; Reiss, A.L.; Bryan, R.N. Autistic features in Joubert syndrome: A genetic disorder with agenesis of the cerebellar vermis. Biol Psychiatry 1991, 29, 287–294. [Google Scholar] [CrossRef] [PubMed]
- Porcelli, S.; Pae, C.U.; Han, C.; Lee, S.J.; Patkar, A.A.; Masand, P.S.; Balzarro, B.; Alberti, S.; de Ronchi, D.; Serretti, A. PorcelliAbelson helper integration site-1 gene variants on major depressive disorder and bipolar disorder. Psychiatry Investig. 2014, in press. [Google Scholar]
- CHIP Bioinformatics Tools—SNPper. Available online: http://snpper.chip.org/bio/snpper-enter (accessed on 1 January 2001).
- Sullivan, P.F. Spurious genetic associations. Biol. Psychiatry 2007, 61, 1121–1126. [Google Scholar] [CrossRef] [PubMed]
- Sheehan, D.V.; Lecrubier, Y.; Sheehan, K.H.; Amorim, P.; Janavs, J.; Weiller, E.; Hergueta, T.; Baker, R.; Dunbar, G.C. The Mini-International Neuropsychiatric Interview (M.I.N.I.): The development and validation of a structured diagnostic psychiatric interview for DSM-IV and ICD-10. J. Clin. Psychiatry 1998, 59, 22–33; 34–57. [Google Scholar] [PubMed]
- Kay, S.R.; Fiszbein, A.; Opler, L.A. The positive and negative syndrome scale (PANSS) for schizophrenia. Schizophr. Bull. 1987, 13, 261–276. [Google Scholar] [CrossRef] [PubMed]
- Barrett, J.C.; Fry, B.; Maller, J.; Daly, M.J. Haploview: Analysis and visualization of LD and haplotype maps. Bioinformatics 2005, 21, 263–265. [Google Scholar] [CrossRef] [PubMed]
- Benjamini, Y.; Drai, D.; Elmer, G.; Kafkafi, N.; Golani, I. Controlling the false discovery rate in behavior genetics research. Behav. Brain Res. 2001, 125, 279–284. [Google Scholar] [CrossRef] [PubMed]
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Porcelli, S.; Pae, C.-U.; Han, C.; Lee, S.-J.; Patkar, A.A.; Masand, P.S.; Balzarro, B.; Alberti, S.; De Ronchi, D.; Serretti, A. The Influence of AHI1 Variants on the Diagnosis and Treatment Outcome in Schizophrenia. Int. J. Mol. Sci. 2015, 16, 2517-2529. https://doi.org/10.3390/ijms16022517
Porcelli S, Pae C-U, Han C, Lee S-J, Patkar AA, Masand PS, Balzarro B, Alberti S, De Ronchi D, Serretti A. The Influence of AHI1 Variants on the Diagnosis and Treatment Outcome in Schizophrenia. International Journal of Molecular Sciences. 2015; 16(2):2517-2529. https://doi.org/10.3390/ijms16022517
Chicago/Turabian StylePorcelli, Stefano, Chi-Un Pae, Changsu Han, Soo-Jung Lee, Ashwin A. Patkar, Prakash S. Masand, Beatrice Balzarro, Siegfried Alberti, Diana De Ronchi, and Alessandro Serretti. 2015. "The Influence of AHI1 Variants on the Diagnosis and Treatment Outcome in Schizophrenia" International Journal of Molecular Sciences 16, no. 2: 2517-2529. https://doi.org/10.3390/ijms16022517
APA StylePorcelli, S., Pae, C.-U., Han, C., Lee, S.-J., Patkar, A. A., Masand, P. S., Balzarro, B., Alberti, S., De Ronchi, D., & Serretti, A. (2015). The Influence of AHI1 Variants on the Diagnosis and Treatment Outcome in Schizophrenia. International Journal of Molecular Sciences, 16(2), 2517-2529. https://doi.org/10.3390/ijms16022517





