Cloning of the Major Capsid Protein (MCP) of Grouper Iridovirus of Taiwan (TGIV) and Preliminary Evaluation of a Recombinant MCP Vaccine against TGIV
Abstract
:1. Introduction
2. Results
2.1. Sequence Analysis of TGIV-MCP
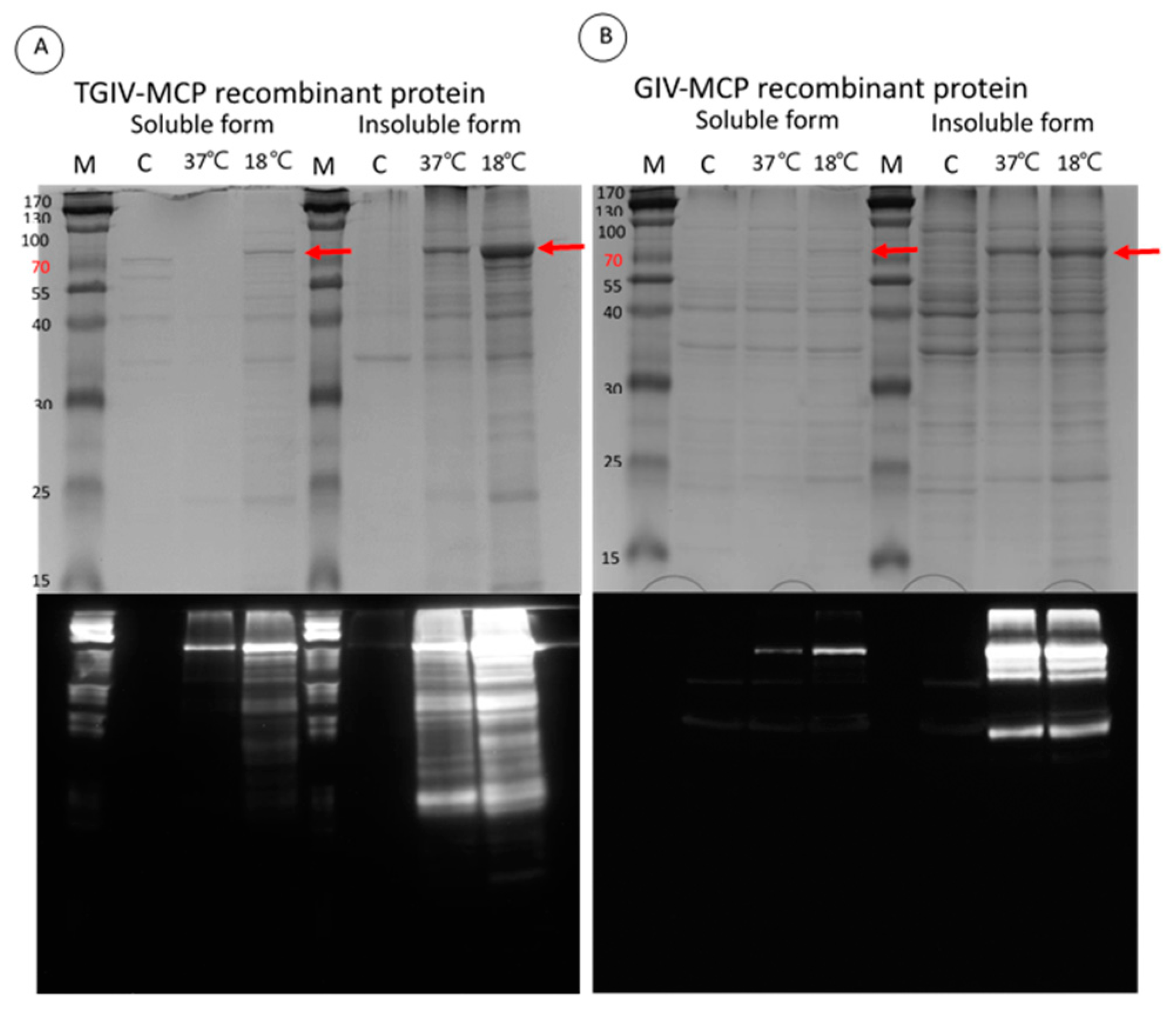
2.2. Expression and Purification of Recombinant TGIV-MCP and GIV-MCP for Production of Polyclonal Antibodies
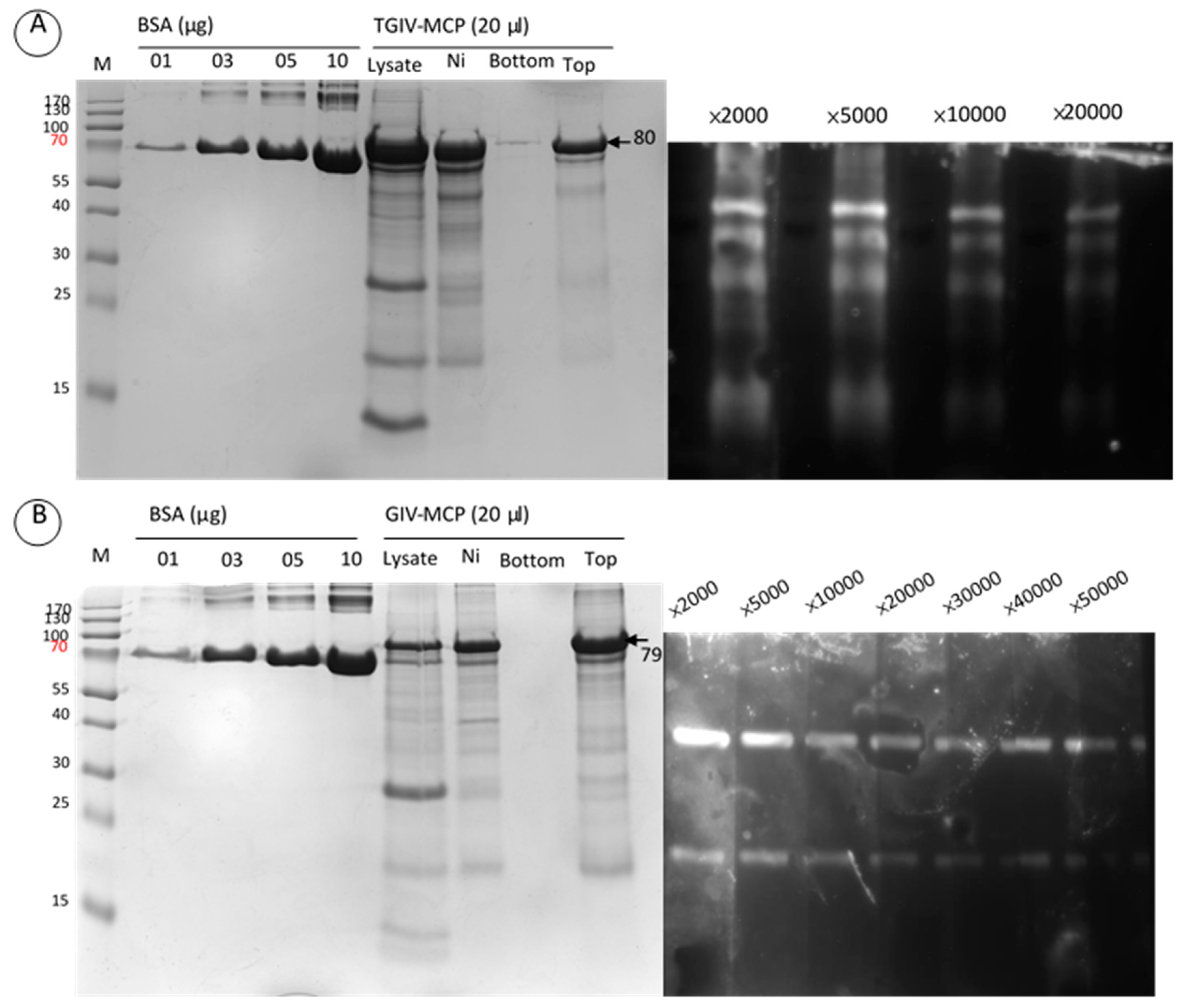
2.3. Specificity of Anti-TGIV-MCP and Anti-GIV-MCP Sera

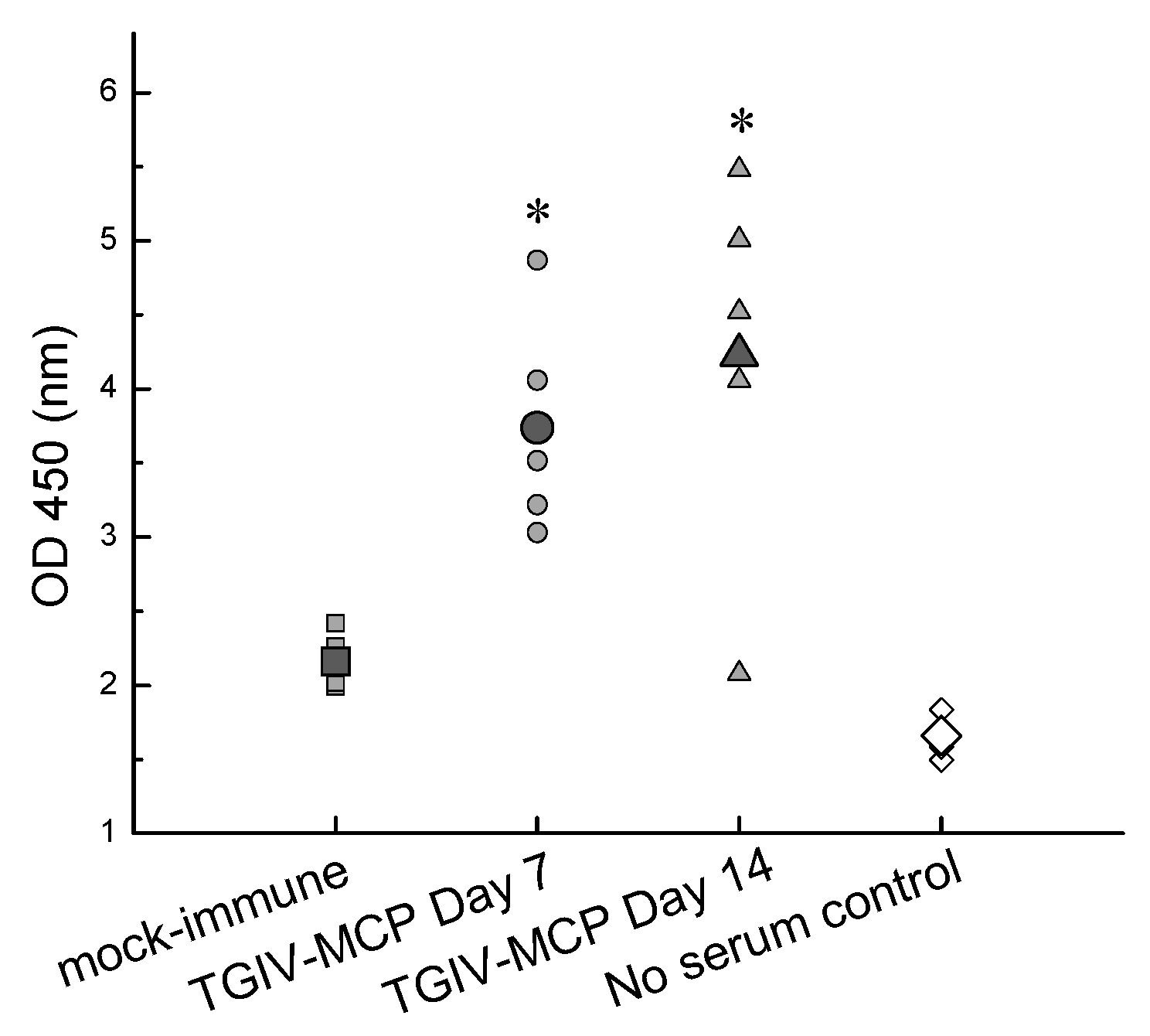
2.4. Immunogenicity of Recombinant TGIV-MCP
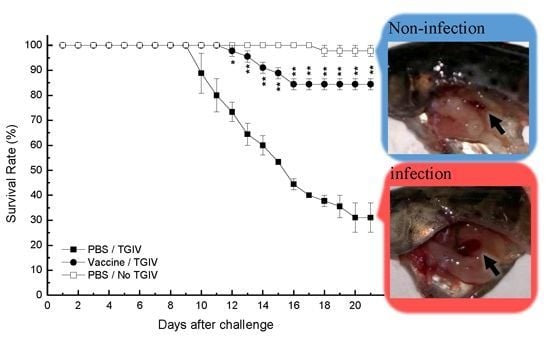
2.5. Efficacy of Recombinant TGIV-MCP Vaccine
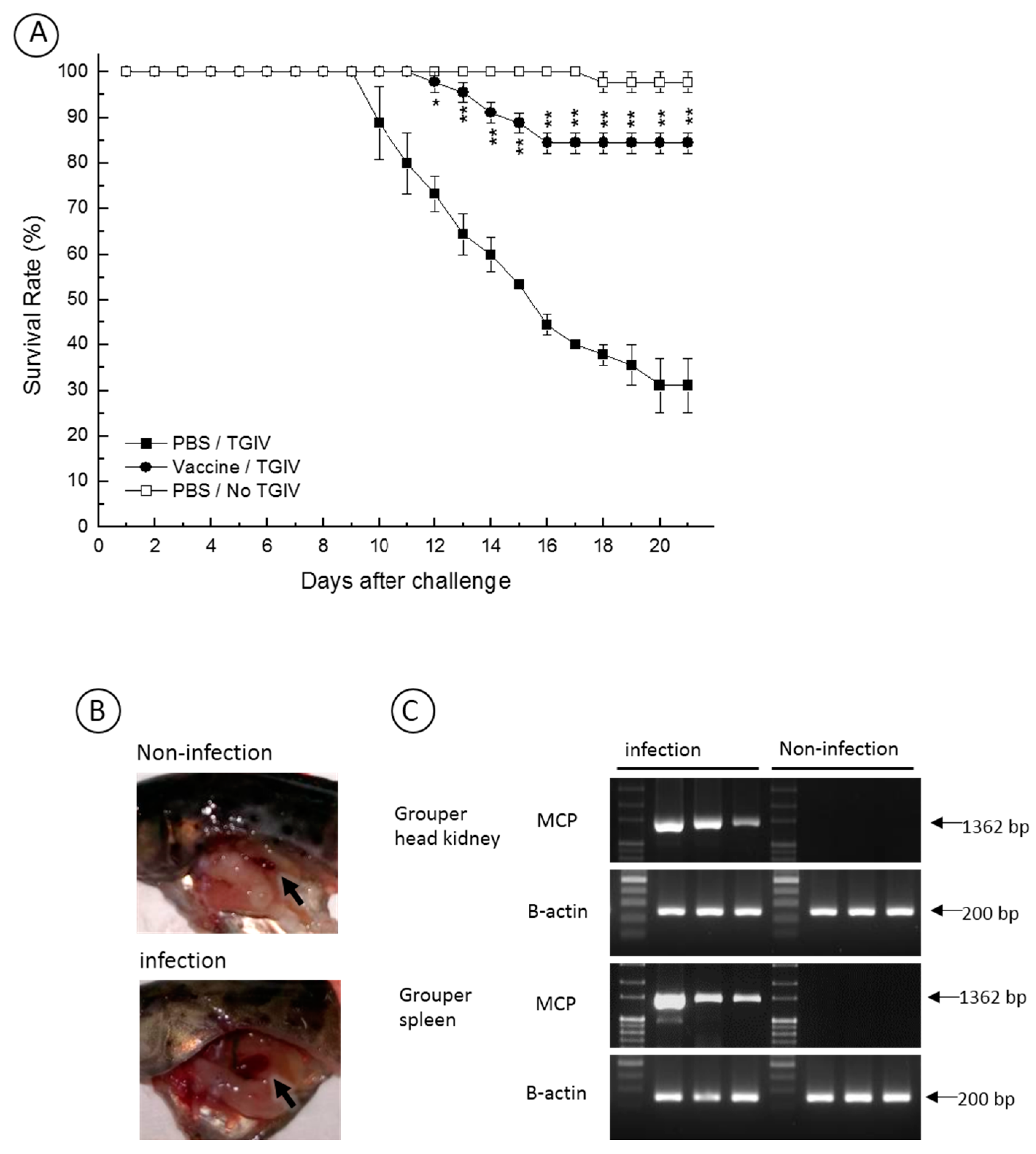
3. Discussion
4. Experimental Section
4.1. Cell Culture
4.2. Grouper
4.3. TGIV Major Capsid Protein Gene Clone
4.4. Expression of Recombinant TGIV Major Capsid Protein
4.5. SDS-PAGE
4.6. Western Blot
4.7. Analysis of Antibody Production in Response to Immunization
4.8. Vaccination and TGIV Challenge
4.9. Reverse Transcription-PCR, RT-PCR
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Council of Agriculture (COA). Agricultural Statistics Yearbook 2014; Council of Agriculture: Taipei, Taiwan, 2014.
- Chinchar, V.G. Ranaviruses (family Iridoviridae): Emerging cold-blooded killers. Arch. Virol. 2002, 147, 447–470. [Google Scholar] [CrossRef] [PubMed]
- Williams, T. The iridoviruses. Adv. Virus Res. 1996, 46, 345–412. [Google Scholar] [PubMed]
- Wang, C.S.; Shih, H.H.; Ku, C.C.; Chen, S.N. Studies on epizootic iridovirus infection among red sea bream, Pagrus major (Temminck & Schlegel), cultured in Taiwan. J. Fish. Dis. 2003, 26, 127–133. [Google Scholar] [PubMed]
- Chua, F.H.C.; Ng, M.L.; Ng, K.L.; Loo, J.J.; Wee, J.Y. Investigation of outbreaks of a novel disease, “Sleepy Grouper Disease”, affecting the brown-spotted grouper, Epinephelus tauvina Forskal. J. Fish. Dis. 1994, 17, 417–427. [Google Scholar] [CrossRef]
- Murali, S.; Wu, M.F.; Guo, I.C.; Chen, S.C.; Yang, H.W.; Chang, C.Y. Molecular characterization and pathogenicity of a grouper iridovirus (GIV) isolated from yellow grouper, Epinephelus awoara (Temminck & Schlegel). J. Fish. Dis. 2002, 25, 91–100. [Google Scholar]
- Kawakami, H.; Nakajima, K. Cultured fish species affected by red sea bream iridoviral disease from 1996 to 2000. Fish. Pathol. 2002, 37, 45–48. [Google Scholar] [CrossRef]
- Mahardika, K.; Zafran; Yamamoto, A.; Miyazaki, T. Susceptibility of juvenile humpback grouper Cromileptes altivelis to grouper sleepy disease iridovirus (GSDIV). Dis. Aquat. Organ. 2004, 59, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Huang, Z.; Tang, J.; Li, M.; Fu, Y.; Dong, C.; Zhong, J.F.; He, J. Immunological evaluation of Vibrio alginolyticus, Vibrio harveyi, Vibrio vulnificus and infectious spleen and kidney necrosis virus (ISKNV) combined-vaccine efficacy in Epinephelus coioides. Vet. Immunol. Immunopathol. 2012, 150, 61–68. [Google Scholar] [CrossRef] [PubMed]
- He, J.G.; Weng, S.P.; Zeng, K.; Huang, Z.J.; Chan, S.M. Systemic disease caused by an iridovirus-like agent in cultured mandarinfish, Siniperca chuatsi (Basilewsky), in China. J. Fish. Dis. 2000, 23, 219–222. [Google Scholar] [CrossRef]
- Wang, Y.Q.; Lü, L.; Weng, S.P.; Huang, J.N.; Chan, S.M.; He, J.G. Molecular epidemiology and phylogenetic analysis of a marine fish infectious spleen and kidney necrosis virus-like (ISKNV-like) virus. Arch. Virol. 2007, 152, 763–773. [Google Scholar] [CrossRef] [PubMed]
- Chou, H.Y.; Hsu, C.C.; Peng, T.Y. Isolation and characterization of a pathogenic iridovirus from cultured grouper (Epinephelus sp.) in Taiwan. Fish. Pathol. 1998, 33, 201–206. [Google Scholar] [CrossRef]
- Black, P.N.; Blair, C.D.; Butcher, A.; Capinera, J.L.; Happ, G.M. Biochemistry and ultrastructure of iridescent virus type 29. J. Invertebr. Pathol. 1981, 38, 12–21. [Google Scholar] [CrossRef]
- Willis, D.B.; Goorha, R.; Miles, M.; Granoff, A. Macromolecular synthesis in cells infected by frog virus 3. VII. Transcriptional and post-transcriptional regulation of virus gene expression. J. Virol. 1977, 24, 326–342. [Google Scholar] [PubMed]
- Sommerset, I.; Krossoey, B.; Biering, E.; Frost, P. Vaccines for fish in aquaculture. Expert Rev. Vaccines 2005, 4, 89–101. [Google Scholar] [CrossRef] [PubMed]
- Caipang, C.M.A.; Takano, T.; Hirono, I.; Aoki, T. Genetic vaccines protect red seabream, Pagrus major, upon challenge with red seabream iridovirus (RSIV). Fish. Shellfish Immunol. 2006, 21, 130–138. [Google Scholar] [CrossRef] [PubMed]
- Kim, T.J.; Jang, E.J.; Lee, J.I. Vaccination of rock bream, Oplegnathus fasciatus (Temminck & Schlegel), using a recombinant major capsid protein of fish iridovirus. J. Fish. Dis. 2008, 31, 547–551. [Google Scholar] [PubMed]
- Hill, B.J.; Way, K. Serological classification of infectious pancreatic necrosis (IPN) virus and other aquatic birnaviruses. Annu. Rev. Fish. Dis. 1995, 5, 55–77. [Google Scholar] [CrossRef]
- Nakajima, K.; Maeno, Y.; Kurita, J.; Inui, Y. Vaccination against red sea bream iridoviral disease in red sea bream. Fish. Pathol. 1997, 32, 205–209. [Google Scholar] [CrossRef]
- Nakajima, K.; Maeno, Y.; Honda, A.; Yokoyama, K.; Tooriyama, T.; Manabe, S. Effectiveness of a vaccine against red sea bream iridoviral disease in a field trial test. Dis. Aquat. Organ. 1999, 36, 73–75. [Google Scholar] [CrossRef] [PubMed]
- Christie, K.E. Immunization with viral antigens: Infectious pancreatic necrosis. Dev. Biol. Stand. 1997, 90, 191–199. [Google Scholar] [PubMed]
- Anderson, E.; Clouthier, S.; Shewmaker, W.; Weighall, A.; LaPatra, S. Inactivated infectious haematopoietic necrosis virus (IHNV) vaccines. J. Fish. Dis. 2008, 31, 729–745. [Google Scholar] [CrossRef] [PubMed]
- Kai, Y.-H.; Chi, S.-C. Efficacies of inactivated vaccines against betanodavirus in grouper larvae (Epinephelus coioides) by bath immunization. Vaccine 2008, 26, 1450–1457. [Google Scholar] [CrossRef] [PubMed]
- Caipang, C.M.A.; Hirono, I.; Aoki, T. Immunogenicity, retention and protective effects of the protein derivatives of formalin-inactivated red seabream iridovirus (RSIV) vaccine in red seabream, Pagrus major. Fish. Shellfish Immunol. 2006, 20, 597–609. [Google Scholar] [CrossRef] [PubMed]
- Shin, Y.J.; Kwon, T.H.; Seo, J.Y.; Kim, T.J. Oral immunization of fish against iridovirus infection using recombinant antigen produced from rice callus. Vaccine 2013, 31, 5210–5215. [Google Scholar] [CrossRef] [PubMed]
- Seo, J.Y.; Chung, H.J.; Kim, T.J. Codon-optimized expression of fish iridovirus capsid protein in yeast and its application as an oral vaccine candidate. J. Fish. Dis. 2013, 36, 763–768. [Google Scholar] [CrossRef] [PubMed]
- Fu, X.; Li, N.; Lin, Q.; Guo, H.; Zhang, D.; Liu, L.; Wu, S. Protective immunity against infectious spleen and kidney necrosis virus induced by immunization with DNA plasmid containing mcp gene in Chinese perch Siniperca chuatsi. Fish. Shellfish Immunol. 2014, 40, 259–266. [Google Scholar] [CrossRef] [PubMed]
- Fernandez, R.D.; Yoshimizu, M.; Kimura, T.; Ezura, Y.; Inouye, K.; Takami, I. Characterization of three continuous cell lines from marine fish. J. Aquat. Anim Health 1993, 5, 127–136. [Google Scholar] [CrossRef]
- Saitou, N.; Nei, M. The neighbor-joining method: A new method for reconstructing phylogenetic trees. Mol. Biol. Evol. 1987, 4, 406–425. [Google Scholar] [PubMed]
- Kumar, S.; Tamura, K.; Nei, M. MEGA3: Integrated software for Molecular Evolutionary Genetics Analysis and sequence alignment. Brief. Bioinform. 2004, 5, 150–163. [Google Scholar] [CrossRef] [PubMed]
- Laemmli, U.K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 1970, 227, 680–685. [Google Scholar] [CrossRef] [PubMed]
- Amend, D.F. Potency testing of fish vaccines. Dev. Biol Stand. 1980, 447–454. [Google Scholar]
- Norušis, M.J. SPSS Inc. In SPSS Advanced Statistics User's Guide; SPSS: Chicago, IL, USA, 1990; p. 285. [Google Scholar]
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons by Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, H.-I.; Chiou, P.P.; Gong, H.-Y.; Chou, H.-Y. Cloning of the Major Capsid Protein (MCP) of Grouper Iridovirus of Taiwan (TGIV) and Preliminary Evaluation of a Recombinant MCP Vaccine against TGIV. Int. J. Mol. Sci. 2015, 16, 28647-28656. https://doi.org/10.3390/ijms161226118
Liu H-I, Chiou PP, Gong H-Y, Chou H-Y. Cloning of the Major Capsid Protein (MCP) of Grouper Iridovirus of Taiwan (TGIV) and Preliminary Evaluation of a Recombinant MCP Vaccine against TGIV. International Journal of Molecular Sciences. 2015; 16(12):28647-28656. https://doi.org/10.3390/ijms161226118
Chicago/Turabian StyleLiu, Hsin-I, Pinwen Peter Chiou, Hong-Yi Gong, and Hsin-Yiu Chou. 2015. "Cloning of the Major Capsid Protein (MCP) of Grouper Iridovirus of Taiwan (TGIV) and Preliminary Evaluation of a Recombinant MCP Vaccine against TGIV" International Journal of Molecular Sciences 16, no. 12: 28647-28656. https://doi.org/10.3390/ijms161226118
APA StyleLiu, H.-I., Chiou, P. P., Gong, H.-Y., & Chou, H.-Y. (2015). Cloning of the Major Capsid Protein (MCP) of Grouper Iridovirus of Taiwan (TGIV) and Preliminary Evaluation of a Recombinant MCP Vaccine against TGIV. International Journal of Molecular Sciences, 16(12), 28647-28656. https://doi.org/10.3390/ijms161226118