Production of Two Novel Methoxy-Isoflavones from Biotransformation of 8-Hydroxydaidzein by Recombinant Escherichia coli Expressing O-Methyltransferase SpOMT2884 from Streptomyces peucetius
Abstract
:1. Introduction
2. Results and Discussion
2.1. Expression of SpOMT2884 in Escherichia coli
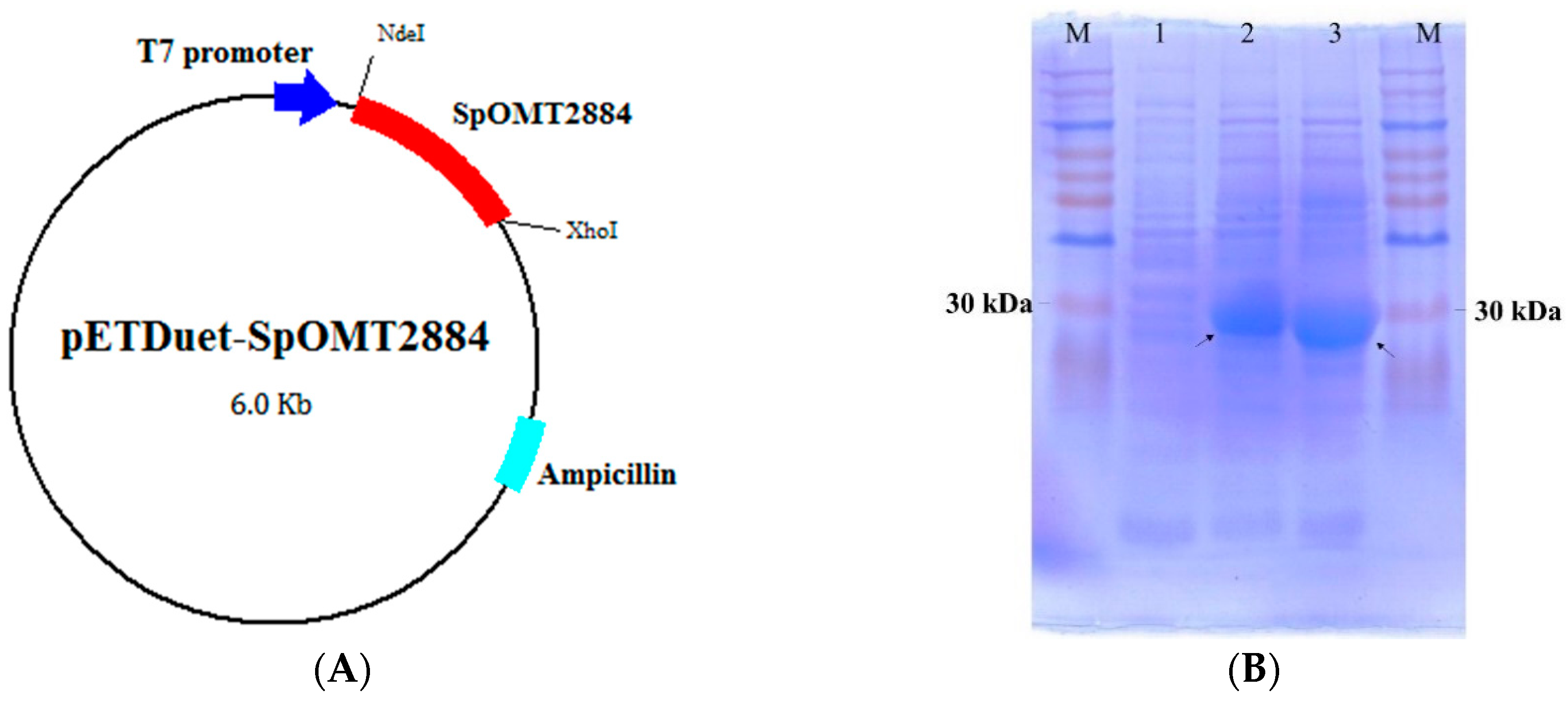
2.2. Biotransformation of 8-Hydroxydaidzein by the Recombinant E. coli
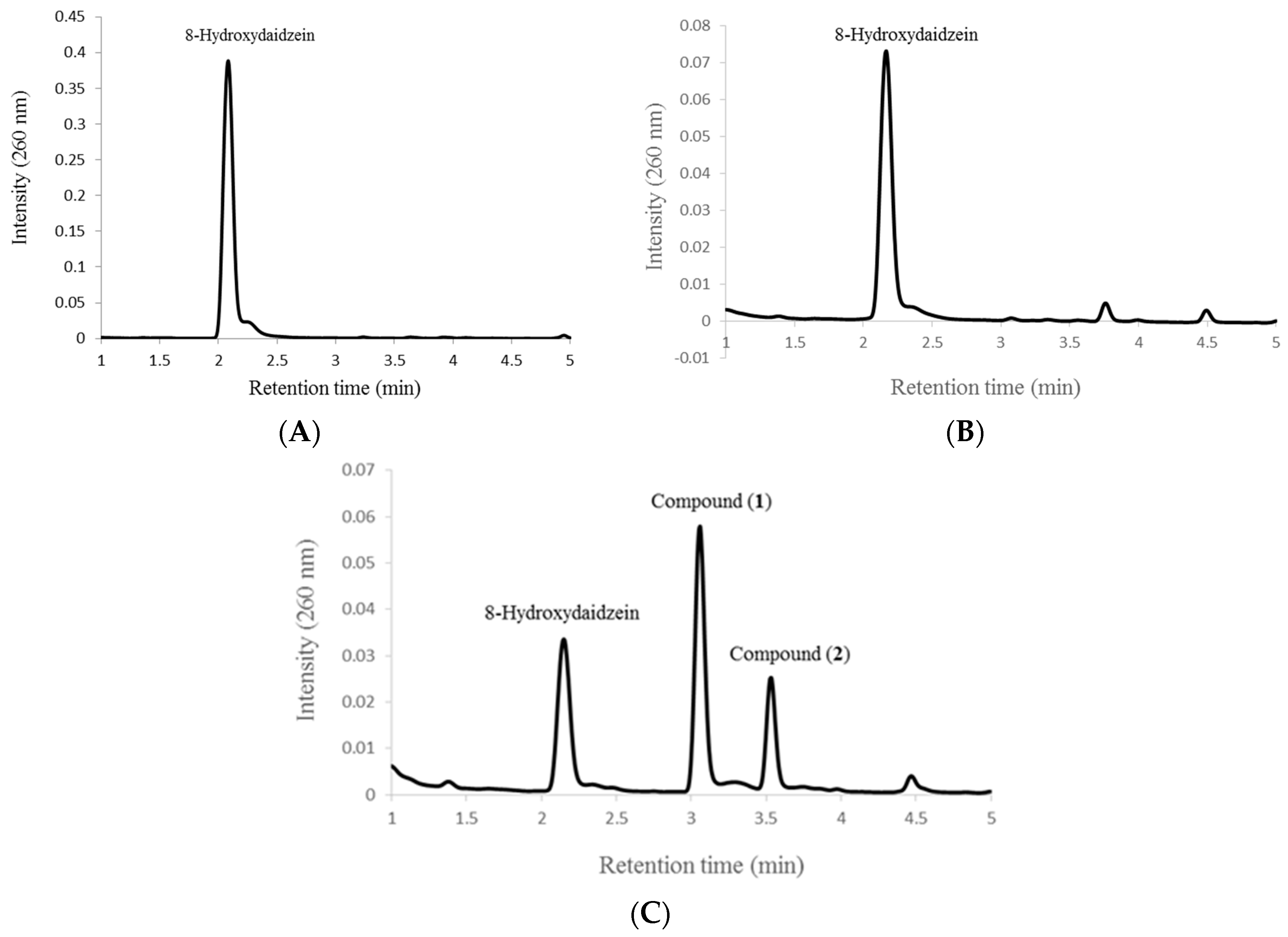
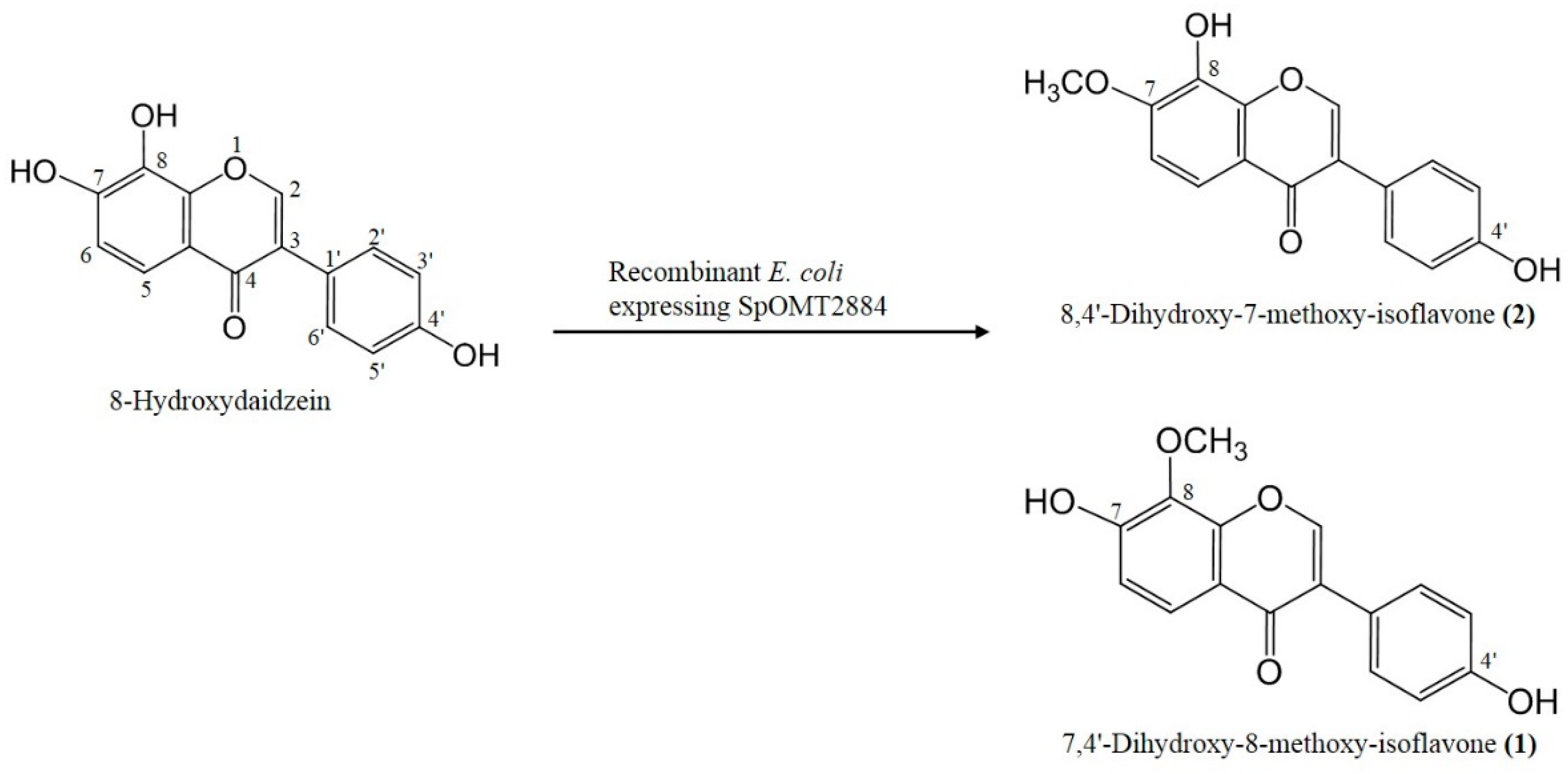
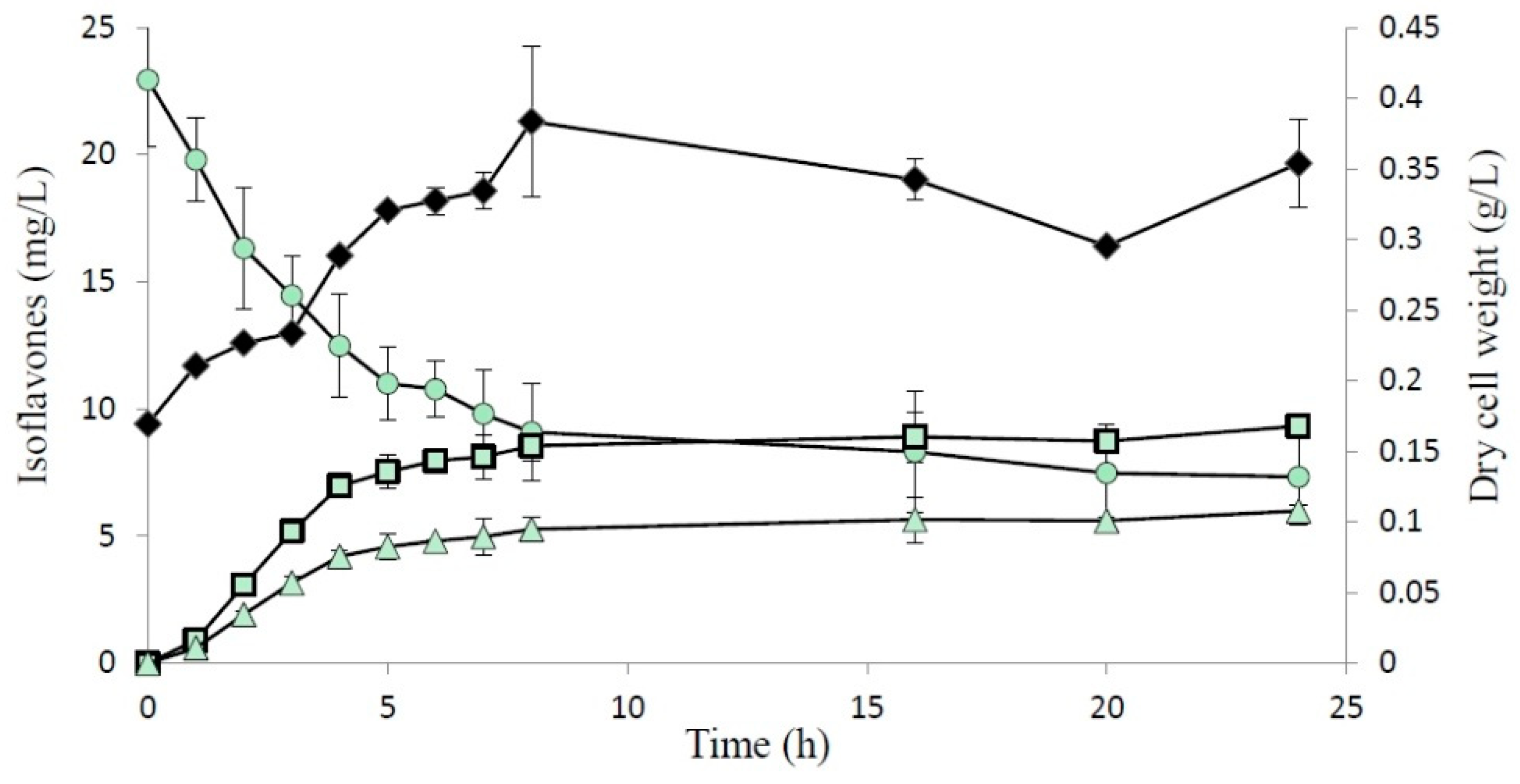
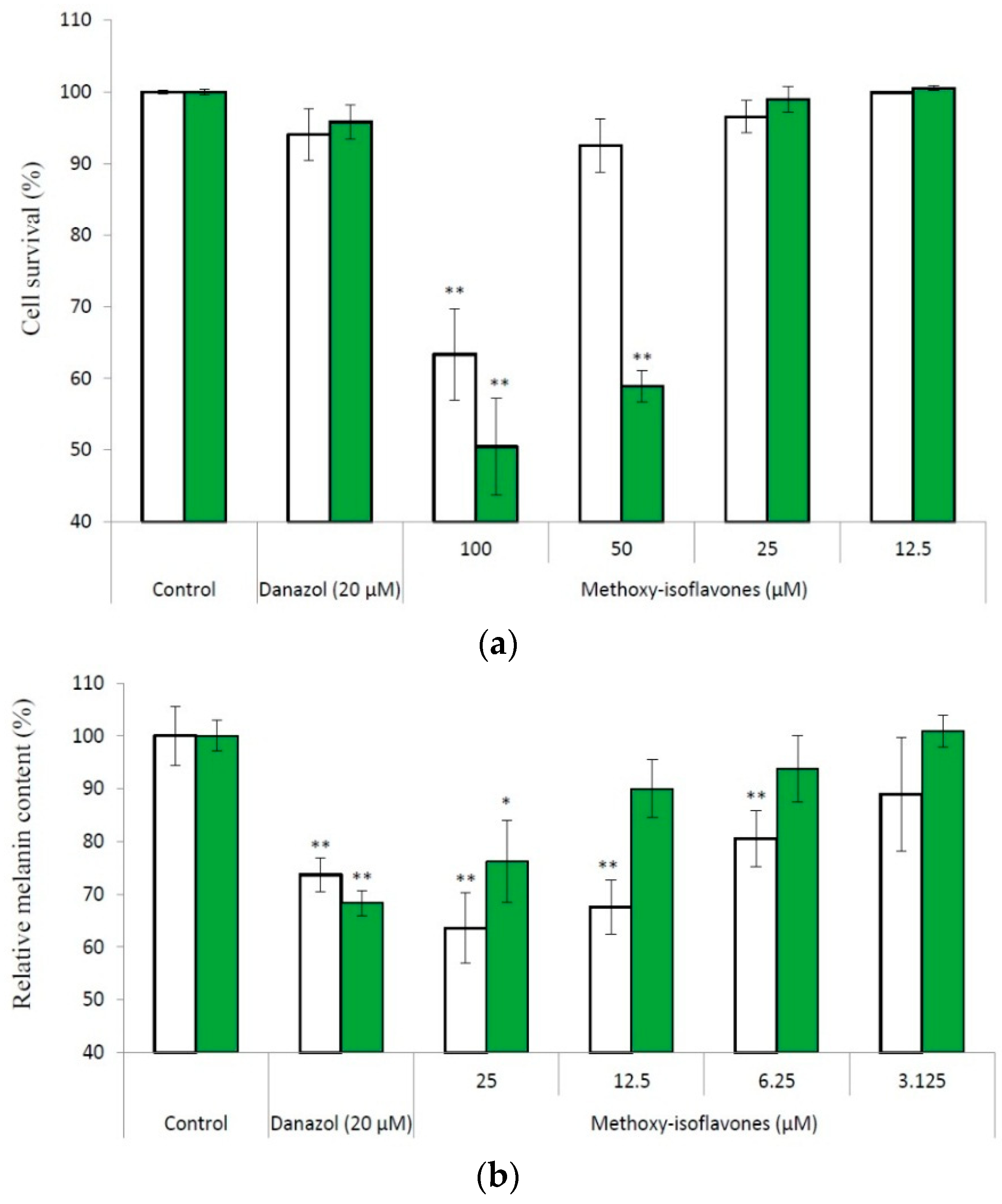
2.3 Inhibitory Activity on Melanogenesis of B16 Cells
3. Materials and Methods
3.1. Microorganisms, Cells and Chemicals
3.2. Expression of SpOMT2884 in E. coli
3.3. Fermentation and UPLC
3.4. Scale-up Fermentation, Isolation and Identification of Biotransformation Products
3.5. Determination of Cell Viability and Melanin Content
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Franke, A.A.; Custer, L.J.; Cerna, C.M.; Narala, K.K. Quantitation of phytoestrogens in legumes by HPLC. J. Agric. Food Chem. 1994, 42, 1905–1913. [Google Scholar] [CrossRef]
- Messina, M. A brief historical overview of the past two decades of soy and isoflavone research. J. Nutr. 2010, 140, 1350S–1354S. [Google Scholar] [CrossRef] [PubMed]
- Lee, D.E.; Lee, K.W.; Jung, S.K.; Lee, E.J.; Hwang, J.A.; Lim, T.G.; Kim, B.Y.; Bode, A.M.; Lee, H.J.; Dong, Z. 6,7,4′-Trihydroxyisoflavone inhibits HCT-116 human colon cancer cell proliferation by targeting CDK1 and CDK2. Carcinogenesis 2011, 32, 629–635. [Google Scholar] [CrossRef] [PubMed]
- Fujita, T.; Funako, T.; Hayashi, H. 8-Hydroxydaidzein, an aldose reductase inhibitor from okara fermented with Aspergillus sp. HK-388. Biosci. Biotechnol. Biochem. 2004, 68, 1588–1590. [Google Scholar] [CrossRef] [PubMed]
- Tai, S.S.K.; Lin, C.G.; Wu, M.H.; Chang, T.S. Evaluation of depigmenting activity by 8-hydroxydaidzein in mouse B16 melanoma cells and human volunteers. Int. J. Mol. Sci. 2009, 10, 4257–4266. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.C.; Inaba, M.; Abe, N.; Hirota, A. Antimutagenic activity of 8-hydroxyisoflavones and 6-hydroxydaidzein from soybean miso. Biosci. Biotechnol. Biochem. 2003, 67, 903–906. [Google Scholar] [CrossRef] [PubMed]
- Goh, M.J.; Park, J.S.; Bae, J.H.; Kim, D.H.; Kim, H.K.; Na, Y.J. Effects of ortho-dihydroxyisoflavone derivatives from Korean fermented soybean paste on melanogenesis in B16 melanoma cells and human skin equivalents. Phytother. Res. 2012, 26, 1107–1112. [Google Scholar] [CrossRef] [PubMed]
- Lo, Y.L.; Wang, W.; Ho, C.T. 7,3′,4′-Trihydroxyisoflavone modulates multidrug resistance transporters and induces apoptosis via production of reactive oxygen species. Toxicology 2012, 302, 221–232. [Google Scholar] [CrossRef] [PubMed]
- Chang, T.S.; Ding, H.Y.; Tai, S.S.K.; Wu, C.Y. Metabolism of the soy isoflavone daidzein and genistein by the fungi used for the preparation of various fermented soybean foods. Biosci. Biotechnol. Biochem. 2007, 71, 1330–1333. [Google Scholar] [CrossRef] [PubMed]
- Chang, T.S.; Chao, S.Y.; Chen, Y.C. Production of ortho-hydroxydaidzein derivatives by a recombinant strain of Pichia pastoris harboring a cytochrome P450 fusion gene. Process Biochem. 2013, 48, 426–429. [Google Scholar] [CrossRef]
- Ding, H.Y.; Chiang, C.M.; Chen, Y.C.; Chang, T.S. Identification of 3′-hydroxygenistein as a potent melanogenesis inhibitor from biotransformation of genistein by recombinant Pichia pastoris. Process Biochem. 2015, 50, 1614–1617. [Google Scholar] [CrossRef]
- Kim, B.G.; Sung, S.H.; Chong, S.Y.; Lim, Y.; Ahn, J.H. Plant flavonoid O-methyltransferase: substrate specificity and application. J. Plant Biol. 2010, 53, 321–329. [Google Scholar] [CrossRef]
- Lin, V.C.; Ding, H.Y.; Tsai, P.C.; Wu, J.Y.; Lu, Y.H.; Chang, T.S. In vitro and in vivo melanogenesis inhibition by biochanin A from Trifolium pretense. Biosci. Biotechnol. Biochem. 2011, 75, 914–918. [Google Scholar] [CrossRef] [PubMed]
- Walle, T. Methylation of dietary flavones increases their metabolic stability and chemopreventive effects. Int. J. Mol. Sci. 2009, 10, 5002–5019. [Google Scholar] [CrossRef] [PubMed]
- Bernini, R.; Crisante, F.; Ginnasi, M.C. A convenient and safe O-methylation of flavonoids with dimethyl carbonate (DMC). Molecules 2011, 16, 1418–1425. [Google Scholar] [CrossRef] [PubMed]
- Koirala, N.; Pandey, R.P.; Parajuli, P.; Jung, H.J.; Sohng, J.K. Methylation and subsequent glycosylation of 7,8-dihydroxyflavone. J. Biotechnol. 2014, 184, 128–137. [Google Scholar] [CrossRef] [PubMed]
- Ren, F.Z.; Gao, Y.Q.; Cheng, X.X.; Li, L.H.; Chen, S.H.; Zhang, Y.I. Study on chemical constituents of Zornia diphylla. Chin. Pharm. J. 2012, 47, 179–181. [Google Scholar]
- Huang, W.Z.; Duan, J.; Li, Z.L. Studies on chemical constituents of Maackia amurensis Rupr. Et. Maxim. Chin. J. Chin. Mater. Med. 2001, 26, 403–404. [Google Scholar]
- Choi, K.Y.; Jung, E.; Yang, Y.H.; Kim, B.G. Production of a novel O-methyl-isoflavone by regioselective sequential hydroxylation and O-methylation reactions in Streptomyces avermitilis host system. Biotechnol. Bioeng. 2013, 110, 2591–2599. [Google Scholar] [CrossRef] [PubMed]
- Chang, T.S.; Lin, J.J. Inhibitory effect of danazol on melanogenesis in mouse B16 melanoma cells. Arch. Pharm. Res. 2010, 33, 1959–1965. [Google Scholar] [CrossRef] [PubMed]
- Shi, H.M.; Huang, Z.Q.; Wen, J.; Tu, P.F. A new isoflavone from Abrus mollis. Chin. J. Nat. Med. 2006, 4, 30–31. [Google Scholar]
- Tang, R.N.; Qu, X.B.; Guan, S.H.; Xu, P.P.; Shi, Y.Y.; Guo, D.A. Chemical constituents of Spatholobus suberectus. Chin. J. Nat. Med. 2012, 10, 32–35. [Google Scholar] [CrossRef] [PubMed]
- Paliy, O.; Gunasekera, T.S. Growth of E. coli BL21 in minimal media with different gluconeogenic carbon sources and salt contents. Appl. Microbiol. Biotechnol. 2007, 73, 1169–1172. [Google Scholar] [CrossRef] [PubMed]
- Chang, T.S.; Tsai, Y.H. Inhibition of melanogenesis by yeast extracts from cultivations of recombinant Pichia pastoris catalyzing ortho-hydroxylation of flavonoids. Curr. Pharma. Biotechnol. 2015, 16, 1085–1093. [Google Scholar] [CrossRef]
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons by Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chiang, C.-M.; Ding, H.-Y.; Tsai, Y.-T.; Chang, T.-S. Production of Two Novel Methoxy-Isoflavones from Biotransformation of 8-Hydroxydaidzein by Recombinant Escherichia coli Expressing O-Methyltransferase SpOMT2884 from Streptomyces peucetius. Int. J. Mol. Sci. 2015, 16, 27816-27823. https://doi.org/10.3390/ijms161126070
Chiang C-M, Ding H-Y, Tsai Y-T, Chang T-S. Production of Two Novel Methoxy-Isoflavones from Biotransformation of 8-Hydroxydaidzein by Recombinant Escherichia coli Expressing O-Methyltransferase SpOMT2884 from Streptomyces peucetius. International Journal of Molecular Sciences. 2015; 16(11):27816-27823. https://doi.org/10.3390/ijms161126070
Chicago/Turabian StyleChiang, Chien-Min, Hsiou-Yu Ding, Ya-Ting Tsai, and Te-Sheng Chang. 2015. "Production of Two Novel Methoxy-Isoflavones from Biotransformation of 8-Hydroxydaidzein by Recombinant Escherichia coli Expressing O-Methyltransferase SpOMT2884 from Streptomyces peucetius" International Journal of Molecular Sciences 16, no. 11: 27816-27823. https://doi.org/10.3390/ijms161126070
APA StyleChiang, C.-M., Ding, H.-Y., Tsai, Y.-T., & Chang, T.-S. (2015). Production of Two Novel Methoxy-Isoflavones from Biotransformation of 8-Hydroxydaidzein by Recombinant Escherichia coli Expressing O-Methyltransferase SpOMT2884 from Streptomyces peucetius. International Journal of Molecular Sciences, 16(11), 27816-27823. https://doi.org/10.3390/ijms161126070




