Effect of Human Adipose Tissue Mesenchymal Stem Cells on the Regeneration of Ovine Articular Cartilage
Abstract
:1. Introduction
2. Results
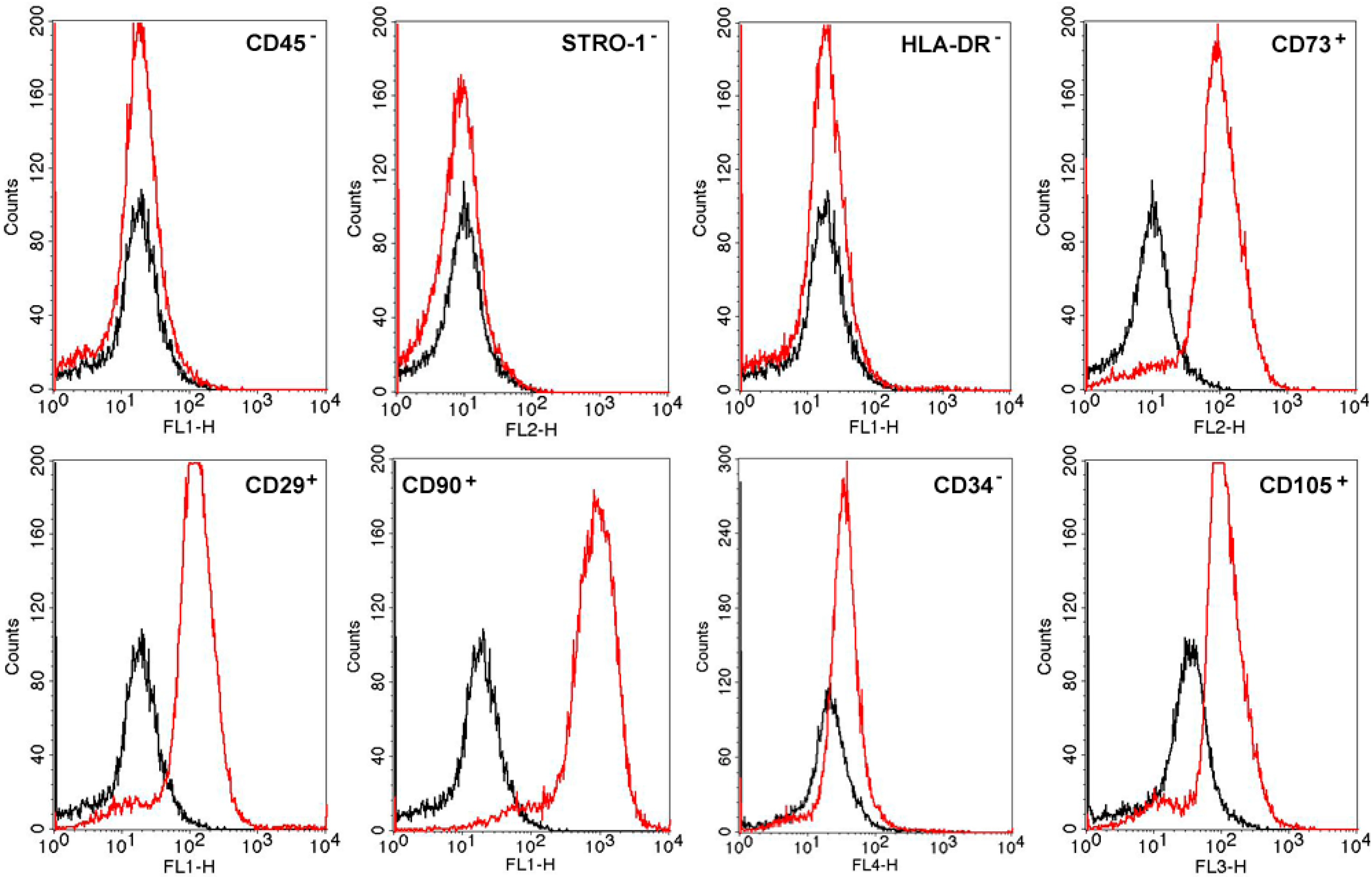
2.1. MSC Characterization and Differentiation

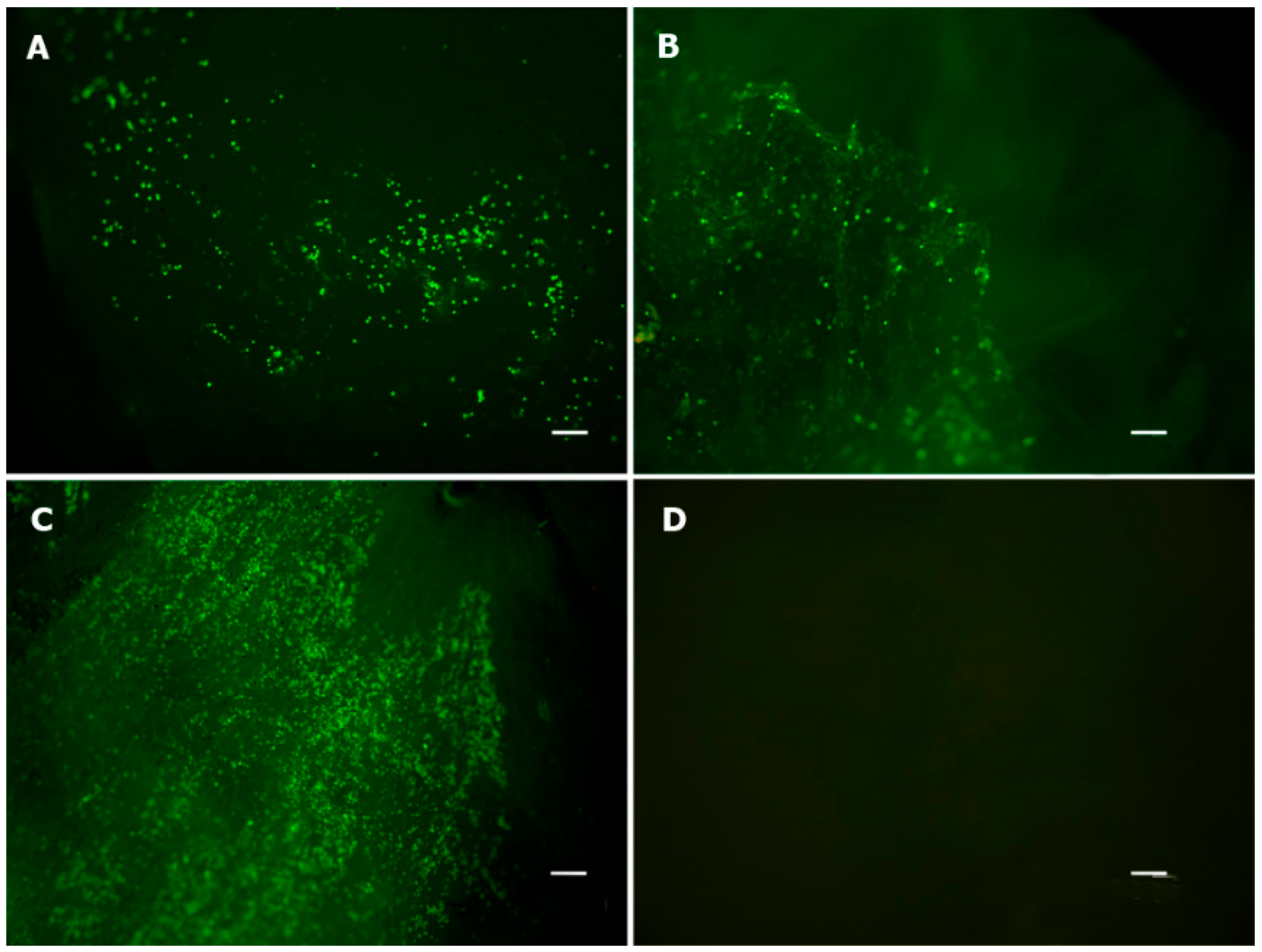
2.2. Viability Assay
2.3. Animal Experiment

2.4. Macroscopic Findings

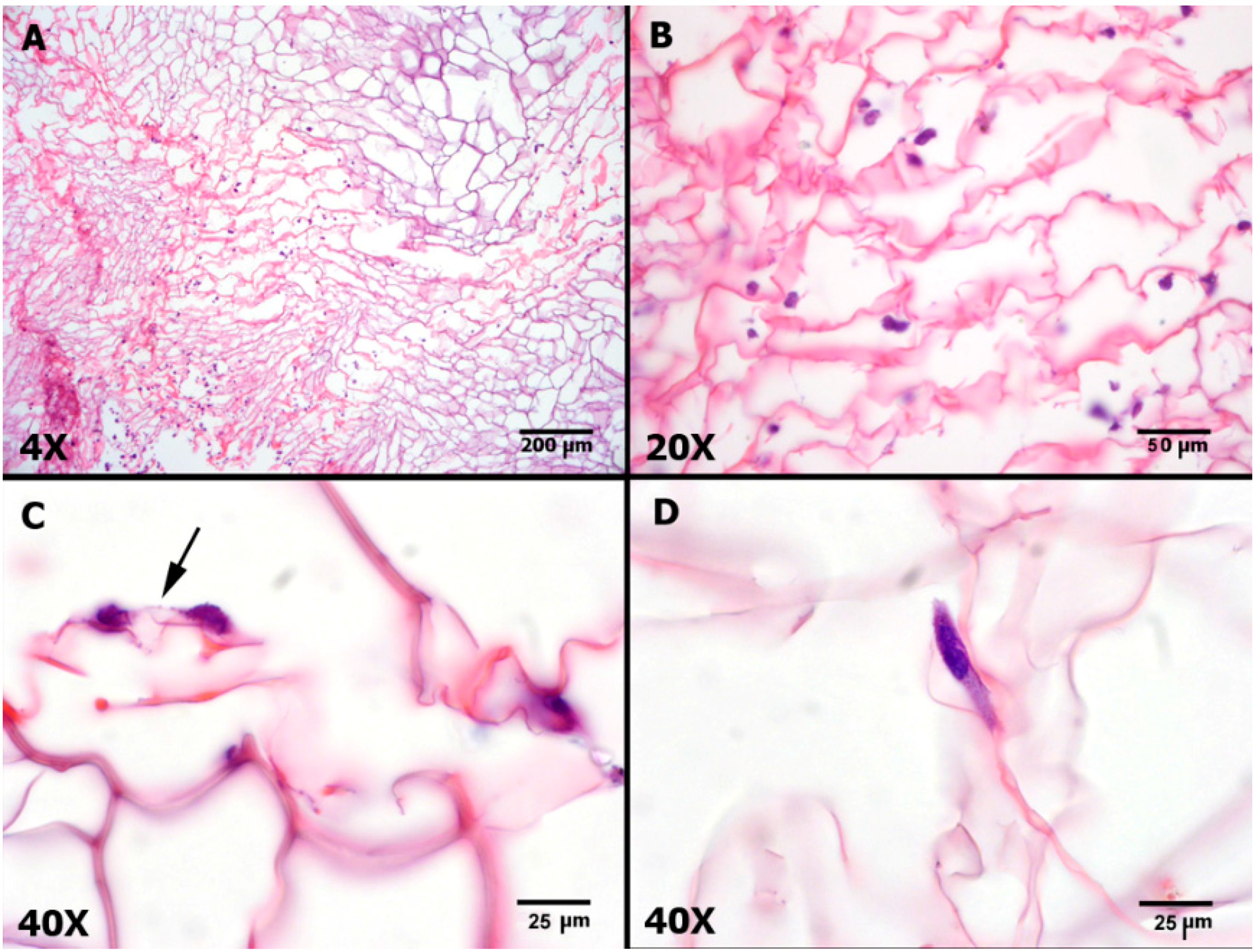
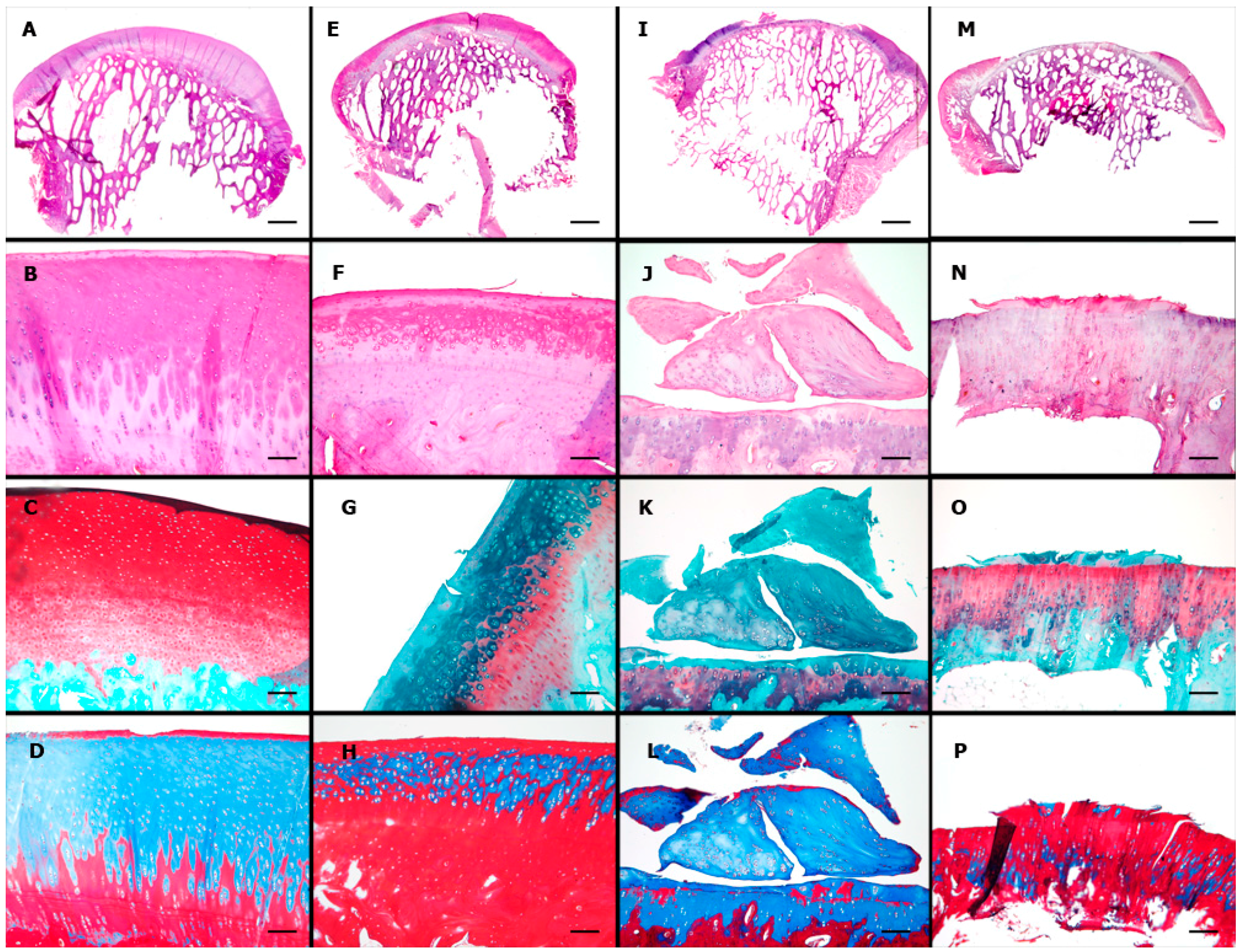
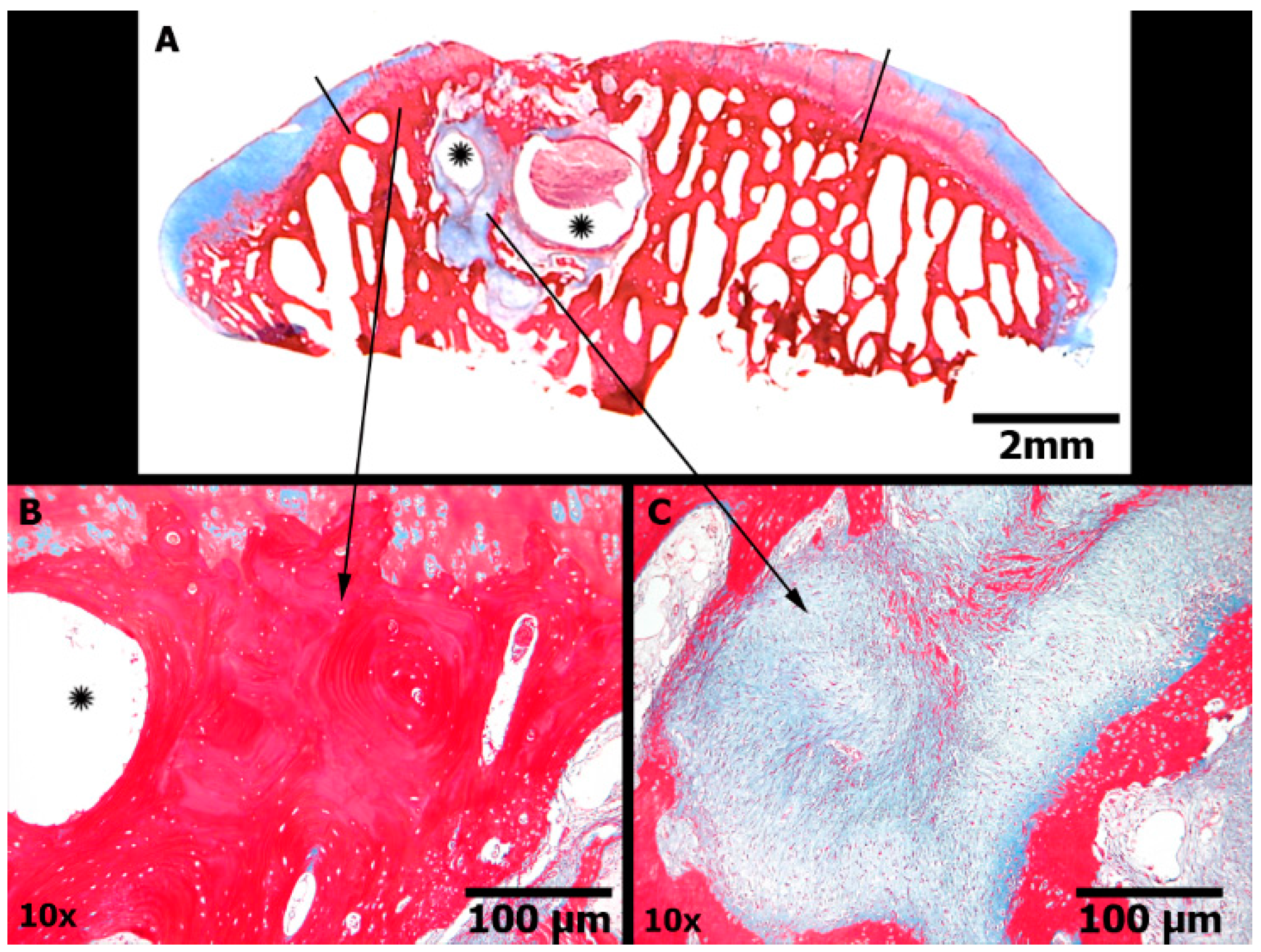
2.5. Microscopic Findings
| Sub-Item | Score | CELLS (n =10) | SCAFFOLD (n = 10) | EMPTY (n = 10) | p | |
|---|---|---|---|---|---|---|
| I | Surface | 3 | 3 | 1 | 0 | 0.089 |
| 0 | 7 | 9 | 10 | |||
| II | Matrix | 2 or 3 | 5 | 2 | 0 | 0.013 * |
| 0 or 1 | 5 | 8 | 10 | |||
| II | Cell Distribution | 2 or 3 | 5 | 3 | 1 | 0.131 |
| 0 or 1 | 5 | 7 | 9 | |||
| IV | Cell Viability | 3 | 5 | 2 | 3 | 0.349 |
| 0 or 1 | 5 | 8 | 7 | |||
| V | Subchondral Bone | 2 or 3 | 6 | 1 | 4 | 0.050 * |
| 0 or 1 | 4 | 9 | 6 |
| Sub-Item | CELLS (n = 10) | SCAFFOLD (n = 10) | EMPTY (n = 10) | p |
|---|---|---|---|---|
| Horizontal filling | 2.0 ± 0.7 | 1.8 ± 0.6 | 1.3 ± 0.5 | 0.044 * |
| Vertical filling | 1.6 ± 0.7 | 1.6 ± 0.7 | 1.4 ± 0.8 | 0.541 |
| Cellularity | 1.3 ± 0.5 | 1.0 ± 0.5 | 0.7 ± 0.5 | 0.038 * |
| Safranin | 0.7 ± 0.5 | 0.7 ± 0.5 | 0.6 ± 0.7 | 0.801 |
| Borders | 1.9 ± 0.3 | 1.6 ± 0.5 | 1.6 ± 0.5 | 0.251 |
| Residual calcified cartilage | 2.2 ± 0.9 | 1.4 ± 0.8 | 2.1 ± 1.1 | 0.132 |
| Subchondral bone | 3.0 ± 0.7 | 2.3 ± 0.7 | 3.1 ± 1.0 | 0.090 |
| Total | 12.7 ± 2.2 | 10.5 ± 2.1 | 11.2 ± 3.6 | 0.167 |
3. Discussion
4. Experimental Section
4.1. Human Adipose Tissue Mesenchymal Stem Cells (hAT-MSCs)
4.1.1. Flow Cytometry Analysis
4.1.2. Mesodermal Lineage Differentiation
4.2. Scaffold Preparation
4.3. Viability Assay
4.4. Experimental Design
- (1)
- CELLS: hAT-MSCs were seeded in a collagen/chitosan scaffold that was used to fill the lesions;
- (2)
- SCAFFOLD: the same scaffold was used to fill the lesion, but no cells were included;
- (3)
- EMPTY: the lesion was left untreated.
4.5. Surgical Procedure
4.6. Macroscopic Analysis
4.7. Microscopic Analysis
| Feature | Score | |
|---|---|---|
| I | Surface | |
| Smooth/continuous | 3 | |
| Discontinuities/irregularities | 0 | |
| II | Matrix | |
| Hyaline | 3 | |
| Mixture: hyaline/fibrocartilage | 2 | |
| Fibrocartilage | 1 | |
| Fibrous tissue | 0 | |
| III | Cell distribution | |
| Columnar | 3 | |
| Mixed columnar/clusters | 2 | |
| Clusters | 1 | |
| Individual cells/ disorganized | 0 | |
| IV | Cell viability | |
| Predominantly viable | 3 | |
| Partially viable | 1 | |
| <10% viable | 0 | |
| V | Subchondral bone | |
| Normal | 3 | |
| Increased remodeling | 2 | |
| Bone necrosis/granulation tissue | 1 | |
| Detached/callus/fracture | 0 | |
| VI | Calcified cartilage | |
| Normal | 3 | |
| Abnormal/ inappropriate location | 0 |
| Repair Tissue inside the Lesion | Feature | Score | |
|---|---|---|---|
| I | Horizontal filling | 75%–100% | 4 |
| 50%–74% | 3 | ||
| 20%–49% | 2 | ||
| 1%–19% | 1 | ||
| 0 | 0 | ||
| II | Vertical filling | 75%–100% | 4 |
| 50%–74% | 3 | ||
| 20%–49% | 2 | ||
| 1%–19% | 1 | ||
| 0 | 0 | ||
| III | Cellularity | Normal | 2 |
| Mild hypocellularity, <25% clusters | 1 | ||
| Moderate or intense hypocellularity, >25% clusters | 0 | ||
| IV | Safranin staining | Homogeneous | 2 |
| Heterogeneous | 1 | ||
| Negative | 0 | ||
| Cartilage Around the Lesion | |||
| V | Borders ingrowth or integration | Bilateral | 2 |
| Unilateral | 1 | ||
| None | 0 | ||
| Base of the Lesion | |||
| VI | Residual calcified cartilage | Intact or integrated | 3 |
| Fibrillation or fissure | 2 | ||
| Focal erosion | 1 | ||
| Severe disruption | 0 | ||
| VII | Subchondral bone | Normal | 4 |
| Mild cystic lesions or granulation tissue | 3 | ||
| Moderate or severe cystic lesions or granulation tissue | 2 | ||
| Remodeling, sclerosis, callus | 1 | ||
| Fracture, necrosis | 0 |
4.8. Statistical Analysis
4.8.1. Sample Size
4.8.2. Data Analysis
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Poole, C.A. Articular cartilage chondrons: Form, function and failure. J. Anat. 1997, 191, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Iwamoto, M.; Ohta, Y.; Larmour, C.; Enomoto-Iwamoto, M. Towards regeneration of articular cartilage. Birth Defects Res. C Embryo Today 2014, 3, 192–202. [Google Scholar] [CrossRef] [PubMed]
- Hjelle, K.; Solheim, E.; Strand, T.; Muri, R.; Brittberg, M. Articular cartilage defects in 1000 knee arthroscopies. Arthroscopy 2002, 18, 730–734. [Google Scholar] [CrossRef] [PubMed]
- Widuchowski, W.; Widuchowski, J.; Koczy, B.; Szyluk, K. Untreated asymptomatic deep cartilage lesions associated with anterior cruciate ligament injury: Results at 10- and 15-year follow-up. Am. J. Sports Med. 2009, 37, 688–692. [Google Scholar] [CrossRef] [PubMed]
- Widuchowski, W.; Widuchowski, J.; Faltus, R.; Lukasik, P.; Kwiatkowski, G.; Szyluk, K.; Koczy, B. Long-term clinical and radiological assessment of untreated severe cartilage damage in the knee: A natural history study. Scand. J. Med. Sci. Sports 2011, 21, 106–110. [Google Scholar] [CrossRef] [PubMed]
- Oussedik, S.; Tsitskaris, K.; Parker, D. Treatment of articular cartilage lesions of the knee by microfracture or autologous chondrocyte implantation: A systematic review. Arthroscopy 2015, 31, 732–744. [Google Scholar] [CrossRef] [PubMed]
- Schinhan, M.; Gruber, M.; Vavken, P.; Dorotka, R.; Samouh, L.; Chiari, C.; Gruebl-Barabas, R.; Nehrer, S. Critical-size defect induces unicompartmental osteoarthritis in a stable ovine knee. J. Orthop. Res. 2011, 30, 214–220. [Google Scholar] [CrossRef] [PubMed]
- Saris, D.B.; Dhert, W.J.; Verbout, A.J. Joint homeostasis. J. Bone Jt. Surg. Br. 2003, 85, 1067–1076. [Google Scholar] [CrossRef]
- Saris, D.; Price, A.; Widuchowski, W.; Bertrand-Marchand, M.; Caron, J.; Drogset, J.O.; Emans, P.; Podskubka, A.; Tsuchida, A.; Kili, S.; et al. On behalf of the SUMMIT study group. Matrix-applied characterized autologous cultured chondrocytes versus microfracture: Two-year follow-up of a prospective randomized trial. Am. J. Sports Med. 2014, 42, 1384–1394. [Google Scholar] [CrossRef] [PubMed]
- Jacobi, M.; Villa, V.; Magnussen, R.A.; Neyret, P. MACI—A new era? Sports Med. Arthrosc. Rehabil. Ther. Technol. 2011, 3. [Google Scholar] [CrossRef] [PubMed]
- Bornes, T.D.; Adesida, A.B.; Jomha, N.M. Mesenchymal stem cells in the treatment of traumatic articular cartilage defects: A comprehensive review. Arthritis Res. Ther. 2014, 16, 1–19. [Google Scholar] [CrossRef]
- Madeira, C.; Santhagunam, A.; Salgueiro, J.B.; Cabral, J.M. Advanced cell therapies for articular cartilage regeneration. Trends Biotechnol. 2015, 33, 35–42. [Google Scholar] [CrossRef] [PubMed]
- Makris, E.A.; Gomoll, A.H.; Malizos, K.N.; Hu, J.C.; Athanasiou, K.A. Repair and tissue engineering techniques for articular cartilage. Nat. Rev. Rheumatol. 2015, 11, 21–34. [Google Scholar] [CrossRef] [PubMed]
- Lim, M.H.; Ong, W.K.; Sugii, S. The current landscape of adipose-derived stem cells in clinical applications. Expert Rev. Mol. Med. 2014, 16. [Google Scholar] [CrossRef] [PubMed]
- Zuk, P.A.; Zhu, M.; Mizuno, H.; Huang, J.; Futrell, J.W.; Katz, A.J.; Benhaim, P.; Lorenz, H.P.; Hedrick, M.H. Multilineage cells from human adipose tissue: Implications for cell-based therapies. Tissue Eng. 2001, 7, 211–228. [Google Scholar] [CrossRef] [PubMed]
- De Francesco, F.; Ricci, G.; D’Andrea, F.; Nicoletti, G.F.; Ferraro, G.A. Human adipose stem cells: From bench to bed-side. Tissue Eng. Part B Rev. 2015. [Google Scholar] [CrossRef] [PubMed]
- Ruetze, M.; Richter, W. Adipose-derived stromal cells for osteoarticular repair: Trophic function versus stem cell activity. Expert Rev. Mol. Med. 2014, 16. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; He, N.; Feng, C.; Liu, V.; Zhang, L.; Wang, F.; He, J.; Zhu, T.; Wang, S.; Qiao, W.; et al. Human adipose-derived mesenchymal progenitor cells engraft into rabbit articular cartilage. Int. J. Mol. Sci. 2015, 16, 12076–12091. [Google Scholar] [CrossRef] [PubMed]
- Bordeaux-Rego, P.; Baratti, M.O.; Duarte, A.S.; Ribeiro, T.B.; Andreoli-Risso, M.F.; Vidal, B.; Miranda, J.B.; Adur, J.; de Thomaz, A.A.; Pelegati, V.B.; et al. Use of the second harmonic generation microscopy to evaluate chondrogenic differentiation of mesenchymal stem cells for cartilage repair. In Multiphoton Microscopy in the Biomedical Sciences XII; Periasamy, A., König, K., Eds.; SPIE: Bellingham, WA, USA, 2012; Volume 8226, p. 82263N. [Google Scholar]
- Mueller, M.B.; Tuan, R.S. Functional characterization of hypertrophy in chondrogenesis of human mesenchymal stem cells. Arthritis Rheumatol. 2008, 58, 1377–1388. [Google Scholar] [CrossRef] [PubMed]
- Augello, A.; de Bari, C. The regulation of differentiation in mesenchymal stem cells. Hum. Gene Ther. 2010, 21, 1226–1238. [Google Scholar] [CrossRef] [PubMed]
- Grässel, S.; Lorenz, J. Tissue-engineering strategies to repair chondral and osteochondral tissue in osteoarthritis: Use of mesenchymal stem cells. Curr. Rheumatol. Rep. 2014, 16, 452. [Google Scholar] [CrossRef] [PubMed]
- Kehoe, O.; Cartwright, A.; Askari, A.; El Haj, A.J.; Middleton, J. Intra-articular injection of mesenchymal stem cells leads to reduced inflammation and cartilage damage in murine antigen-induced arthritis. J. Trans. Med. 2014, 12, 157. [Google Scholar] [CrossRef] [PubMed]
- Guilak, F. Homing in on a biological joint replacement. Stem Cell Res. Ther. 2010, 1. [Google Scholar] [CrossRef] [PubMed]
- Ramallal, M.; Maneiro, E.; López, E.; Fuentes-Boquete, I.; López-Armada, M.J.; Fernández-Sueiro, J.L.; Galdo, F.; de Toro, F.J.; Blanco, F.J. Xeno-implantation of pig chondrocytes into rabbit to treat localized articular cartilage defects: An animal model. Wound Repair Regen. 2004, 12, 337–345. [Google Scholar] [CrossRef] [PubMed]
- Niemietz, T.; Zass, G.; Hagmann, S.; Diederichs, S.; Gotterbarm, T.; Richter, W. Xenogeneic transplantation of articular chondrocytes into full-thickness articular cartilage defects in minipigs: Fate of cells and the role of macrophages. Cell Tissue Res. 2014, 358, 749–761. [Google Scholar] [CrossRef] [PubMed]
- Matsumoto, T.; Okabe, T.; Ikawa, T.; Iida, T.; Yasuda, H.; Nakamura, H.; Wakitani, S. Articular cartilage repair with autologous bone marrow mesenchymal cells. J. Cell. Physiol. 2010, 225, 291–295. [Google Scholar] [CrossRef] [PubMed]
- Wilke, M.M.; Nydam, D.V.; Nixon, A.J. Enhanced early chondrogenesis in articular defects following arthroscopic mesenchymal stem cell implantation in an equine model. J. Orthop. Res. 2007, 25, 913–925. [Google Scholar] [CrossRef] [PubMed]
- Lim, C.T.; Ren, X.; Afizah, M.H.; Tarigan-Panjaitan, S.; Yang, Z.; Wu, Y.; Chian, K.S.; Mikos, A.G.; Hui, J.H. Repair of osteochondral defects with rehydrated freeze-dried oligo[poly(ethylene glycol) fumarate] hydrogels seeded with bone marrow mesenchymal stem cells in a porcine model. Tissue Eng. Part A 2013, 19, 1852–1861. [Google Scholar] [CrossRef] [PubMed]
- Murata, D.; Tokunaga, S.; Tamura, T.; Kawaguchi, H.; Miyoshi, N.; Fujiki, M.; Nakayama, K.; Misumi, K. A preliminary study of osteochondral regeneration using a scaffold-free three-dimensional construct of porcine adipose tissue-derived mesenchymal stem cells. J. Orthop. Surg. Res. 2015, 10. [Google Scholar] [CrossRef] [PubMed]
- Friedman, M.J.; Berasi, C.C.; Fox, J.M.; del Pizzo, W.; Snyder, S.J.; Ferkel, R.D. Preliminary results with abrasion arthroplasty in the osteoarthritic knee. Clin. Orthop. Relat. Res. 1984, 182, 200–205. [Google Scholar] [CrossRef] [PubMed]
- Minas, T.; Gomoll, A.H.; Rosenberger, R.; Royce, R.O.; Bryant, T. Increased failure rate of autologous chondrocyte implantation after previous treatment with marrow stimulation techniques. Am. J. Sports Med. 2009, 37, 902–908. [Google Scholar] [CrossRef] [PubMed]
- Gomoll, A.H.; Madry, H.; Knutsen, G.; van Dijk, N.; Seil, R.; Brittberg, M.; Kon, E. The subchondral bone in articular cartilage repair: Current problems in the surgical management. Knee Surg. Sports Traumatol. Arthrosc. 2010, 18, 434–447. [Google Scholar] [CrossRef] [PubMed]
- Efe, T.; Schofer, M.D.; Füglein, A.; Timmesfeld, N.; Fuchs-Winkelmann, S.; Stein, T.; El-Zayat, B.F.; Paletta, J.R.; Heyse, T.J. An ex vivo continuous passive motion model in a porcine knee for assessing primary stability of cell-free collagen gel plugs. BMC Musculoskelet. Disord. 2010, 11. [Google Scholar] [CrossRef] [PubMed]
- Bekkers, J.E.; Tsuchida, A.I.; Malda, J.; Creemers, L.B.; Castelein, R.J.; Saris, D.B.; Dhert, W.J. Quality of scaffold fixation in a human cadaver knee model. Osteoarthr. Cartil. 2010, 18, 266–272. [Google Scholar] [CrossRef] [PubMed]
- Drobnic, M.; Radosavljevic, D.; Ravnik, D.; Pavlovcic, V.; Hribernik, M. Comparison of four techniques for the fixation of a collagen scaffold in the human cadaveric knee1. Osteoarthr. Cartil. 2006, 14, 337–344. [Google Scholar] [CrossRef] [PubMed]
- O’Driscoll, S.W.; Keeley, F.W.; Salter, R.B. Durability of regenerated articular cartilage produced by free autogenous periosteal grafts in major full-thickness defects in joint surfaces under the influence of continuous passive motion. A follow-up report at one year. J. Bone Jt. Surg. Am. 1988, 70, 595–607. [Google Scholar]
- Hoemann, C.; Kandel, R.; Roberts, S.; Saris, D.B.; Creemers, L.; Mainil-Varlet, P.; Méthot, S.; Hollander, A.P.; Buschmann, M.D. International Cartilage Repair Society (ICRS) recommended guidelines for histological endpoints for cartilage repair studies in animal models and clinical trials. Cartilage 2011, 2, 153–172. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.; Liu, T.; Song, K.; Jiang, B.; Ma, X.; Cui, Z. Collagen-chitosan polymer as a scaffold for the proliferation of human adipose tissue-derived stem cells. J. Mater. Sci. Mater. Med. 2008, 20, 799–808. [Google Scholar] [CrossRef] [PubMed]
- Yan, L.-P.; Wang, Y.-J.; Ren, L.; Wu, G.; Caridade, S.G.; Fan, J.-B.; Wang, L.-Y.; Ji, P.-H.; Oliveira, J.M.; Oliveira, J.T.; et al. Genipin-cross-linked collagen/chitosan biomimetic scaffolds for articular cartilage tissue engineering applications. J. Biomed. Mater. Res. 2010, 95, 465–475. [Google Scholar] [CrossRef] [PubMed]
- Gong, Z.; Xiong, H.; Long, X.; Wei, L.; Li, J.; Wu, Y.; Lin, Z. Use of synovium-derived stromal cells and chitosan/collagen type I scaffolds for cartilage tissue engineering. Biomed. Mater. 2010, 5, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Da Luz Moreira, P.; Genari, S.C.; Goissis, G.; Galembeck, F.; An, Y.H.; Santos, A.R., Jr. Bovine osteoblasts cultured on polyanionic collagen scaffolds: An ultrastructural and immunocytochemical study. J. Biomed. Mater. Res. 2012, 101, 18–27. [Google Scholar] [CrossRef] [PubMed]
- Horn, M.M.; Martins, V.C.A.; de Guzzi Plepis, A.M. Interaction of anionic collagen with chitosan: Effect on thermal and morphological characteristics. Carbohydr. Polym. 2009, 77, 239–243. [Google Scholar] [CrossRef]
- Ma, L.; Gao, C.; Mao, Z.; Zhou, J.; Shen, J.; Hu, X.; Han, C. Collagen/chitosan porous scaffolds with improved biostability for skin tissue engineering. Biomaterials 2003, 24, 4833–4841. [Google Scholar] [CrossRef]
- Jayasinghe, S.N. Cell electrospinning: A novel tool for functionalising fibres, scaffolds and membranes with living cells and other advanced materials for regenerative biology and medicine. Analyst 2013, 138, 2215–2223. [Google Scholar] [CrossRef] [PubMed]
- Townsend-Nicholson, A.; Jayasinghe, S.N. Cell electrospinning: A unique biotechnique for encapsulating living organisms for generating active biological microthreads/scaffolds. Biomacromolecules 2006, 7, 3364–3369. [Google Scholar] [CrossRef] [PubMed]
- Katz, A.J.; Tholpady, A.; Tholpady, S.S.; Shang, H.; Ogle, R.C. Cell surface and transcriptional characterization of Human Adipose-Derived Adherent Stromal (hADAS) Cells. Stem Cells 2005, 23, 412–423. [Google Scholar] [CrossRef] [PubMed]
- Bet, M.R.; Goissis, G.; Vargas, S.; Selistre-de-Araujo, H.S. Cell adhesion and cytotoxicity studies over polyanionic collagen surfaces with variable negative charge and wettability. Biomaterials 2003, 24, 131–137. [Google Scholar] [CrossRef]
- Mainil-Varlet, P.; Aigner, T.; Brittberg, M.; Bullough, P.; Hollander, A.; Hunziker, E.; Kandel, R.; Nehrer, S.; Pritzker, K.; Roberts, S.; et al. Histological assessment of cartilage repair. J. Bone Jt. Surg. 2003, 85, 45–57. [Google Scholar] [CrossRef]
- Power, J.; Hernandez, P.; Guehring, H.; Getgood, A.; Henson, F. Intra-articular injection of rhFGF-18 improves the healing in microfracture treated chondral defects in an ovine model. J. Orthop. Res. 2014, 32, 669–676. [Google Scholar] [CrossRef] [PubMed]
- Sidler, M.; Fouché, N.; Meth, I.; von Hahn, F.; von Rechenberg, B.; Kronen, P. Transcutaneous treatment with vetdrop® sustains the adjacent cartilage in a microfracturing joint defect model in sheep. Open Orthop. J. 2013, 7, 57–66. [Google Scholar] [CrossRef] [PubMed]
- Mika, J.; Clanton, T.O.; Pretzel, D.; Schneider, G.; Ambrose, C.G.; Kinne, R.W. Surgical preparation for articular cartilage regeneration without penetration of the subchondral bone plate: In vitro and in vivo studies in humans and sheep. Am. J. Sports Med. 2011, 39, 624–631. [Google Scholar] [CrossRef] [PubMed]
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons by Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zorzi, A.R.; Amstalden, E.M.I.; Plepis, A.M.G.; Martins, V.C.A.; Ferretti, M.; Antonioli, E.; Duarte, A.S.S.; Luzo, A.C.M.; Miranda, J.B. Effect of Human Adipose Tissue Mesenchymal Stem Cells on the Regeneration of Ovine Articular Cartilage. Int. J. Mol. Sci. 2015, 16, 26813-26831. https://doi.org/10.3390/ijms161125989
Zorzi AR, Amstalden EMI, Plepis AMG, Martins VCA, Ferretti M, Antonioli E, Duarte ASS, Luzo ACM, Miranda JB. Effect of Human Adipose Tissue Mesenchymal Stem Cells on the Regeneration of Ovine Articular Cartilage. International Journal of Molecular Sciences. 2015; 16(11):26813-26831. https://doi.org/10.3390/ijms161125989
Chicago/Turabian StyleZorzi, Alessandro R., Eliane M. I. Amstalden, Ana Maria G. Plepis, Virginia C. A. Martins, Mario Ferretti, Eliane Antonioli, Adriana S. S. Duarte, Angela C. M. Luzo, and João B. Miranda. 2015. "Effect of Human Adipose Tissue Mesenchymal Stem Cells on the Regeneration of Ovine Articular Cartilage" International Journal of Molecular Sciences 16, no. 11: 26813-26831. https://doi.org/10.3390/ijms161125989
APA StyleZorzi, A. R., Amstalden, E. M. I., Plepis, A. M. G., Martins, V. C. A., Ferretti, M., Antonioli, E., Duarte, A. S. S., Luzo, A. C. M., & Miranda, J. B. (2015). Effect of Human Adipose Tissue Mesenchymal Stem Cells on the Regeneration of Ovine Articular Cartilage. International Journal of Molecular Sciences, 16(11), 26813-26831. https://doi.org/10.3390/ijms161125989





