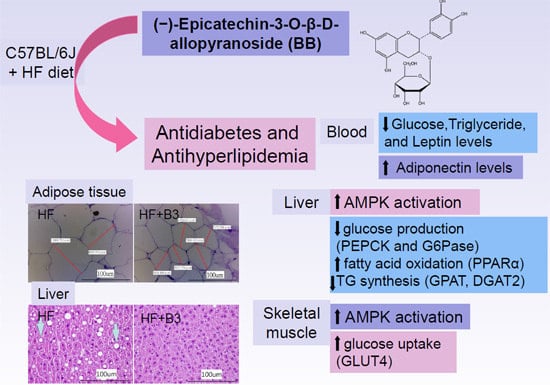
(−)-Epicatechin-3-O-β-d-allopyranoside from Davallia formosana, Prevents Diabetes and Hyperlipidemia by Regulation of Glucose Transporter 4 and AMP-Activated Protein Kinase Phosphorylation in High-Fat-Fed Mice
Abstract
:1. Introduction
2. Results
2.1. Body Weight, Weight Gain, Diet Consumption, and Weights of Tissue
| Parameter | CON | HF | HF + B1 | HF + B2 | HF + B3 | HF + Rosi | HF + Feno |
|---|---|---|---|---|---|---|---|
| Dose (mg/kg/day) | 0 | 0 | 10 | 20 | 40 | 10 | 250 |
| Absolute tissue weight (g) | |||||||
| EWAT | 0.415 ± 0.029 | 1.303 ± 0.123 c | 0.906 ± 0.105 d | 0.902 ± 0.084 d | 0.936 ± 0.075 d | 0.919 ± 0.097 d | 0.763 ± 0.116 f |
| MWAT | 0.139 ± 0.024 | 0.310 ± 0.027 b | 0.293 ± 0.018 | 0.267 ± 0.021 | 0.236 ± 0.030 | 0.302 ± 0.029 | 0.264 ± 0.056 |
| RWAT | 0.089 ± 0.012 | 0.469 ± 0.057 c | 0.280 ± 0.043 d | 0.263 ± 0.034 f | 0.233 ± 0.026 f | 0.224 ± 0.032 f | 0.173 ± 0.032 f |
| Visceral fat | 0.504 ± 0.039 | 1.772 ± 0.173 c | 1.273 ± 0.147 d | 1.199 ± 0.104 e | 1.198 ± 0.075 e | 1.143 ± 0.124 e | 0.936 ± 0.147 f |
| Skeletal muscle | 0.662 ± 0.036 | 0.874 ± 0.078 a | 0.873 ± 0.060 | 0.813 ± 0.077 | 1.156 ± 0.102 d | 0.960 ± 0.078 | 0.990 ± 0.078 |
| BAT | 0.084 ± 0.008 | 0.171 ± 0.014 b | 0.168 ± 0.011 | 0.156 ± 0.017 | 0.144 ± 0.010 | 0.206 ± 0.012 | 0.139 ± 0.011 |
| Liver (g) | 0.879 ± 0.019 | 0.884 ± 0.035 | 0.899 ± 0.036 | 0.866 ± 0.036 | 0.848 ± 0.028 | 0.886 ± 0.031 | 1.699 ± 0.033 f |
| Weight gain (g) | 0.01 ± 0.19 | 0.89 ± 0.16 a | 0.40 ± 0.28 | 0.61 ± 0.43 | −0.05 ± 0.11 d | 0.38 ± 0.32 | −0.58 ± 0.48 d |
| Body weight (g) | 24.37 ± 0.46 | 28.80 ± 0.93 b | 27.85 ± 1.04 | 27.88 ± 1.14 | 27.15 ± 0.84 | 28.61 ± 1.51 | 26.92 ± 0.96 |
| Food intake (g/day/mouse) | 2.40 ± 0.04 | 2.14 ± 0.04 c | 2.08 ± 0.05 | 2.03 ± 0.03 | 2.02 ± 0.03 | 2.11 ± 0.06 | 2.14 ± 0.04 |
| Liver lipids | |||||||
| Total lipid (mg/g) | 55.3 ± 4.5 | 99.1 ± 7.1 b | 71.9 ± 6.5 d | 66.1 ± 6.9 e | 60.2 ± 5.2 f | 70.1 ± 8.2 d | 62.4 ± 3.9 f |
| Triacylglycerol (μmol/g) | 42.1 ± 4.5 | 81.1 ± 7.8 b | 49.9 ± 8.0 d | 42.8 ± 6.5 f | 40.8 ± 4.6 f | 52.1 ± 7.7 d | 43.8 ± 5.6 f |
| Blood profiles | |||||||
| FFA (mEq/L) | 1.02 ± 0.10 | 1.48 ± 0.23 b | 1.11 ± 0.13 d | 0.95 ± 0.04 f | 0.86 ± 0.09 f | 1.08 ± 0.11 e | 0.91 ± 0.08 f |
| TC (mg/dL) | 104.6 ± 8.6 | 163.8 ± 7.2 c | 153.5 ± 7.7 | 144.1 ± 4.0 | 142.9 ± 6.6 | 111.5 ± 2.3 f | 104.5 ± 5.5 f |
| Leptin (ng/mL) | 1.359 ± 0.044 | 2.133 ± 0.044 c | 1.662 ± 0.009 f | 1.372 ± 0.029 f | 1.321 ± 0.008 f | 1.661 ± 0.013 f | 1.206 ± 0.015 f |
| Adiponectin (μg/mL) | 2.737 ± 0.021 | 2.153 ± 0.036 c | 2.895 ± 0.010 f | 3.277 ± 0.052 f | 3.628 ± 0.034 f | 3.058 ± 0.040 f | 3.101 ± 0.028 f |
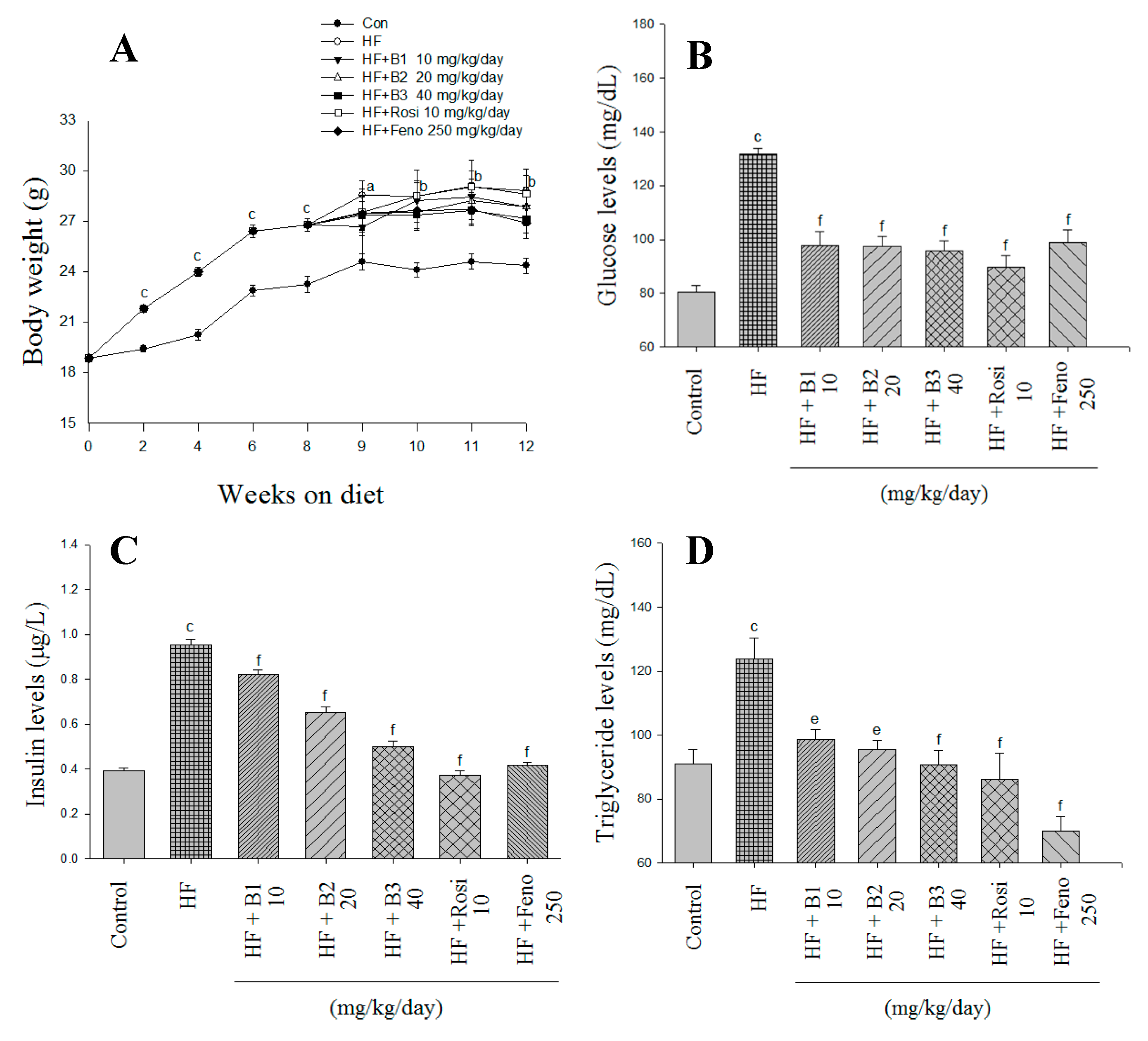
2.2. Glucose and Insulin Levels in Blood
2.3. Blood Lipid, Leptin, and Adiponectin Levels, and Hepatic Lipid
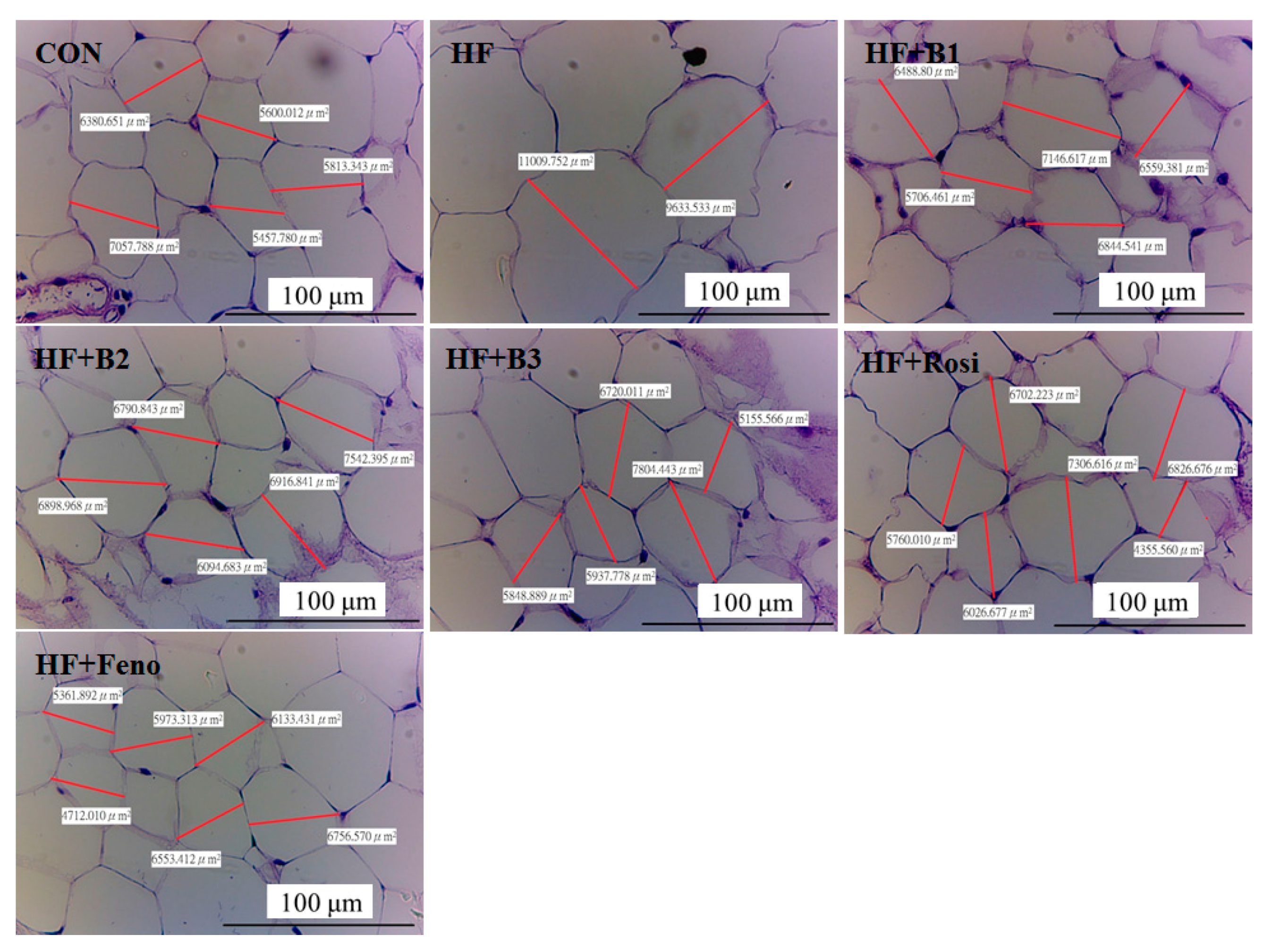
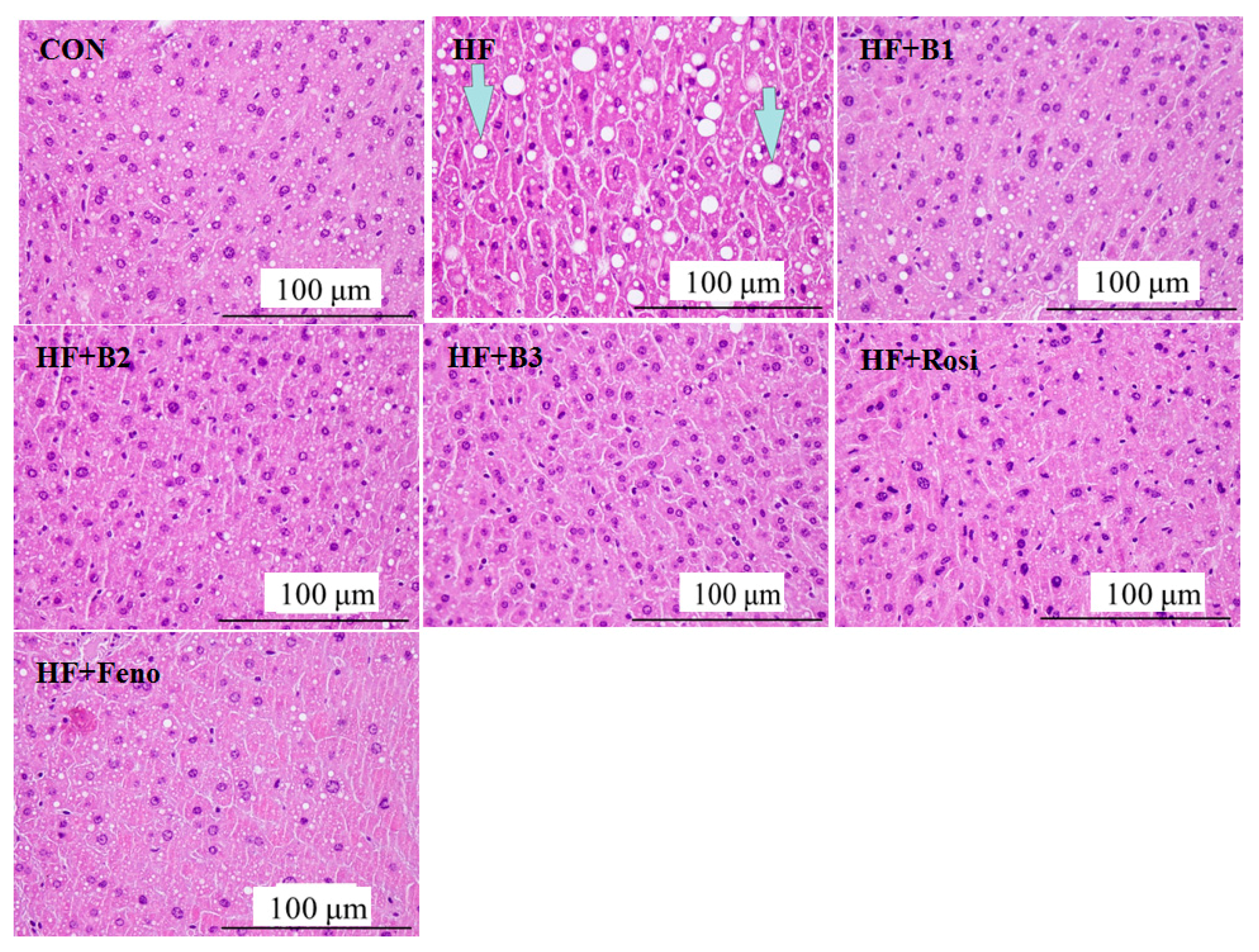
2.4. Pathological Investigation
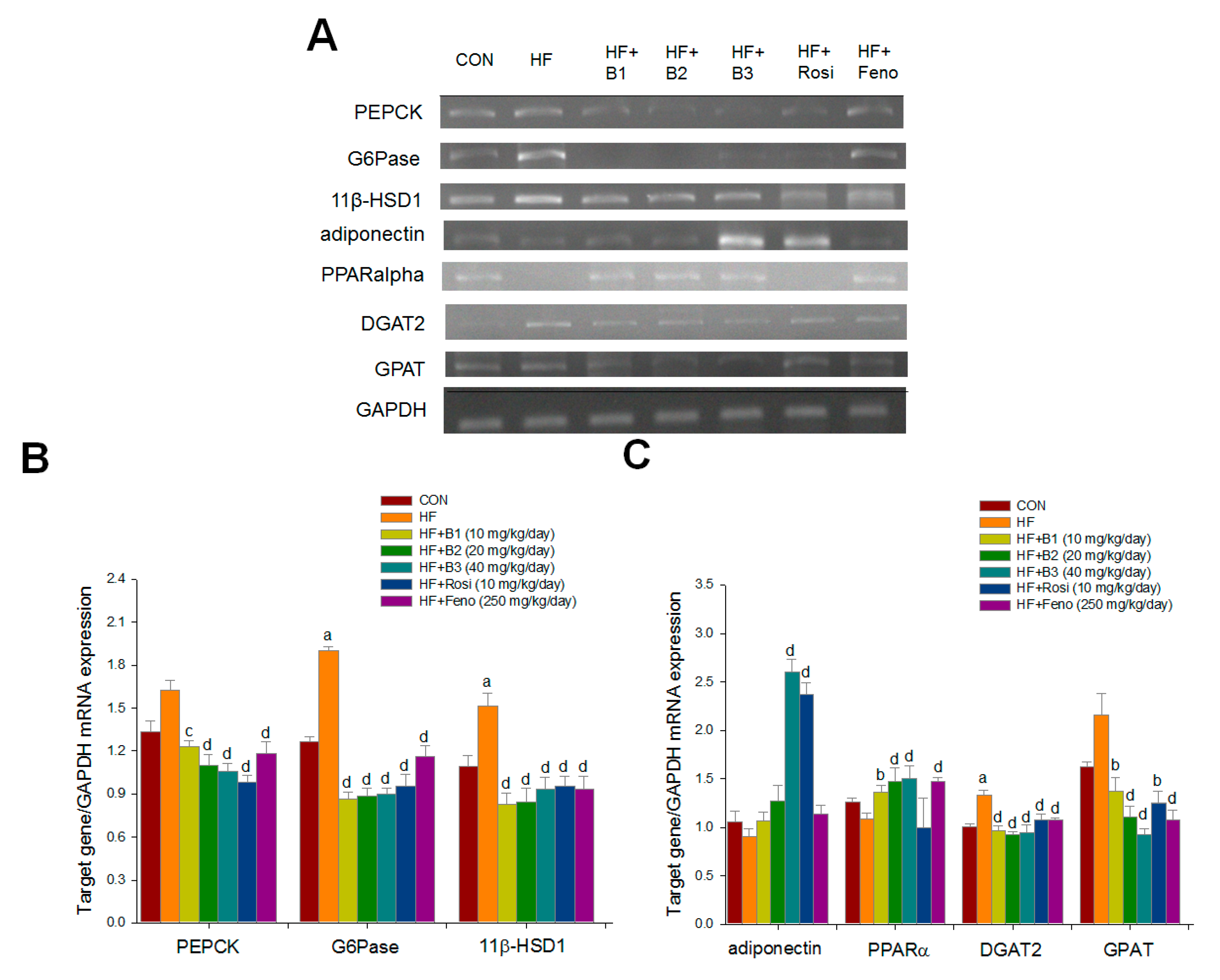
2.5. Hepatic Related Gene mRNA Levels
2.6. Western Blotting

3. Discussion
4. Experimental Section
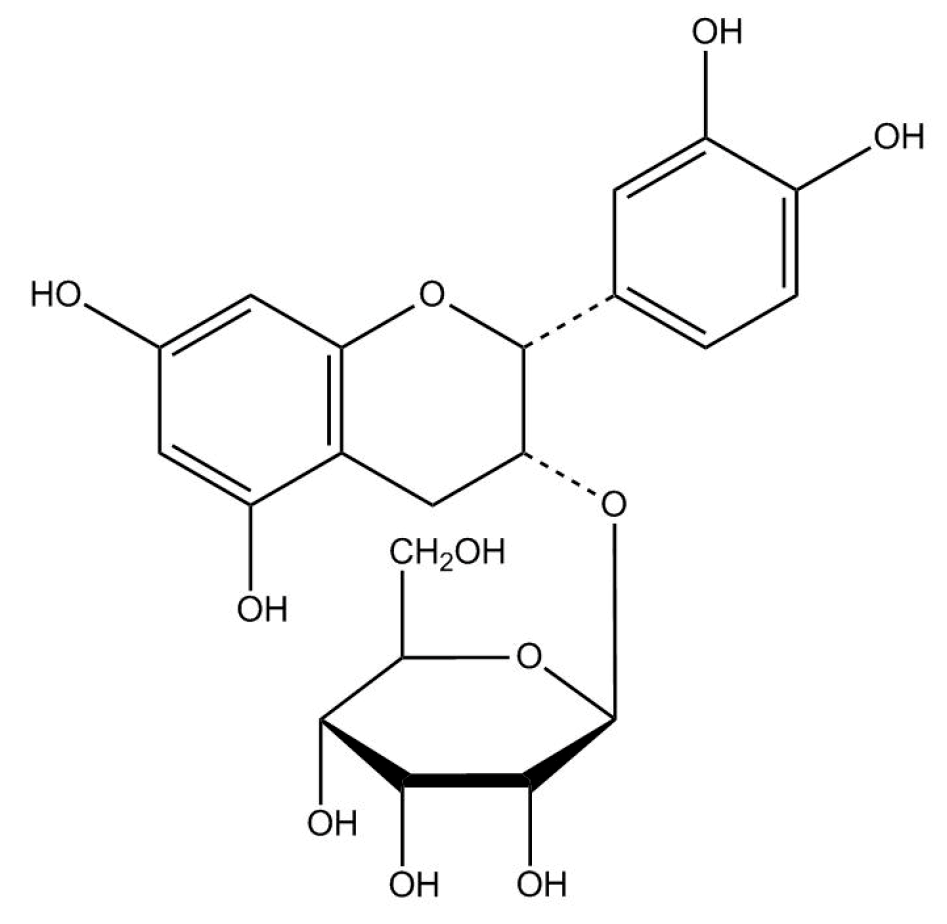
4.1. Preparation of (−)-Epicatechin-3-O-β-d-allopyranoside (BB)
4.1.1. Preparation of Extract of Davallia formosana
| C | H | ||
|---|---|---|---|
| C-2 | 79.19 | H-2 | 5.05 (d, J = 2.2 Hz) |
| C-3 | 73.40 | H-3 | 4.43 (m) |
| C-4 | 24.75 | H-4-2 | 2.74, 2.72 |
| C-5 | 157.85 | - | - |
| C-6 | 96.43 | H-6 | 5.86 (d, J = 2.3 Hz) |
| C-7 | 157.85 | - | - |
| C-8 | 95.68 | H-8 | 5.90 (d, J = 2.3 Hz) |
| C-9 | 157.13 | - | - |
| C-10 | 100.21 | - | - |
| C-1ʹ | 131.67 | - | - |
| C-2ʹ | 115.57 | H-2ʹ | 7.02 (d, J = 2.1 Hz) |
| C-3ʹ | 145.50 | - | - |
| C-4ʹ | 145.72 | - | - |
| C-5ʹ | 116.29 | H-5ʹ | 6.66 (d, J = 8.2 Hz) |
| C-6ʹ | 120.34 | H-6ʹ | 6.78 (dd, J = 2.1 Hz) |
| Allosyl | |||
| C-1ʹʹ | 100.41 | H-11ʹʹ | - |
| C-2ʹʹ | 72.28 | (H-2ʹʹ-6ʹʹ) | - |
| C-3ʹʹ | 72.91 | (H-5) | |
| C-4ʹʹ | 68.96 | ||
| C-5ʹʹ | 75.33 | 4.74 (d, J = 8 Hz) | |
| C-6ʹʹ | 63.26 | 3.22–3.99 | |
4.1.2. Purification of (−)-Epicatechin-3-O-β-d-allopyranoside
4.2. Animal and Diet Treatment
4.3. Measurement of Body Weight, Body Weight Gain, and Diet Consumption
4.4. Analysis of Blood Glucose, Blood Lipid, Insulin, Leptin, and Adiponectin
4.5. Histology
4.6. Hepatic Lipids Analysis
4.7. Relative Quantization of mRNA Analysis
| Gene | Accession Number | Forward Primer and Reverse Primer | PCR Product (bp) | Annealing Temperature (°C) |
|---|---|---|---|---|
| Liver | ||||
| PEPCK | NM_011044.2 | F: CTACAACTTCGGCAAATACC | 330 | 52 |
| R: TCCAGATACCTGTCGATCTC | ||||
| G6Pase | NM_008061.3 | F: GAACAACTAAAGCCTCTGAAAC | 350 | 50 |
| R: TTGCTCGATACATAAAACACTC | ||||
| 11β-HSD1 | NM_008288.2 | F: AAGCAGAGCAATGGCAGCAT | 300 | 50 |
| R: GAGCAATCATAGGCTGGGTCA | ||||
| Adiponectin | NM_009605.4 | F: TCTTCTACAACCAACAGAATCA | 324 | 50.5 |
| R: GTATCATGGTAGAGAAGGAAGC | ||||
| PPARα | NM_011144 | F: CCTGAGATTAACCAGCCTTT | 352 | 55 |
| R: AGGACCTACTCTCATTGCTG | ||||
| GPAT | BC019201.1 | F: CAGTCCTGAATAAGAGGT | 441 | 48 |
| R: TGGACAAAGATGGCAGCAGA | ||||
| GAPDH | NM_008084.3 | F: TGTGTCCGTCGTGGATCTGA | 99 | 55 |
| R: CCTGCTTCACCACCTTCTTGA | ||||
4.8. Western Blotting Analysis
4.9. Statistical Analysis
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- O’Rahilly, S.; Turner, R.C.; Matthews, D.R. Impaired pulsatile secretion of insulin in relatives of patients with non-insulin-dependent diabetes. N. Engl. J. Med. 1988, 318, 1225–1230. [Google Scholar] [CrossRef] [PubMed]
- Jakicic, J.M.; Jaramillo, S.A.; Balasubramanyam, A.; Bancroft, B.; Curtis, J.M.; Mathews, A.; Pereira, M.; Regensteiner, J.G.; Ribisl, P.M. Look AHEAD Study Group. Effect of a lifestyle intervention on change in cardiorespiratory fitness in adults with type 2 diabetes: Results from the Look AHEAD Study. Int. J. Obes. 2009, 33, 305–316. [Google Scholar] [CrossRef] [PubMed]
- Mozaffarian, D.; Kamineni, A.; Carnethon, M.; Djousse, L.; Mukamal, K.J.; Siscovick, D. Lifestyle risk factors and new-onset diabetes mellitus in older adults: The cardiovascular health study. Arch. Intern. Med. 2009, 169, 798–807. [Google Scholar] [CrossRef] [PubMed]
- Petro, A.E.; Cotter, J.; Cooper, D.A.; Peters, J.C.; Surwit, S.J.; Surwit, R.S. Fat, carbohydrate and calories in the development of diabetes and obesity in the C57BL/6J mouse. Metabolism 2004, 53, 454–457. [Google Scholar] [CrossRef] [PubMed]
- Editorial Committee of the Flora of Taiwan; Davallia, S. Flora of Taiwan, 2nd ed.; National Taiwan University: Taipei, Taiwan, 1994; p. 8. [Google Scholar]
- Lin, Y.Y.; Kakisawa, H.; Shiobara, Y.; Nakanishi, K. The structure of Davallic acid. Chem. Pharm. Bull. 1965, 13, 986–995. [Google Scholar] [CrossRef] [PubMed]
- Hwang, T.H.; Kashiwada, Y.; Nonaka, G.I.; Nishioka, I. Flavan-3-ol and proanthocyanidin allosides from Davallia divaricata. Phytochemistry 1989, 28, 891–896. [Google Scholar] [CrossRef]
- Molinuevo, M.S.; Schurman, L.; McCarthy, A.D.; Cortizo, A.M.; Tolosa, M.J.; Gangoiti, M.V.; Arnol, V.; Sedlinsky, C. Efect of metformin on bone marrow progenitor cell differentiation: In vivo and in vitro studies. J. Bone Miner. Res. 2010, 25, 211–221. [Google Scholar] [CrossRef] [PubMed]
- Wongdee, K.; Charoenphandhu, N. Osteoporosis in diabetes mellitus: Possible cellular and molecular mechanisms. World J. Diabetes 2011, 2, 41–48. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.H.; Choung, S.Y. Antihyperglycemic and antihyperlipidemic action of Cinnamomi Cassiae (cinnamon bark) extract in C57BL/Ks db/db mice. Arch. Pharm. Res. 2010, 33, 325–333. [Google Scholar] [CrossRef] [PubMed]
- Huang, S.; Czech, M.P. The GLUT4 glucose transporter. Cell Metab. 2007, 5, 237–252. [Google Scholar] [CrossRef] [PubMed]
- Bryant, N.J.; Govers, R.; James, D.E. Regulated transport of the glucose transporter GLUT4. Nat. Rev. Mol. Cell Biol. 2002, 3, 267–277. [Google Scholar] [CrossRef] [PubMed]
- Holmes, B.; Dohm, G.L. Regulation of GLUT4 gene expression during exercise. Med. Sci. Sports Exerc. 2004, 36, 1202–1206. [Google Scholar] [CrossRef] [PubMed]
- Sriwijitkamol, A.; Coletta, D.K.; Wajcberg, E.; Balbontin, G.B.; Reyna, S.M.; Barrientes, J.; Eagan, P.A.; Jenkinson, C.P.; Cersosimo, E.; DeFronzo, R.A.; et al. Effect of acute exercise on AMPK signaling in skeletal muscle of subjects with type 2 diabetes: A time-course and dose-response study. Diabetes 2007, 56, 836–848. [Google Scholar] [CrossRef] [PubMed]
- Mackenzie, R.W.; Elliot, B.T. Akt/PKB activation and insulin signaling: A novel insulin signaling pathway in the treatment of type 2 diabetes. Diabetes Metab. Syndr. Obes. 2014, 7, 55–64. [Google Scholar] [CrossRef] [PubMed]
- Jing, M.; Cheruvu, V.K.; Ismail-Beigi, F. Stimulation of glucose transport in response to activation of distinct AMPK signaling pathways. Am. J. Physiol. Cell Physiol. 2008, 295, C1071–C1082. [Google Scholar] [CrossRef] [PubMed]
- Zhou, G.; Myers, R.; Li, Y.; Chen, Y.; Shen, X.; Fenyk-Melody, J.; Wu, M.; Ventre, J.; Doebber, T.; Fujii, N.; et al. Role of AMP-activated protein kinase in mechanism of metformin action. J. Clin. Investig. 2001, 108, 1167–1174. [Google Scholar] [CrossRef] [PubMed]
- Yu, S.; Matsusue, K.; Kashireddy, P.; Cao, W.Q.; Yeldandi, V.; Yeldandi, A.V.; Rao, M.S.; Gonzalez, F.J.; Reddy, J.K. Adipocyte-specific gene expression and adipogenic steatosis in the mouse liver due to peroxisome proliferator-activated receptor γ1 (PPARγ1) overexpression. Biol. Chem. 2003, 278, 498–505. [Google Scholar] [CrossRef] [PubMed]
- Yki-Jȁrvinen, H. Thiazolidines. N. Engl. J. Med. 2004, 351, 1106–1118. [Google Scholar] [CrossRef] [PubMed]
- Martinez, L.; Berenguer, M.; Bruce, M.C.; Le Marchand-Brustel, Y.; Govers, R. Rosiglitazone increases cell surface GLUT4 levels in 3T3-L1 adipocytes through an enhancement of endosomal recycling. Biochem. Pharmacol. 2010, 79, 1300–1309. [Google Scholar] [CrossRef] [PubMed]
- Damci, T.; Tatliagac, S.; Osar, Z.; Ilkova, H. Fenofibrate treatment is associated with better glycemic control and lower serum leptin and insulin levels in type 2 diabetic patients with hypertriglyceridemia. Eur. J. Intern. Med. 2003, 14, 357–360. [Google Scholar] [CrossRef]
- Farnier, M.; Bonnefous, F.; Debbas, N.; Irvine, A. Comparative efficacy and safety of micronised fenofibrate and simvastatin in patients with primary type Ha or IIb hyperlipidemia. Arch. Intern. Med. 1994, 154, 441–449. [Google Scholar] [CrossRef] [PubMed]
- Hsu, S.C.; Huang, C.J. Reduced fat mass in rats fed a high oleic acid-rich safflower oil diet is associated with changes in expression of hepatic PPARα and adipose SREBP-1c-regulated genes. J. Nutr. 2006, 136, 1779–1785. [Google Scholar] [PubMed]
- Srivastava, R.A.K.; Jahagirdar, R.; Azhar, S.; Sharma, S.; Bisgaier, C.L. Peroxisome proliferator-activated receptor-α selective ligand reduces adiposity, improves insulin sensitivity, and inhibits atherosclerosis in LDL receptor-deficient mice. Mol. Cell. Biochem. 2006, 285, 35–50. [Google Scholar] [CrossRef] [PubMed]
- Fryer, L.G.; Parbu-Patel, A.; Patel, A. The anti-diabetic drugs rosiglitazone and metformin stimulate AMP-activated protein kinase through distinct signaling pathways. J. Biol. Chem. 2002, 277, 25226–25232. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, M.H.; Byrne, C.D. Modulation of sterol regulatory element binding proteins (SREBPs) as potential treatments for non-alcoholic fatty liver disease (NAFLD). Drug Discov. Today 2007, 12, 740–747. [Google Scholar] [CrossRef] [PubMed]
- Stein, S.C.; Woods, A.; Jones, N.A.; Davison, M.D.; Carling, D. The regulation of AMP-activated protein kinase by phosphorylation. Biochem. J. 2000, 345, 437–443. [Google Scholar] [CrossRef] [PubMed]
- Kleiner, D.E.; Brunt, E.M.; van Natta, M.; Sanyal, A.J. Design and validation of a histological scoring system for nonalcoholic fatty liver disease. Hepatology 2005, 41, 1313–1321. [Google Scholar] [CrossRef] [PubMed]
- Harte, R.A.; Kirk, E.A.; Rosenfeld, M.E.; LeBoeuf, R.C. Initiation of hyperinsulinemia and hyperleptinemia is diet dependent in C57BL/6 mice. Horm. Metab. Res. 1999, 31, 570–575. [Google Scholar] [CrossRef] [PubMed]
- Guo, S. Insulin signaling, resistance, and metabolic syndrome: Insights from mouse models into disease mechanisms. J. Endocrinol. 2014, 220, T1–T23. [Google Scholar] [CrossRef] [PubMed]
- Zhu, W.; Cheng, K.K.; Vanhoutte, P.M.; Lam, K.S.; Xu, A. Vascular effects of adiponectin: Molecular mechanisms and potential therapeutic intervention. Clin. Sci. 2008, 114, 361–374. [Google Scholar] [CrossRef] [PubMed]
- Welsh, G.I.; Hers, I.; Berwick, D.C.; Dell, G.; Wherlock, M.; Birkin, R.; Leney, S.; Tavaré, J.M. Role of protein kinase B in insulin-regulated glucose uptake. Biochem. Soc. Trans. 2005, 33, 346–349. [Google Scholar] [CrossRef] [PubMed]
- Barthel, A.; Schmoll, D. Novel concepts in insulin regulation of hepatic gluconeogenesis. Am. J. Physiol. Endocrinol. Metab. 2003, 285, E685–E692. [Google Scholar] [CrossRef] [PubMed]
- O’Brien, R.M.; Printz, R.L.; Halmi, N.; Tiesinga, J.J.; Granner, D.K. Structural and functional analysis of the human phosphoenolpyruvate carboxykinase gene promoter. Biochim. Biophys. Acta 1995, 1264, 284–288. [Google Scholar] [CrossRef]
- Sutherland, C.; O’Brien, R.M.; Granner, D.K. New connections in the regulation of PEPCK gene expression by insulin. Philos. Trans. R. Soc. Lond. B Biol. Sci. 1996, 351, 191–199. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.D.; Park, K.G.; Lee, Y.S.; Park, Y.Y.; Kim, D.K.; Nedumaran, B.; Jang, W.G.; Cho, W.J.; Ha, J.; Lee, I.K.; et al. Metformin inhibits hepatic gluconeogenesis through AMP-activated protein kinase-dependent regulation of the orphan nuclear receptor SHP. Diabetes 2008, 57, 306–314. [Google Scholar] [CrossRef] [PubMed]
- Koeilevtsev, Y.; Holmes, M.C.; Burchell, A.; Houston, P.M.; Schmoll, D.; Jamieson, P.; Best, R.; Brown, R.; Edwards, C.R.; Seckl, J.R.; et al. 11β-hydroxysteroid dehydrogenase type 1 knockout mice show attenuated glucocorticoid-inducible responses and resist hyperglycemia on obesity or stress. Proc. Natl. Acad. Sci. USA 1997, 94, 14924–14929. [Google Scholar] [CrossRef]
- Cases, S.; Smith, S.J.; Zheng, Y.W.; Myers, H.M.; Lear, S.R.; Sande, E.; Novak, S.; Collin, C.; Welch, C.B.; Lusis, A.J.; et al. Identification of a gene encoding an acyl CoA: Diacylglycerol acyltransferase, a key enzyme in triacylglycerol synthesis. Proc. Natl. Acad. Sci. USA 1998, 95, 13018–13023. [Google Scholar] [CrossRef] [PubMed]
- Oelkers, P.; Behari, A.; Cromley, D.; Billheimer, J.T.; Sturley, S.L. Characterization of two human genes encoding acyl coenzyme A: Cholesterol acyltransferase-related enzymes. J. Biol. Chem. 1998, 273, 26765–26771. [Google Scholar] [CrossRef] [PubMed]
- Stone, S.J.; Levin, M.C.; Zhou, P.; Han, J.; Walther, T.C.; Farese, R.V., Jr. The endoplasmic reticulum enzyme DGAT2 is found in mitochondria-associated membranes and has a mitochondrial targeting signal that promotes its association with mitochondria. J. Biol. Chem. 2009, 284, 5352–5361. [Google Scholar] [CrossRef] [PubMed]
- Meegalla, R.L.; Billheimer, J.T.; Cheng, D. Concerted elevation of acyl-coenzyme A: Diacylglycerol acyltransferase (DGAT) activity through independent stimulation of mRNA expression of DGAT1 and DGAT2 by carbohydrate and insulin. Biochem. Biophys. Res. Commun. 2002, 298, 317–323. [Google Scholar] [CrossRef]
- Yen, C.L.; Stone, S.J.; Koliwad, S.; Harris, C.; Farese, R.V., Jr. Thematic Review Series: Glycerolipids. DGAT enzymes and triacylglycerol biosynthesis. J. Lipid Res. 2008, 49, 2283–2301. [Google Scholar] [CrossRef] [PubMed]
- Lewin, T.M.; Granger, D.A.; Kim, J.H.; Coleman, R.A. Regulation of mitochondrial sn-glycerol-3-phosphate acyltransferase activity: Response to feeding status is unique in various rat tissues and is discordant with protein expression. Arch. Biochem. Biophys. 2001, 396, 119–127. [Google Scholar] [CrossRef] [PubMed]
- Hotta, K.; Funahashi, T.; Arita, Y.; Takahashi, M.; Matsuda, M.; Okamoto, Y.; Iwahashi, H.; Kuriyama, H.; Ouchi, N.; Maeda, K.; et al. Plasma concentrations of a novel, adipose-specific protein, adiponectin, in type 2 diabetic patients. Arterioscler. Thromb. Vasc. Biol. 2000, 20, 1595–1599. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.; Motoshima, H.; Mahadev, K.; Stalker, T.J.; Scalia, R.; Goldstein, B.J. Involvement of AMP-activated protein kinase in glucose uptake stimulated by globular domain of adiponectin in primary rat adipocytes. Diabetes 2003, 52, 1355–1363. [Google Scholar] [CrossRef] [PubMed]
- Minokoshi, Y.; Kim, Y.-B.; Kahn, B.B. Leptin stimulates fatty acid oxidation directly in white adipose tissue through activation of AMP-activated protein kinase. Diabetes 2002, 51, 1375. [Google Scholar]
- Rommel, C.; Bodine, S.C.; Clarke, B.A.; Rossman, R.; Nunez, L.; Stitt, T.N.; Yancopoulos, G.D.; Glass, D.J. Mediation of IGF-1-induced skeletal myotube hypertrophy by PI(3)K/Akt/mTOR and PI(3)/Akt/GSK3 pathways. Nat. Cell Biol. 2001, 3, 1009–1013. [Google Scholar] [CrossRef] [PubMed]
- Izumiya, Y.; Hopkins, T.; Morris, C.; Sato, K.; Zeng, L.; Viereck, J.; Hamilton, J.A.; Ouchi, N.; LeBrasseur, N.K.; Walsh, K. Fast/Glycolytic muscle fiber growth reduces fat mass and improves metabolic parameters in obese mice. Cell Metab. 2008, 7, 159–172. [Google Scholar] [CrossRef] [PubMed]
- George, S.; Rochford, J.J.; Wolfrum, C.; Gray, S.L.; Schinner, S.; Wilson, J.C.; Soos, M.A.; Murgatroyd, P.R.; Williams, R.M.; Acerini, C.L.; et al. A family with severe insulin resistance and diabetes due to a mutation in AKT2. Science 2004, 304, 1325–1328. [Google Scholar] [CrossRef] [PubMed]
- Ko, Y.J.; Wu, J.B.; Ho, H.Y.; Lin, W.C. Antiosteoporotic activity of Davallia formosana. J. Ethnopharmacol. 2012, 139, 558–565. [Google Scholar] [CrossRef] [PubMed]
- Li, F.; Xiong, F.M.; Li, X.; Lin, H. Stimulative activity of Drynaria fortune (Kunez) J. Sm. Extracts and two of its flavonoids on the proliferation of osteoblastic like cells. Pharmazie 2006, 61, 962–965. [Google Scholar] [PubMed]
- Kuo, Y.H.; Lin, C.H.; Shih, C.C. Ergostatrien-3β-ol from Antrodia camphorata inhibits diabetes and hyperlipidemia in high-fat-diet treated mice via regulation of hepatic related genes, glucose transporter 4, and AMP-Activated protein kinase phosphorylation. J. Agric. Food Chem. 2015, 63, 2479–2489. [Google Scholar] [CrossRef] [PubMed]
- Shih, C.C.; Shlau, M.T.; Lin, C.H.; Wu, J.B. Momordica charantia ameliorates insulin resistance and dyslipidemia with altered hepatic glucose production and fatty acid synthesis and AMPK phosphorylation in high-fat-fed mice. Phytother. Res. 2014, 28, 363–371. [Google Scholar] [CrossRef] [PubMed]
- Shih, C.C.; Lin, C.H.; Wu, J.B. Eriobotrya japonica improves hyperlipidaemia and reverses insulin resistance in high-fat-fed mice. Phytother. Res. 2010, 24, 1769–1780. [Google Scholar] [CrossRef] [PubMed]
- Shih, C.C.; Ciou, J.L.; Lin, C.H.; Wu, J.B.; Ho, H.Y. Cell suspension culture of Eriobotrya japonica regulates the diabetic and hyperlipidemic signs of high-fat-fed mice. Molecules 2013, 18, 2726–2753. [Google Scholar] [CrossRef] [PubMed]
- Lin, C.H.; Kuo, Y.H.; Shih, C.C. Effects of Bofu-tsusho-san on diabetes and hyperlipidemia associated with AMP-activated protein kinase and glucose transporter 4 in high-fat-fed mice. Int. J. Mol. Sci. 2014, 15, 20022–20044. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.B.; Kuo, Y.H.; Lin, C.H.; Ho, Y.H.; Shih, C.C. Tormentic acid, a major component of suspension cells of Eriobotrya japonica, suppresses high-fat diet-induced diabetes and hyperlipidemia by glucose transporter 4 and AMP-activated protein kinase phosphorylation. J. Agric. Food Chem. 2014, 62, 10717–10726. [Google Scholar] [CrossRef] [PubMed]
- Shih, C.C.; Lin, C.H.; Lin, W.L.; Wu, J.B. Momordica charantia extract on insulin resistance and the skeletal muscle GLUT4 protein in fructose-fed rats. J. Ethnopharmacol. 2009, 123, 82–90. [Google Scholar] [CrossRef] [PubMed]
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shih, C.-C.; Wu, J.-B.; Jian, J.-Y.; Lin, C.-H.; Ho, H.-Y. (−)-Epicatechin-3-O-β-d-allopyranoside from Davallia formosana, Prevents Diabetes and Hyperlipidemia by Regulation of Glucose Transporter 4 and AMP-Activated Protein Kinase Phosphorylation in High-Fat-Fed Mice. Int. J. Mol. Sci. 2015, 16, 24983-25001. https://doi.org/10.3390/ijms161024983
Shih C-C, Wu J-B, Jian J-Y, Lin C-H, Ho H-Y. (−)-Epicatechin-3-O-β-d-allopyranoside from Davallia formosana, Prevents Diabetes and Hyperlipidemia by Regulation of Glucose Transporter 4 and AMP-Activated Protein Kinase Phosphorylation in High-Fat-Fed Mice. International Journal of Molecular Sciences. 2015; 16(10):24983-25001. https://doi.org/10.3390/ijms161024983
Chicago/Turabian StyleShih, Chun-Ching, Jin-Bin Wu, Jia-Ying Jian, Cheng-Hsiu Lin, and Hui-Ya Ho. 2015. "(−)-Epicatechin-3-O-β-d-allopyranoside from Davallia formosana, Prevents Diabetes and Hyperlipidemia by Regulation of Glucose Transporter 4 and AMP-Activated Protein Kinase Phosphorylation in High-Fat-Fed Mice" International Journal of Molecular Sciences 16, no. 10: 24983-25001. https://doi.org/10.3390/ijms161024983
APA StyleShih, C.-C., Wu, J.-B., Jian, J.-Y., Lin, C.-H., & Ho, H.-Y. (2015). (−)-Epicatechin-3-O-β-d-allopyranoside from Davallia formosana, Prevents Diabetes and Hyperlipidemia by Regulation of Glucose Transporter 4 and AMP-Activated Protein Kinase Phosphorylation in High-Fat-Fed Mice. International Journal of Molecular Sciences, 16(10), 24983-25001. https://doi.org/10.3390/ijms161024983