Effects of Pulsed Electric Fields (PEF) on Vitamin C and Its Antioxidant Properties
Abstract
:1. Introduction
2. Results and Discussion
2.1. Fluorescence of VIT-C
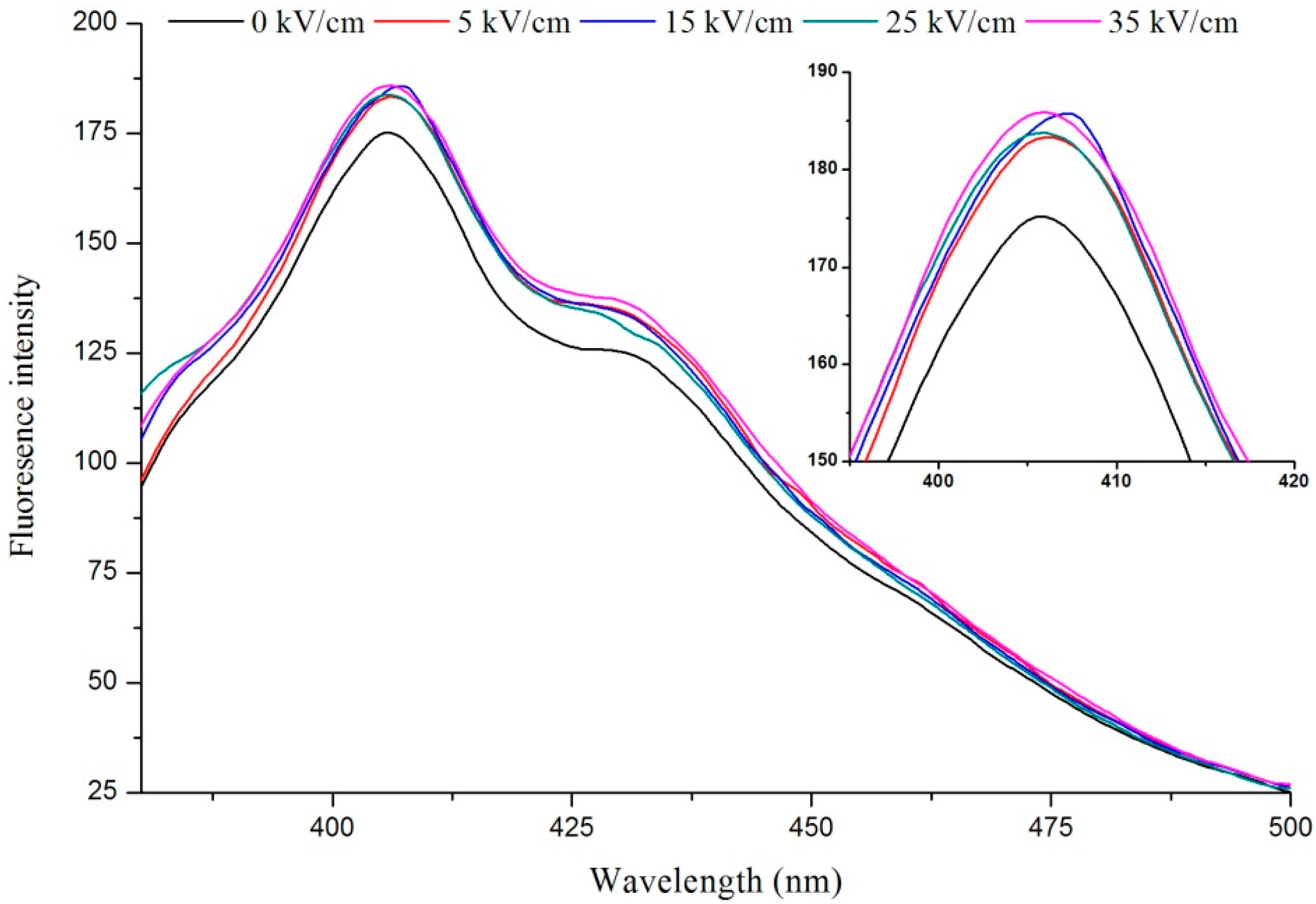
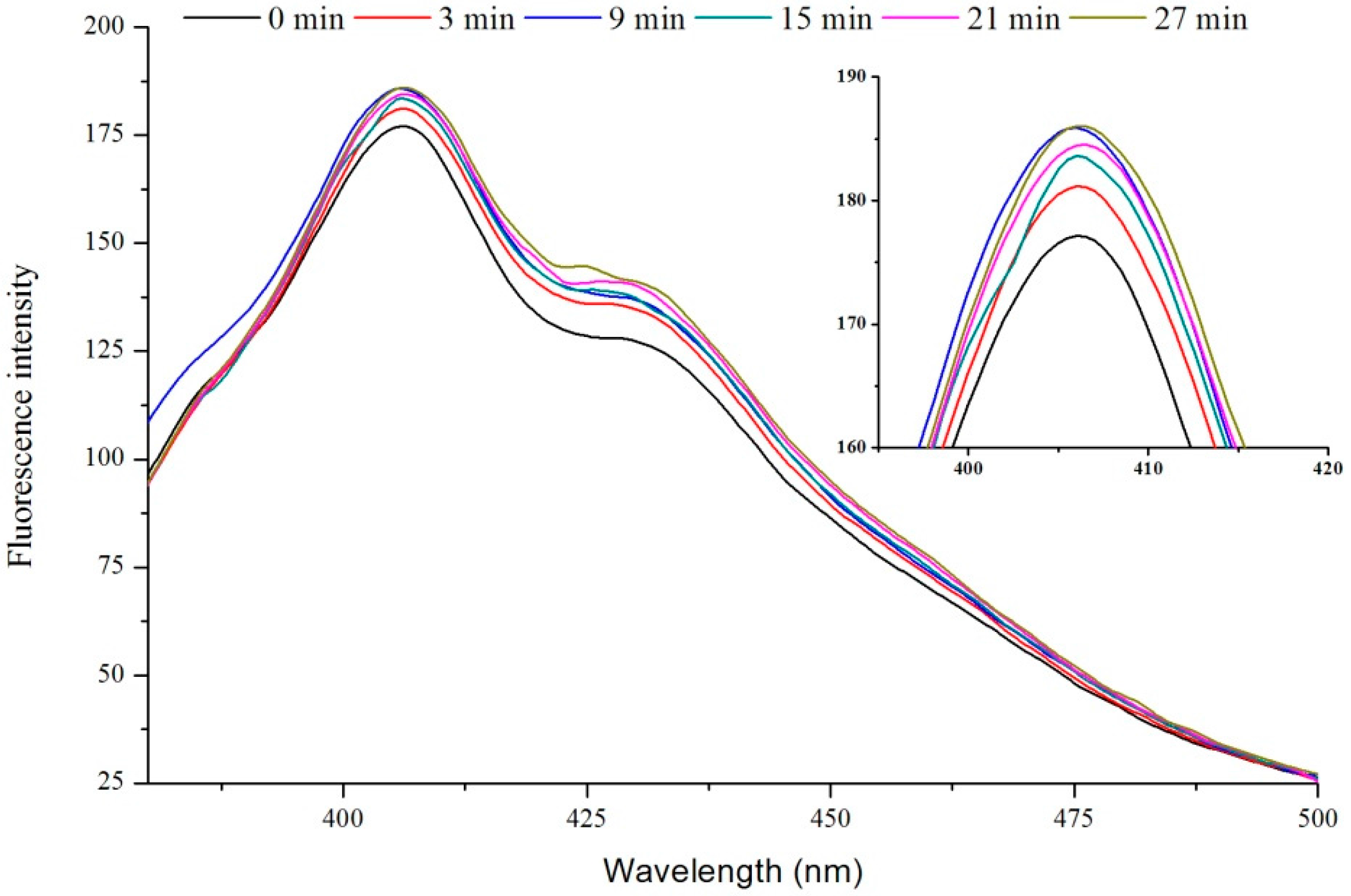
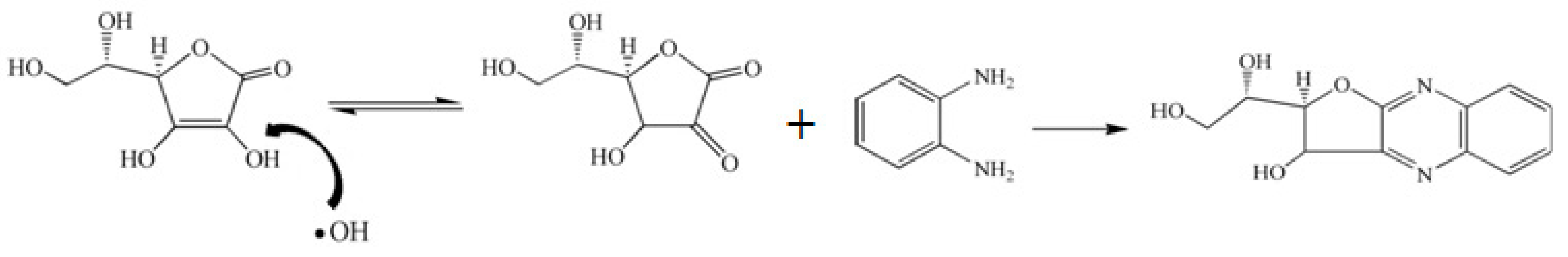
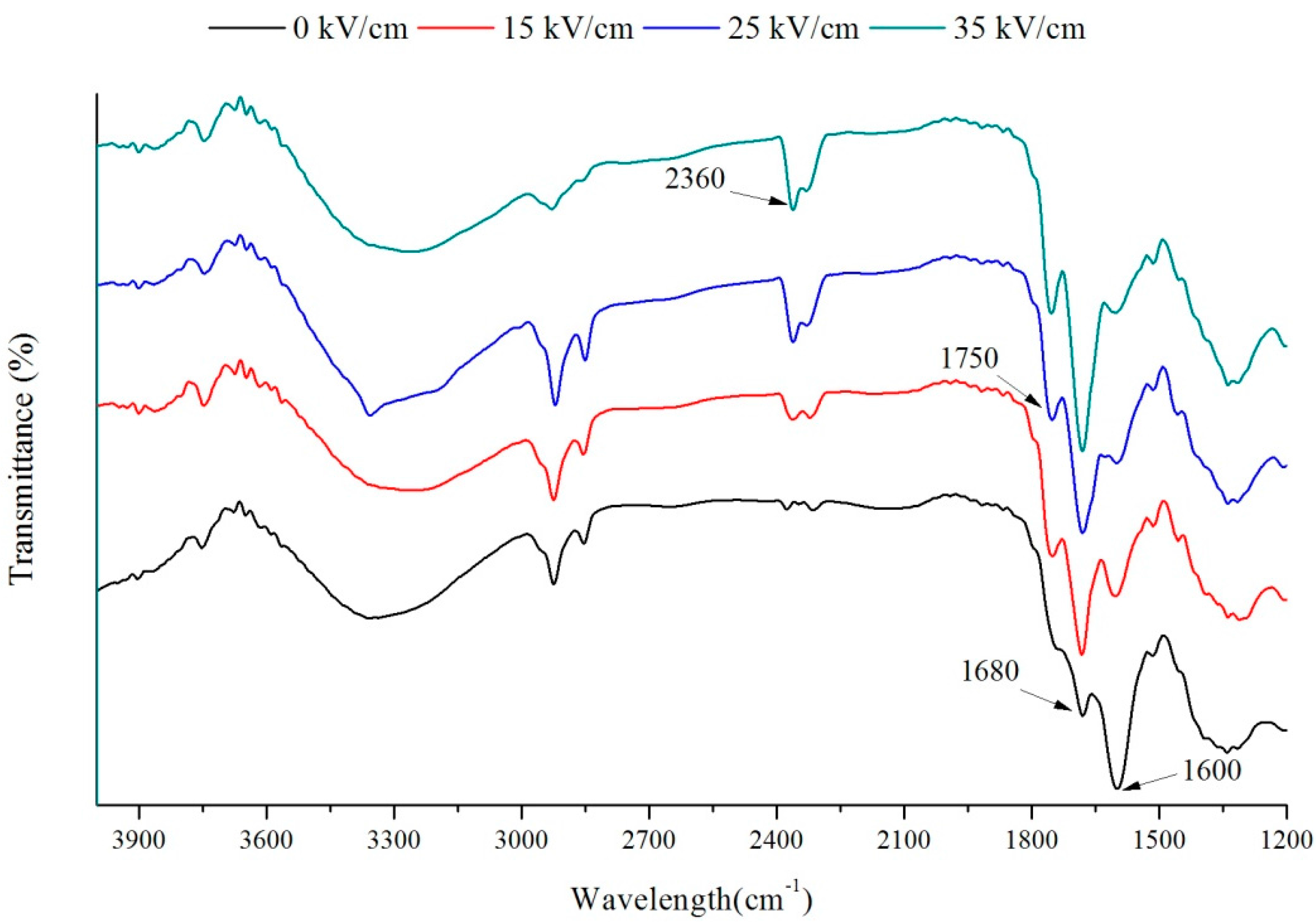
2.2. FT-IR Analysis
2.3. The Relative Content of VIT-C
| Relative Content (mg/L) | 0 kV/cm | 5 kV/cm | 15 kV/cm | 25 kV/cm | 35 kV/cm |
|---|---|---|---|---|---|
| 3 min | 93.69 ± 0.12 A a | 97.56 ±0.10 B C a | 97.98 ± 0.38 C a | 93.60 ± 0.81 A a | 95.28 ± 0.24 D a |
| 9 min | 92.85 ± 0.23 A b | 98.12 ± 0.31 B a b | 97.96 ± 0.50 B a | 96.77 ± 0.24 C b | 96.30 ± 0.56 C a |
| 15 min | 91.72 ± 0.41 A c | 98.53 ± 0.27 B b | 97.77 ± 0.43 B a | 98.45 ± 0.62 B c | 96.50 ± 0.70 C a |
| 21 min | 91.51 ± 0.15 A c | 97.43 ± 0.42 B a | 97.59 ± 0.41 B a | 98.33 ± 0.47 B c | 98.19 ± 0.53 B b |
| 27 min | 91.38 ± 0.22 A c | 95.12 ± 0.51 B c | 97.32 ± 0.35 C a | 98.01 ± 0.53 C b c | 98.14 ± 0.42 C b |
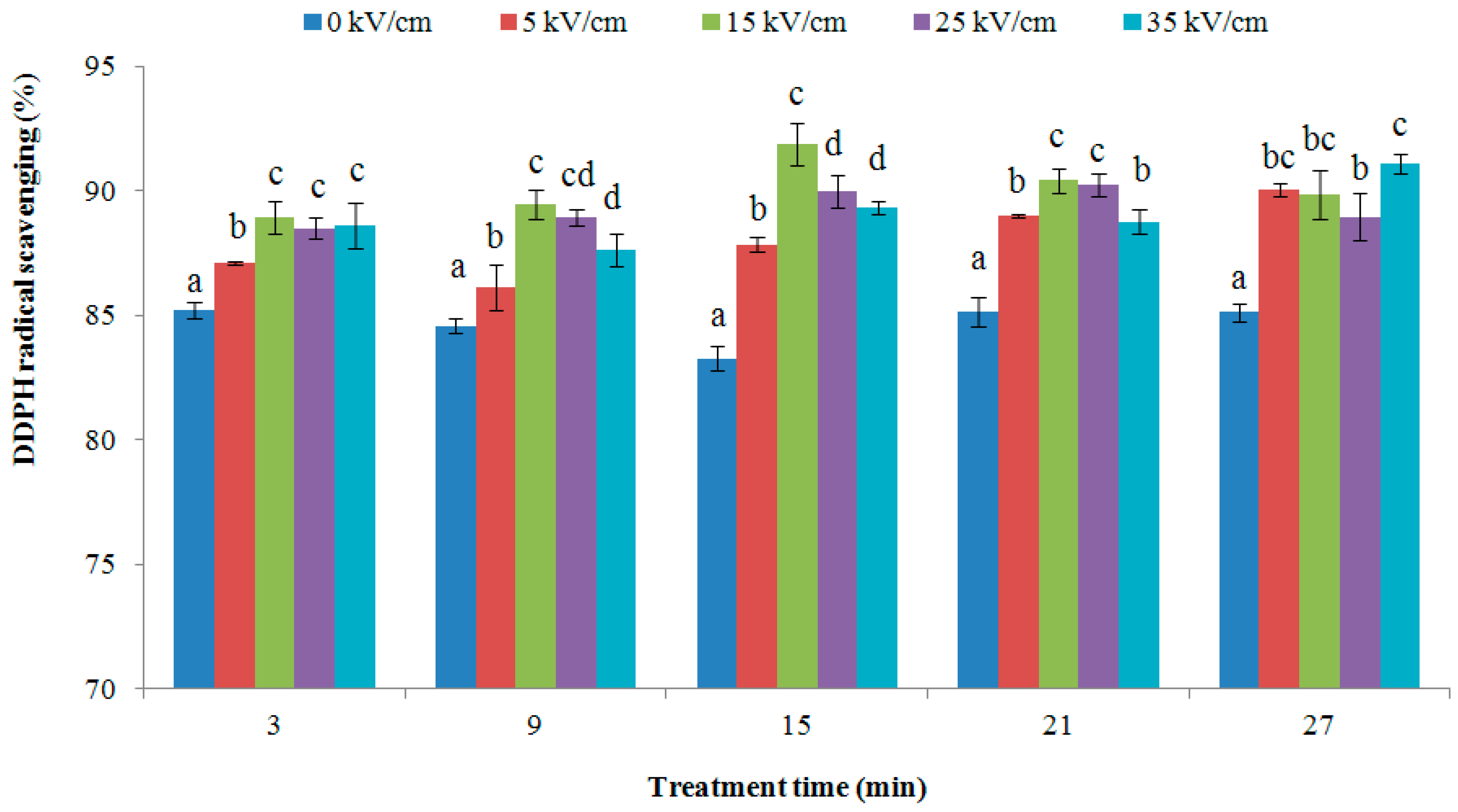
2.4. 1,1-Diphenyl-2-picrylhydrazyl (DDPH) Radical Scavenging Activity
2.5. Reducing Power Ability

3. Materials and Methods
3.1. Materials
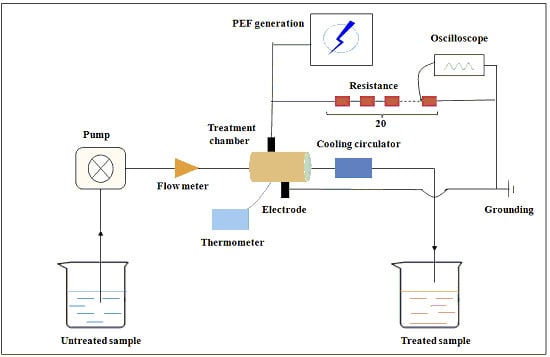
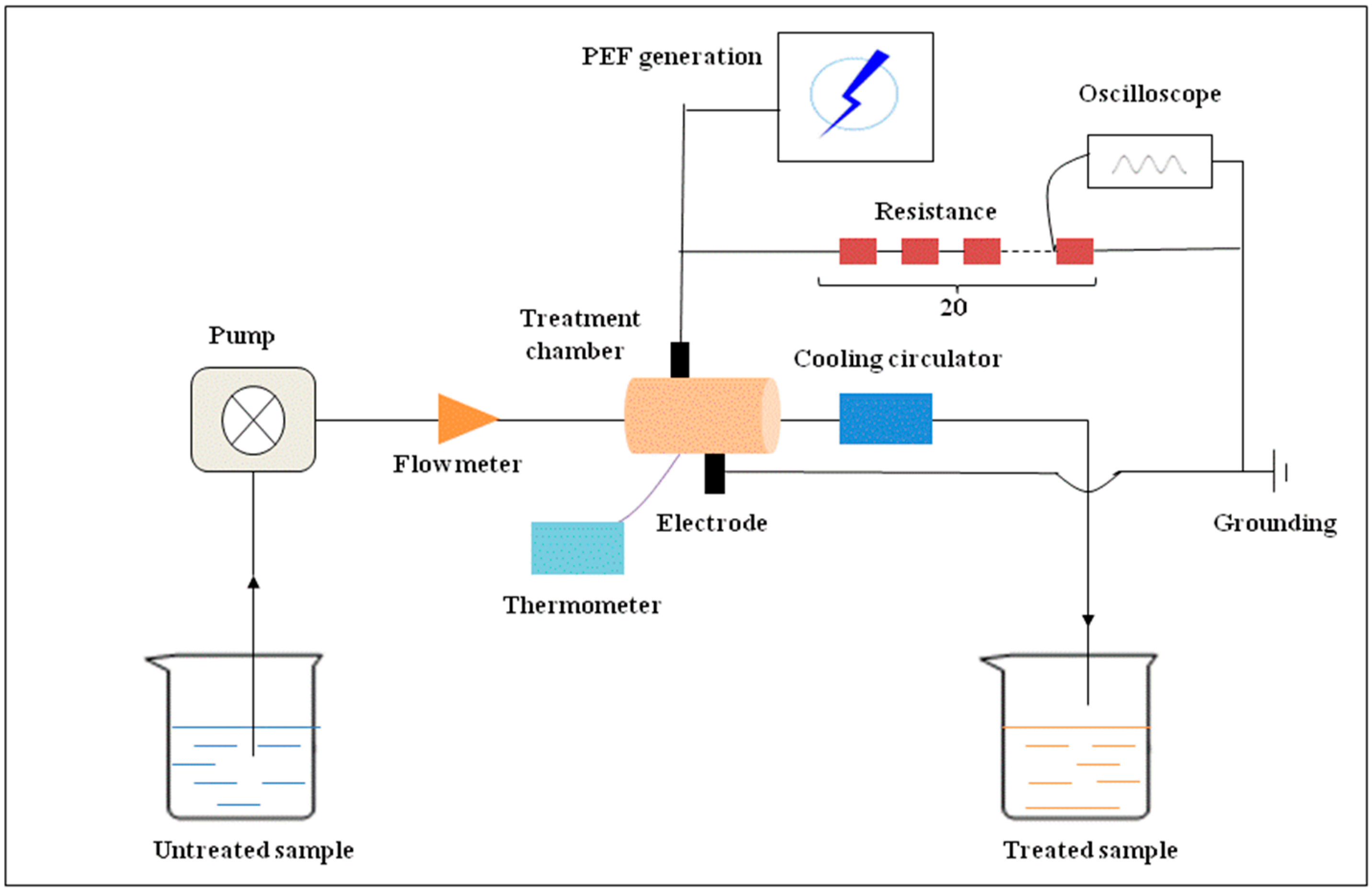
3.2. PEF Treatment System
| Electric Field Strength (kV/cm) | Treatment Time (ms) | ||||
|---|---|---|---|---|---|
| 0.8 | 2.4 | 4.0 | 5.6 | 7.2 | |
| 5 | 0.05 | 0.15 | 0.25 | 0.35 | 0.45 |
| 15 | 0.45 | 1.35 | 2.25 | 3.15 | 4.05 |
| 25 | 1.25 | 3.75 | 6.25 | 8.75 | 11.25 |
| 35 | 2.45 | 7.35 | 12.25 | 17.15 | 22.05 |
3.3. Fluorescence Measurement
3.4. FT-IR Analysis
3.5. Determination of VIT-C by HPLC
3.6. DDPH Radical Scavenging Activity
3.7. Reducing Power Ability
3.8. Statistical Analysis
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Engel, R.; Stefanovits-Bányai, É.; Abrankó, L. LC simultaneous determination of the free forms of B group vitamins and vitamin C in various fortified food products. Chromatographia 2010, 71, 1069–1074. [Google Scholar] [CrossRef]
- Reis, S.F.; Rai, D.K.; Abu-Ghannam, N. Apple pomace as a potential ingredient for the development of new functional foods. Int. J. Food Sci. Technol. 2014, 49, 1743–1750. [Google Scholar] [CrossRef]
- Carr, A.C.; Vissers, M. Synthetic or food-derived vitamin C–Are they equally bioavailable? Nutrients 2013, 5, 4284–4304. [Google Scholar] [CrossRef] [PubMed]
- Gallie, D.R. Increasing vitamin C content in plant foods to improve their nutritional value—Successes and challenges. Nutrients 2011, 5, 3424–3446. [Google Scholar] [CrossRef] [PubMed]
- Fenoll, J.; Martínez, A.; Hellín, P.; Flores, P. Simultaneous determination of ascorbic and dehydroascorbic acids in vegetables and fruits by liquid chromatography with tandem-mass spectrometry. Food Chem. 2011, 127, 340–344. [Google Scholar] [CrossRef]
- Shashirekha, M.N.; Mallikarjuna, S.E.; Rajarathnam, S. Status of bioactive compounds in foods, with focus on fruits and vegetables. Crit. Rev. Food Sci. 2015, 55, 1324–1339. [Google Scholar] [CrossRef] [PubMed]
- Cui, J.; Yuan, X.; Wang, L.; Jones, G.; Zhang, S. Recent loss of vitamin C biosynthesis ability in bats. PLoS ONE 2011, 6, e27114. [Google Scholar] [CrossRef]
- Davey, M.W.; Montagu, M.V.; Inzé, D.; Sanmartin, M.; Kanellis, A.; Smirnoff, N.; Benzie, I.J.J.; Strain, J.J.; Favell, D.; Fletcher, J. Plant l-ascorbic acid: Chemistry, function, metabolism, bioavailability and effects of processing. J. Sci. Food Agric. 2000, 80, 825–860. [Google Scholar] [CrossRef]
- Valente, A.; Albuquerque, T.G.; Sanches-Silva, A.; Costa, H.S. Ascorbic acid content in exotic fruits: A contribution to produce quality data for food composition databases. Food Res. Int. 2011, 44, 2237–2242. [Google Scholar] [CrossRef]
- Spínola, V.; Mendes, B.; Câmara, J.S.; Castilho, P.C. Effect of time and temperature on vitamin C stability in horticultural extracts. UHPLC-PDA vs. iodometric titration as analytical methods. LWT-Food Sci. Technol. 2013, 50, 489–495. [Google Scholar] [CrossRef]
- Roohinejad, S.; Everett, D.W.; Oey, I. Effect of pulsed electric field processing on carotenoid extractability of carrot purée. Int. J. Food Sci. Technol. 2014, 49, 2120–2127. [Google Scholar] [CrossRef]
- Pedras, M.M.; Tribst, A.A.L.; Cristianini, M. Effects of high-pressure homogenisation on physicochemical characteristics of partially skimmed milk. Int. J. Food Sci. Technol. 2014, 49, 861–866. [Google Scholar] [CrossRef]
- Jabbar, S.; Abid, M.; Hu, B.; Hashim, M.M.; Saeeduddin, M.; Lei, S.; Wu, T.; Zeng, X. Influence of sonication and high hydrostatic pressure on the quality of carrot juice. Int. J. Food Sci. Technol. 2014, 49, 2449–2457. [Google Scholar]
- Unni, L.E.; Chauhan, O.P.; Raju, P.S. High pressure processing of garlic paste: Effect on the quality attributes. Int. J. Food Sci. Technol. 2014, 49, 1579–1585. [Google Scholar] [CrossRef]
- Lafuente, M.T.; Ballester, A.R.; Calejero, J.; González-Candelas, L. Effect of high-temperature-conditioning treatments on quality, flavonoid composition and vitamin C of cold stored “Fortune” mandarins. Food Chem. 2011, 128, 1080–1086. [Google Scholar] [CrossRef]
- Polydera, A.; Stoforos, N.; Taoukis, P. Comparative shelf life study and vitamin C loss kinetics in pasteurised and high pressure processed reconstituted orange juice. J. Food Eng. 2003, 60, 21–29. [Google Scholar] [CrossRef]
- Patras, A.; Brunton, N.P.; da Pieve, S.; Butler, F. Impact of high pressure processing on total antioxidant activity, phenolic, ascorbic acid, anthocyanin content and colour of strawberry and blackberry purées. Innov. Food Sci. Emerg. Technol. 2009, 10, 308–313. [Google Scholar] [CrossRef]
- Suárez-Jacobo, Á.; Rüfer, C.E.; Gervilla, R.; Guamis, B.; Roig-Sagués, A.X.; Saldo, J. Influence of ultra-high pressure homogenisation on antioxidant capacity, polyphenol and vitamin content of clear apple juice. Food Chem. 2011, 127, 447–454. [Google Scholar]
- Barba, F.; Jäger, H.; Meneses, N.; Esteve, M.; Frígola, A.; Knorr, D. Evaluation of quality changes of blueberry juice during refrigerated storage after high-pressure and pulsed electric fields processing. Innov. Food Sci. Emerg. Technol. 2012, 14, 18–24. [Google Scholar] [CrossRef]
- Odriozola-Serrano, I.; Soliva-Fortuny, R.; Martín-Belloso, O. Impact of high-intensity pulsed electric fields variables on vitamin C, anthocyanins and antioxidant capacity of strawberry juice. LWT-Food Sci. Technol. 2009, 42, 93–100. [Google Scholar] [CrossRef]
- Odriozola-Serrano, I.; Aguilo-Aguayo, I.; Soliva-Fortuny, R.; Martin-Belloso, O. Pulsed electric fields processing effects on quality and health-related constituents of plant-based foods. Trends Food Sci. Technol. 2013, 29, 98–107. [Google Scholar] [CrossRef]
- Meneses, N.; Jaeger, H.; Knorr, D. pH-changes during pulsed electric field treatments—Numerical simulation and in situ impact on polyphenoloxidase inactivation. Innov. Food Sci. Emerg. Technol. 2011, 12, 499–504. [Google Scholar] [CrossRef]
- Siemer, C.; Toepfl, S.; Heinz, V. Inactivation of Bacillus subtilis spores by pulsed electric fields (PEF) in combination with thermal energy—I. Influence of process- and product parameters. Food Control 2014, 39, 163–171. [Google Scholar] [CrossRef]
- Oms-Oliu, G.; Odriozola-Serrano, I.; Soliva-Fortuny, R.; Elez-Martinez, P.; Martin-Belloso, O. Stability of health-related compounds in plant foods through the application of non thermal processes. Trends Food Sci. Technol. 2012, 23, 111–123. [Google Scholar] [CrossRef]
- Ade-Omowaye, B.; Taiwo, K.; Eshtiaghi, N.; Angersbach, A.; Knorr, D. Comparative evaluation of the effects of pulsed electric field and freezing on cell membrane permeabilisation and mass transfer during dehydration of red bell peppers. Innov. Food Sci. Emerg. Technol. 2003, 4, 177–188. [Google Scholar] [CrossRef]
- Marsellés-Fontanet, Á.R.; Puig-Pujol, A.; Olmos, P.; Mínguez-Sanz, S.; Martín-Belloso, O. A comparison of the effects of pulsed electric field and thermal treatments on grape juice. Food Bioprocess Technol. 2013, 6, 978–987. [Google Scholar]
- Quitão-Teixeira, L.J.; Odriozola-Serrano, I.; Soliva-Fortuny, R.; Mota-Ramos, A.; Martín-Belloso, O. Comparative study on antioxidant properties of carrot juice stabilised by high-intensity pulsed electric fields or heat treatments. J. Sci. Food Agric. 2009, 89, 2636–2642. [Google Scholar] [CrossRef]
- Wu, X.; Diao, Y.; Sun, C.; Yang, J.; Wang, Y.; Sun, S. Fluorimetric determination of ascorbic acid with o-phenylenediamine. Talanta 2003, 59, 95–99. [Google Scholar] [CrossRef]
- Boussetta, N.; Soichi, E.; Lanoisellé, J.-L.; Vorobiev, E. Valorization of oilseed residues: Extraction of polyphenols from flaxseed hulls by pulsed electric fields. Ind. Crop. Prod. 2014, 52, 347–353. [Google Scholar] [CrossRef]
- Zhang, S.; Yang, R.; Zhao, W.; Liang, Q.; Zhang, Z. The first ESR observation of radical species generated under pulsed electric fields processing. LWT-Food Sci. Technol. 2011, 44, 1233–1235. [Google Scholar]
- Torregrosa, F.; Esteve, M.J.; Frígola, A.; Cortés, C. Ascorbic acid stability during refrigerated storage of orange-carrot juice treated by high pulsed electric field and comparison with pasteurized juice. J. Food Eng. 2006, 73, 339–345. [Google Scholar] [CrossRef]
- Han, Z.; Zeng, X.A.; Fu, N.; Yu, S.J.; Chen, X.D.; Kennedy, J.F. Effects of pulsed electric field treatments on some properties of tapioca starch. Carbohydr. Polym. 2012, 89, 1012–1017. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.Y.; Zeng, X. A.; Deng, Z.; Yu, S.J.; Yamasaki, S. Effect of pulsed electric field on the secondary structure and thermal properties of soy protein isolate. Eur. Food Res. Technol. 2011, 233, 841–850. [Google Scholar] [CrossRef]
- Perez, O.E.; Pilosof, A.M. Pulsed electric fields effects on the molecular structure and gelation of β-lactoglobulin concentrate and egg white. Food Res. Int. 2004, 37, 102–110. [Google Scholar] [CrossRef]
- Dabbagh, H.A.; Azami, F.; Farrokhpour, H.; Chermahini, A.N. UV-vis, NMR and FT-IR spectra of tautomers of vitamin C. Experimental and DFT calculations. J. Chil. Chem. Soc. 2014, 59, 2588–2594. [Google Scholar] [CrossRef]
- Panicker, C.Y.; Varghese, H.T.; Philip, D. FT-IR, FT-Raman and SERS spectra of vitamin C. Spectrochim. Acta Part A: Mol. Biomol. Spectrosc. 2006, 65, 802–804. [Google Scholar] [CrossRef]
- Luo, W.B.; Han, Z.; Zeng, X.A.; Yu, S.J.; Kennedy, J.F. Study on the degradation of chitosan by pulsed electric fields treatment. Innov. Food Sci. Emerg. Technol. 2010, 11, 587–591. [Google Scholar] [CrossRef]
- Wang, J.; Wang, K.; Wang, Y.; Lin, S.; Zhao, P.; Jones, G. A novel application of pulsed electric field (PEF) processing for improving glutathione (GSH) antioxidant activity. Food Chem. 2014, 161, 361–366. [Google Scholar] [CrossRef] [PubMed]
- Plaza, L.; Sánchez-Moreno, C.; Elez-Martínez, P.; de Ancos, B.; Martín-Belloso, O.; Cano, M.P. Effect of refrigerated storage on vitamin C and antioxidant activity of orange juice processed by high-pressure or pulsed electric fields with regard to low pasteurization. Eur. Food Res. Technol. 2006, 223, 487–493. [Google Scholar] [CrossRef]
- Yeom, H.W.; Streaker, C.B.; Zhang, Q.H.; Min, D.B. Effects of pulsed electric fields on the quality of orange juice and comparison with heat pasteurization. J. Agric. Food Chem. 2000, 48, 4597–4605. [Google Scholar] [CrossRef] [PubMed]
- Elez-Martinez, P.; Martin-Belloso, O. Effects of high intensity pulsed electric field processing conditions on vitamin C and antioxidant capacity of orange juice and gazpacho, a cold vegetable soup. Food Chem. 2007, 102, 201–209. [Google Scholar] [CrossRef]
- Lin, S.; Guo, Y.; You, Q.; Yin, Y.; Liu, J. Preparation of antioxidant peptide from egg white protein and improvement of its activities assisted by high-intensity pulsed electric field. J. Sci. Food Agric. 2012, 92, 1554–1561. [Google Scholar] [CrossRef] [PubMed]
- Wang, K.; Wang, J.; Zhao, P.; Lin, S.Y.; Liu, B.L.; Liu, J.B.; Jones, G.; Huang, H.C. Optimized PEF treatment for antioxidant polypeptides with MW 10–30 kDa and preliminary analysis of structure change. Int. J. Biol. Macromol. 2012, 51, 819–825. [Google Scholar] [CrossRef] [PubMed]
- Lin, S.Y.; Jin, Y.; Liu, M.Y.; Yang, Y.; Zhang, M.S.; Guo, Y.; Jones, G.; Liu, J.B.; Yin, Y.G. Research on the preparation of antioxidant peptides derived from egg white with assisting of high-intensity pulsed electric field. Food Chem. 2013, 139, 300–306. [Google Scholar] [CrossRef]
- Sun, J.; Bai, W.; Zhang, Y.; Liao, X.; Hu, X. Effects of electrode materials on the degradation, spectral characteristics, visual colour, and antioxidant capacity of cyanidin-3-glucoside and cyanidin-3-sophoroside during pulsed electric field (PEF) treatment. Food Chem. 2011, 128, 742–747. [Google Scholar] [CrossRef]
- Liu, Z.W.; Zeng, X.A.; Sun, D.W.; Han, Z. Effects of pulsed electric fields on the permeabilization of calcein-filled soybean lecithin vesicles. J. Food Eng. 2014, 131, 26–32. [Google Scholar] [CrossRef]
- Wang, M.S.; Zeng, X.A.; Sun, D.W.; Han, Z. Quantitative analysis of sublethally injured Saccharomyces cerevisiae cells induced by pulsed electric fields. LWT-Food Sci. Technol. 2015, 60, 672–677. [Google Scholar] [CrossRef]
- Klimczak, I.; Gliszczyńska-Świgło, A. Comparison of UPLC and HPLC methods for determination of vitamin C. Food Chem. 2015, 175, 100–105. [Google Scholar] [CrossRef] [PubMed]
- Gu, F.; Kim, J.M.; Hayat, K.; Xia, S.; Feng, B.; Zhang, X. Characteristics and antioxidant activity of ultrafiltrated Maillard reaction products from a casein-glucose model system. Food Chem. 2009, 117, 48–54. [Google Scholar] [CrossRef]
- Yen, W.J.; Wang, B.S.; Chang, L.W.; Duh, P.D. Antioxidant properties of roasted coffee residues. J. Agric. Food Chem. 2005, 53, 2658–2663. [Google Scholar] [CrossRef] [PubMed]
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, Z.-H.; Zeng, X.-A.; Brennan, C.S.; Brennan, M.; Han, Z.; Xiong, X.-Y. Effects of Pulsed Electric Fields (PEF) on Vitamin C and Its Antioxidant Properties. Int. J. Mol. Sci. 2015, 16, 24159-24173. https://doi.org/10.3390/ijms161024159
Zhang Z-H, Zeng X-A, Brennan CS, Brennan M, Han Z, Xiong X-Y. Effects of Pulsed Electric Fields (PEF) on Vitamin C and Its Antioxidant Properties. International Journal of Molecular Sciences. 2015; 16(10):24159-24173. https://doi.org/10.3390/ijms161024159
Chicago/Turabian StyleZhang, Zhi-Hong, Xin-An Zeng, Charles S. Brennan, Margaret Brennan, Zhong Han, and Xia-Yu Xiong. 2015. "Effects of Pulsed Electric Fields (PEF) on Vitamin C and Its Antioxidant Properties" International Journal of Molecular Sciences 16, no. 10: 24159-24173. https://doi.org/10.3390/ijms161024159
APA StyleZhang, Z.-H., Zeng, X.-A., Brennan, C. S., Brennan, M., Han, Z., & Xiong, X.-Y. (2015). Effects of Pulsed Electric Fields (PEF) on Vitamin C and Its Antioxidant Properties. International Journal of Molecular Sciences, 16(10), 24159-24173. https://doi.org/10.3390/ijms161024159