Early and Degressive Putamen Atrophy in Multiple Sclerosis
Abstract
:1. Introduction
2. Results
2.1. No Difference of Age and Gender Distribution between Patients and Healthy Controls (HC)
2.2. Significant Reduced Absolute Putamen Volume (APV) in Patients Compared to HC
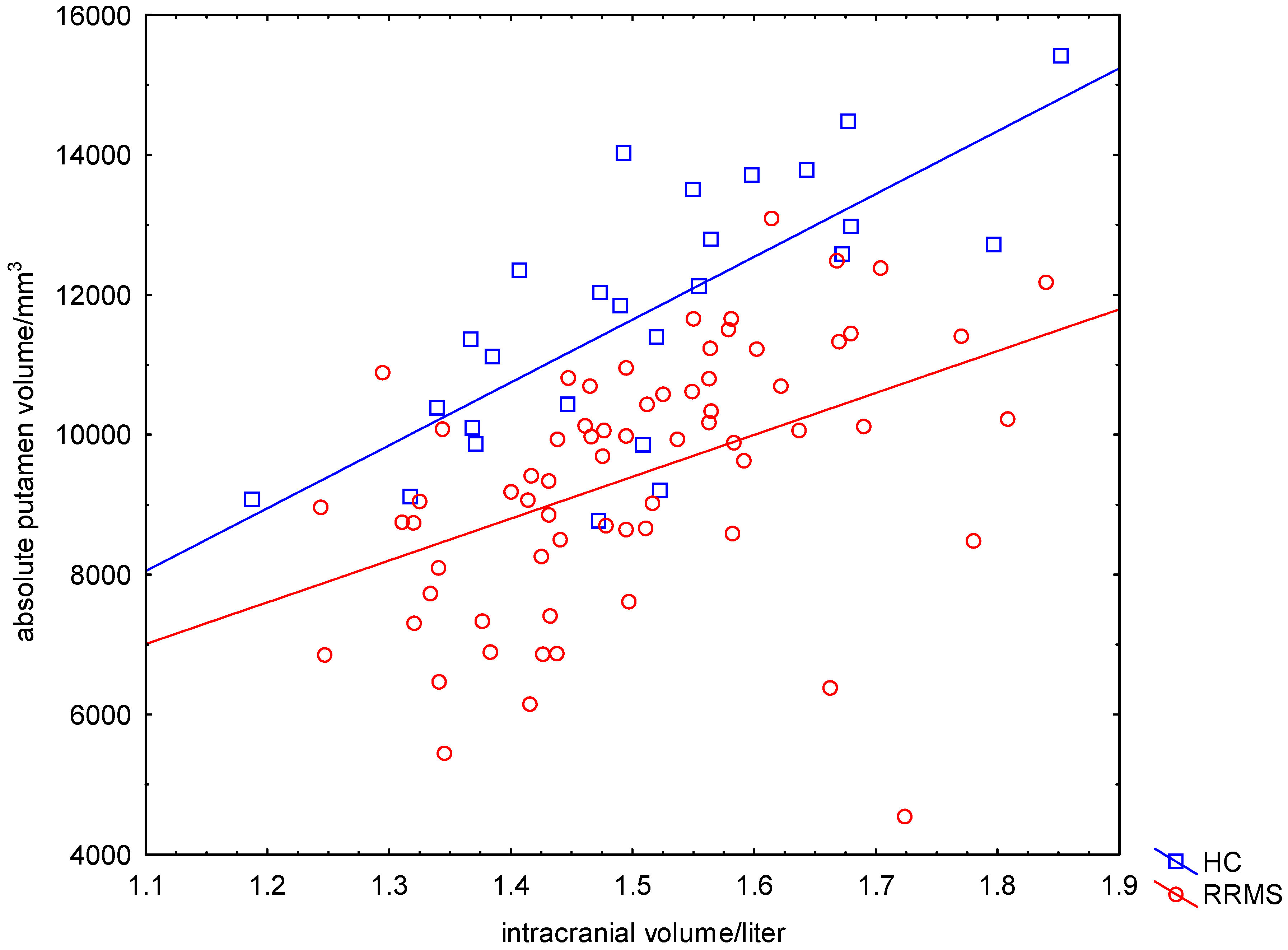
2.3. Significant Reduced Relative Putamen Volume (RPV) in Patients Compared to HC
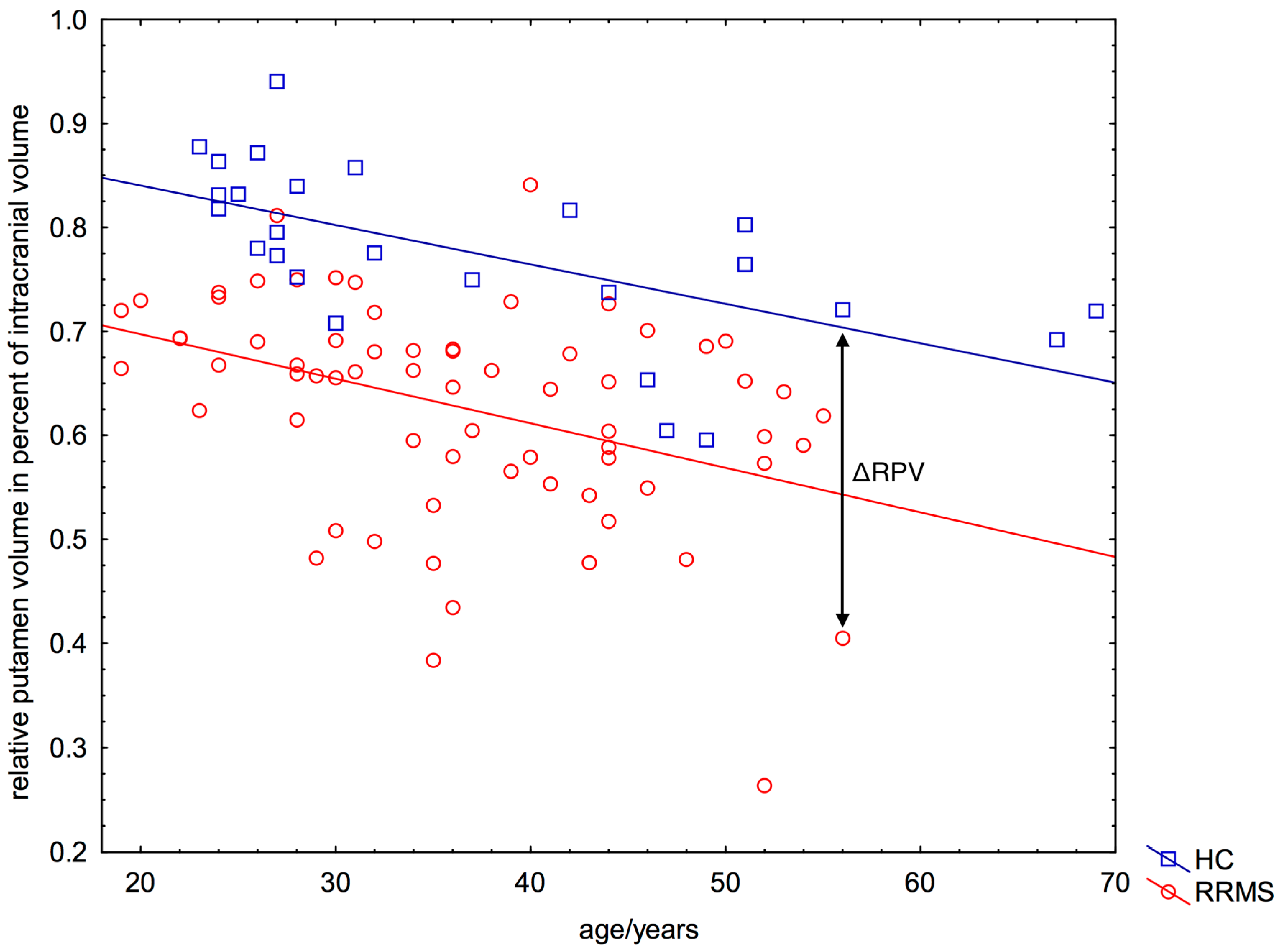
2.4. Negative ΔRPV and ΔRPV% in Nearly All Patients
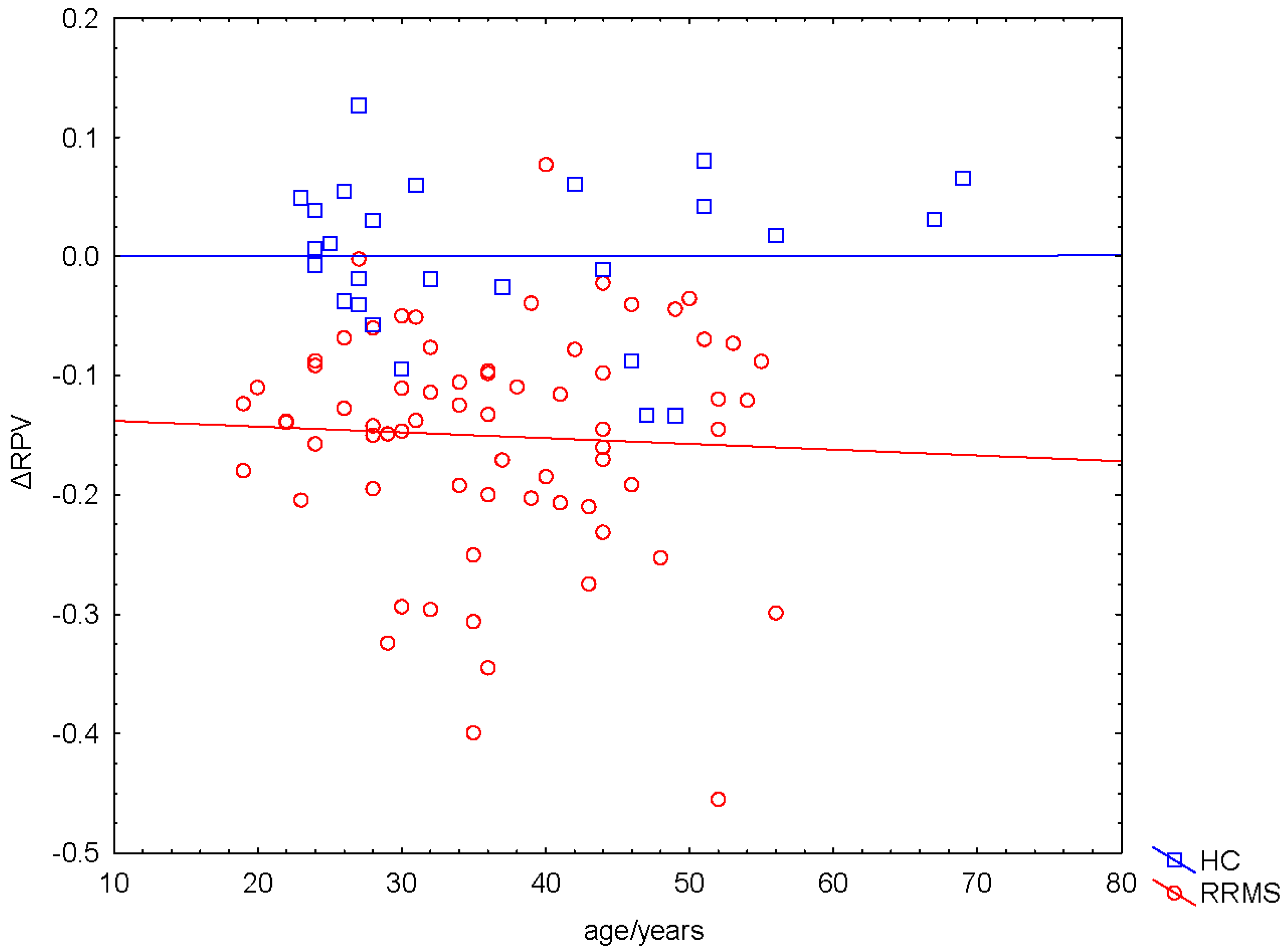
2.5. Correlation between ΔRPV% and Volume of WM Lesions
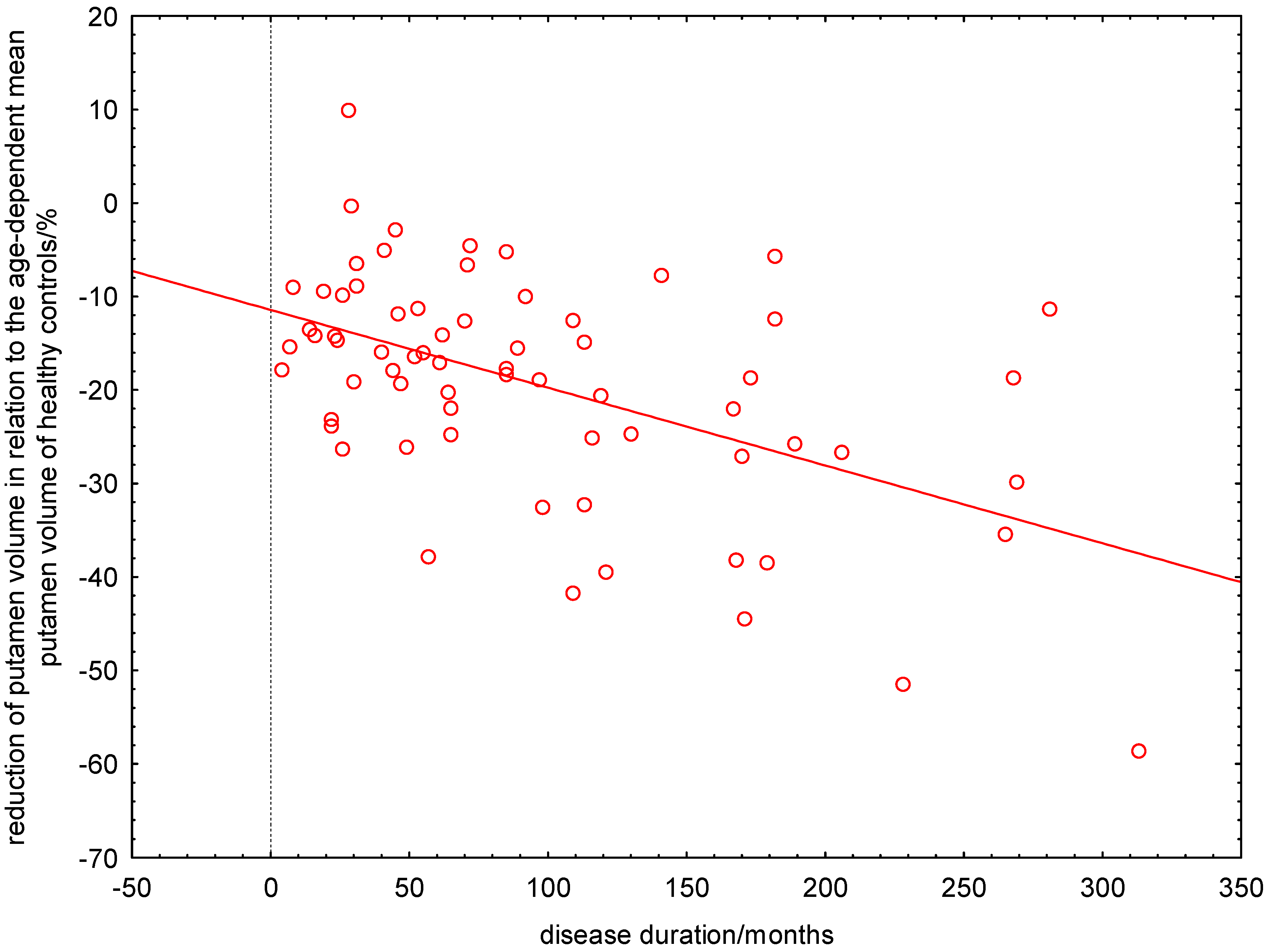
2.6. Linear or Non-Linear Dependence of ΔRPV% on Disease Duration
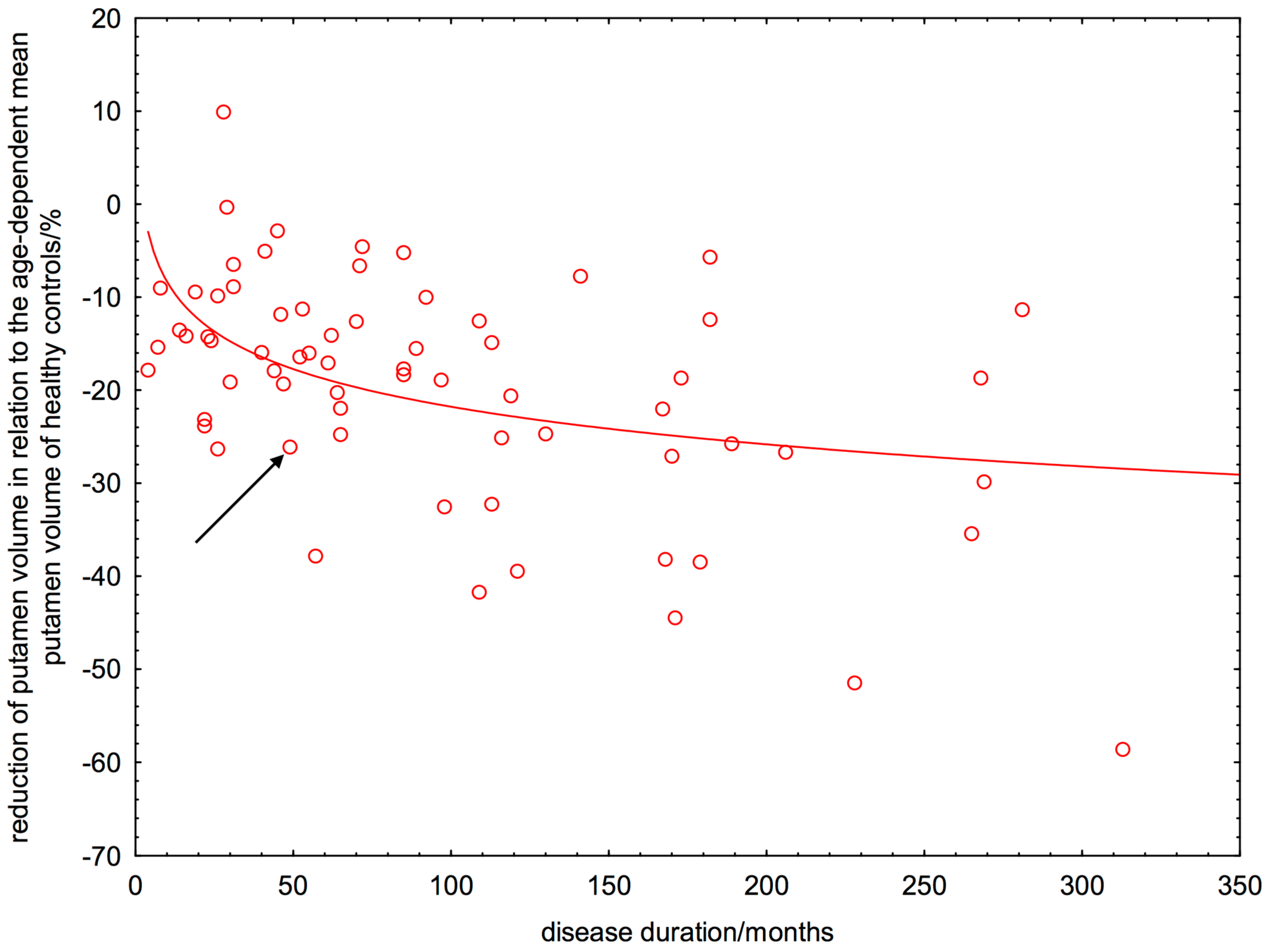
3. Discussion
3.1. Early and Degressive Putamen Atrophy in Patients with Relapsing-Remitting Multiple Sclerosis (RRMS)
3.2. Interpretation of Results in Perspective of Previous Studies
3.3. Limitations and Future Research
4. Experimental Section
4.1. Subjects and Their Main Clinical and Imaging Features
| Patients | Mean | Median | Min. | Max. | Lower Quartile | Upper Quartile | Standard Deviation |
|---|---|---|---|---|---|---|---|
| Age/years | 36.5 | 36.0 | 19.0 | 56.0 | 29.0 | 44.0 | 9.8 |
| White matter volume/L | 0.46 | 0.45 | 0.31 | 0.63 | 0.41 | 0.49 | 0.06 |
| Grey matter volume/L | 0.68 | 0.68 | 0.54 | 0.81 | 0.62 | 0.71 | 0.06 |
| Intracranial volume/L | 1.5 | 1.5 | 1.2 | 1.8 | 1.4 | 1.6 | 0.1 |
| Disease duration/months | 96.1 | 70.5 | 4.0 | 313.0 | 35.5 | 135.5 | 76.5 |
| Expanded Disability Status Scale | 2.1 | 2.0 | 0.0 | 6.0 | 1.5 | 2.5 | 1.3 |
| White matter lesion volume in Fluid-attenuated inversion recovery images/mL | 9.6 | 4.9 | 0.0 | 100.6 | 1.4 | 11.7 | 15.1 |
| White matter lesion volume in T1-weighted images/mL | 4.0 | 2.7 | 0.4 | 30.8 | 1.6 | 4.3 | 5.0 |
| Healthy Controls | |||||||
| Age/years | 37.0 | 30.5 | 23.0 | 69.0 | 26.0 | 47.0 | 13.8 |
| White matter volume/L | 0.52 | 0.52 | 0.41 | 0.70 | 0.47 | 0.57 | 0.07 |
| Grey matter volume/L | 0.73 | 0.73 | 0.54 | 0.86 | 0.68 | 0.80 | 0.09 |
| Intracranial volume/L | 1.5 | 1.5 | 1.2 | 1.9 | 1.4 | 1.6 | 0.2 |
4.2. Magnetic Resonance Imaging
4.3. Automated Volumetric Analysis of Cerebral Structures
4.4. Statistical Analysis
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
Abbreviations
References
- Cifelli, A.; Arridge, M.; Jezzard, P.; Esiri, M.M.; Palace, J.; Matthews, P.M. Thalamic neurodegeneration in multiple sclerosis. Ann. Neurol. 2002, 52, 650–653. [Google Scholar] [CrossRef] [PubMed]
- Geurts, J.J.; Calabrese, M.; Fisher, E.; Rudick, R.A. Measurement and clinical effect of grey matter pathology in multiple sclerosis. Lancet Neurol. 2012, 11, 1082–1092. [Google Scholar] [CrossRef]
- Hannoun, S.; Durand-Dubief, F.; Confavreux, C.; Ibarrola, D.; Streichenberger, N.; Cotton, F. Diffusion tensor-MRI evidence for extra-axonal neuronal degeneration in caudate and thalamic nuclei of patients with multiple sclerosis. AJNR Am. J. Neuroradiol. 2012, 33, 1363–1368. [Google Scholar] [CrossRef] [PubMed]
- Horakova, D.; Kalincik, T.; Dusankova, J.B.; Dolezal, O. Clinical correlates of grey matter pathology in multiple sclerosis. BMC Neurol. 2012, 12, 10. [Google Scholar] [CrossRef] [PubMed]
- Hulst, H.E.; Geurts, J.J. Gray matter imaging in multiple sclerosis: what have we learned? BMC Neurol. 2011, 11, 153. [Google Scholar] [CrossRef] [PubMed]
- Popescu, B.F.; Lucchinetti, C.F. Meningeal and cortical grey matter pathology in multiple sclerosis. BMC Neurol. 2012, 12, 11. [Google Scholar] [CrossRef] [PubMed]
- Jacobsen, C.; Hagemeier, J.; Myhr, K.M.; Nyland, H.; Lode, K.; Bergsland, N. Brain atrophy and disability progression in multiple sclerosis patients: A 10-year follow-up study. J. Neurol. Neurosurg. Psychiatr. 2014, 85, 1109–1115. [Google Scholar] [CrossRef] [PubMed]
- Roosendaal, S.D.; Bendfeldt, K.; Vrenken, H.; Polman, C.H.; Borgwardt, S.; Radue, E.W. Grey matter volume in a large cohort of MS patients: relation to MRI parameters and disability. Mult. Scler. 2011, 17, 1098–1106. [Google Scholar] [CrossRef] [PubMed]
- Minagar, A.; Barnett, M.H.; Benedict, R.H.; Pelletier, D.; Pirko, I.; Sahraian, M.A. The thalamus and multiple sclerosis: Modern views on pathologic, imaging, and clinical aspects. Neurology 2013, 80, 210–219. [Google Scholar] [CrossRef] [PubMed]
- Ashburner, J.; Friston, K.J. Voxel-based morphometry—The methods. Neuroimage 2000, 11, 805–821. [Google Scholar] [CrossRef] [PubMed]
- Wright, I.C.; McGuire, P.K.; Poline, J.B.; Travere, J.M.; Murray, R.M.; Frith, C.D.; Frackowiak, R.S.; Friston, K.J. A voxel-based method for the statistical analysis of gray and white matter density applied to schizophrenia. Neuroimage 1995, 2, 244–252. [Google Scholar] [CrossRef] [PubMed]
- FreeSurfer Software Suite. Available online: http://surfer.nmr.mgh.harvard.edu/ (accessed on 21 September 2015).
- FSL FIRST. Available online: http://www.fmrib.ox.ac.uk/fsl/first (accessed on 21 September 2015).
- Bergsland, N.; Horakova, D.; Dwyer, M.G.; Dolezal, O.; Seidl, Z.K.; Vaneckova, M. Subcortical and cortical gray matter atrophy in a large sample of patients with clinically isolated syndrome and early relapsing-remitting multiple sclerosis. AJNR Am. J. Neuroradiol. 2012, 33, 1573–1578. [Google Scholar] [CrossRef] [PubMed]
- Henry, R.G.; Shieh, M.; Okuda, D.T.; Evangelista, A.; Gorno-Tempini, M.L.; Pelletier, D. Regional grey matter atrophy in clinically isolated syndromes at presentation. J. Neurol. Neurosurg. Psychiatr. 2008, 79, 1236–1244. [Google Scholar] [CrossRef] [PubMed]
- Hagemeier, J.; Yeh, E.A.; Brown, M.H.; Bergsland, N.; Dwyer, M.G.; Carl, E. Iron content of the pulvinar nucleus of the thalamus is increased in adolescent multiple sclerosis. Mult. Scler. 2013, 19, 567–576. [Google Scholar] [CrossRef] [PubMed]
- Sepulcre, J.; Sastre-Garriga, J.; Cercignani, M.; Ingle, G.T.; Miller, D.H.; Thompson, A.J. Regional gray matter atrophy in early primary progressive multiple sclerosis: A voxel-based morphometry study. Arch. Neurol. 2006, 63, 1175–1180. [Google Scholar] [CrossRef] [PubMed]
- Batista, S.; Zivadinov, R.; Hoogs, M.; Bergsland, N.; Heininen-Brown, M.; Dwyer, M.G. Basal ganglia, thalamus and neocortical atrophy predicting slowed cognitive processing in multiple sclerosis. J. Neurol. 2012, 259, 139–146. [Google Scholar] [CrossRef] [PubMed]
- Ramasamy, D.P.; Benedict, R.H.; Cox, J.L.; Fritz, D.; Abdelrahman, N.; Hussein, S. Extent of cerebellum, subcortical and cortical atrophy in patients with MS: a case-control study. J. Neurol. Sci. 2009, 282, 47–54. [Google Scholar] [CrossRef] [PubMed]
- Ciccarelli, O.; Werring, D.J.; Wheeler-Kingshott, C.A.; Barker, G.J.; Parker, G.J.; Thompson, A.J. Investigation of MS normal-appearing brain using diffusion tensor MRI with clinical correlations. Neurology 2001, 56, 926–933. [Google Scholar] [CrossRef] [PubMed]
- Dolezal, O.; Gabelic, T.; Horakova, D.; Bergsland, N.; Dwyer, M.G.; Seidl, Z. Development of gray matter atrophy in relapsing-remitting multiple sclerosis is not gender dependent: Results of a 5-year follow-up study. Clin. Neurol. Neurosurg. 2013, 115, 42–48. [Google Scholar] [CrossRef] [PubMed]
- Calabrese, M.; Rinaldi, F.; Mattisi, I.; Bernardi, V.; Favaretto, A.; Perini, P. The predictive value of gray matter atrophy in clinically isolated syndromes. Neurology 2011, 77, 257–263. [Google Scholar] [CrossRef] [PubMed]
- Modica, C.M.; Zivadinov, R.; Dwyer, M.G.; Bergsland, N.; Weeks, A.R.; Benedict, R.H. Iron and volume in the deep gray matter: association with cognitive impairment in multiple sclerosis. AJNR Am. J. Neuroradiol. 2015, 36, 57–62. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Metz, L.M.; Yong, V.W.; Mitchell, J.R. 3T deep gray matter T2 hypointensity correlates with disability over time in stable relapsing-remitting multiple sclerosis: A 3-year pilot study. J. Neurol. Sci. 2010, 297, 76–81. [Google Scholar] [CrossRef] [PubMed]
- Ge, Y.; Jensen, J.H.; Lu, H.; Helpern, J.A.; Miles, L.; Inglese, M. Quantitative assessment of iron accumulation in the deep gray matter of multiple sclerosis by magnetic field correlation imaging. AJNR Am. J. Neuroradiol. 2007, 28, 1639–1644. [Google Scholar] [CrossRef] [PubMed]
- Neema, M.; Arora, A.; Healy, B.C.; Guss, Z.D.; Brass, S.D.; Duan, Y. Deep gray matter involvement on brain MRI scans is associated with clinical progression in multiple sclerosis. J. Neuroimaging 2009, 19, 3–8. [Google Scholar] [CrossRef] [PubMed]
- Bonilha, L.; Kobayashi, E.; Cendes, F.; Min, L.L. Protocol for volumetric segmentation of medial temporal structures using high-resolution 3-D magnetic resonance imaging. Hum. Brain Mapp. 2004, 22, 145–154. [Google Scholar] [CrossRef] [PubMed]
- Collins, D.L.; Pruessner, J.C. Towards accurate, automatic segmentation of the hippocampus and amygdala from MRI by augmenting ANIMAL with a template library and label fusion. Neuroimage 2010, 52, 1355–1366. [Google Scholar] [CrossRef] [PubMed]
- Crum, W.R.; Scahill, R.I.; Fox, N.C. Automated hippocampal segmentation by regional fluid registration of serial MRI: validation and application in Alzheimer's disease. Neuroimage 2001, 13, 847–855. [Google Scholar] [CrossRef] [PubMed]
- Pruessner, J.C.; Li, L.M.; Serles, W.; Pruessner, M.; Collins, D.L.; Kabani, N. Volumetry of hippocampus and amygdala with high-resolution MRI and three-dimensional analysis software: minimizing the discrepancies between laboratories. Cereb. Cortex. 2000, 10, 433–442. [Google Scholar] [CrossRef] [PubMed]
- Keller, S.S.; Gerdes, J.S.; Mohammadi, S.; Kellinghaus, C.; Kugel, H.; Deppe, K. Volume estimation of the thalamus using freesurfer and stereology: consistency between methods. Neuroinformatics 2012, 10, 341–350. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, D.; Voevodskaya, O.; Imrell, K.; Stawiarz, L.; Spulber, G.; Wahlund, L.O.; Hillert, J.; Westman, E.; Karrenbauer, V.D. Multiple sclerosis patients lacking oligoclonal bands in the cerebrospinal fluid have less global and regional brain atrophy. J. Neuroimmunol. 2014, 274, 149–154. [Google Scholar] [CrossRef] [PubMed]
- Polman, C.H.; Reingold, S.C.; Banwell, B.; Clanet, M.; Cohen, J.A.; Filippi, M. Diagnostic criteria for multiple sclerosis: 2010 revisions to the McDonald criteria. Ann. Neurol. 2011, 69, 292–302. [Google Scholar] [CrossRef] [PubMed]
- Dale, A.M.; Fischl, B.; Sereno, M.I. Cortical surface-based analysis. I. Segmentation and surface reconstruction. Neuroimage 1999, 9, 179–194. [Google Scholar] [CrossRef] [PubMed]
- Fischl, B. FreeSurfer. Neuroimage 2012, 62, 774–781. [Google Scholar] [CrossRef] [PubMed]
- Fischl, B.; Dale, A.M. Measuring the thickness of the human cerebral cortex from magnetic resonance images. Proc. Natl. Acad. Sci. USA 2000, 97, 11050–11055. [Google Scholar] [CrossRef] [PubMed]
- Fischl, B.; Sereno, M.I.; Dale, A.M. Cortical surface-based analysis. II: Inflation, flattening, and a surface-based coordinate system. Neuroimage 1999, 9, 195–207. [Google Scholar] [CrossRef] [PubMed]
- Patenaude, B.; Smith, S.M.; Kennedy, D.; Jenkinson, M. A Bayesian Model of Shape and Appearance for Subcortical Brain. NeuroImage 2011, 56, 907–922. [Google Scholar] [CrossRef] [PubMed]
- LST: Lesion Segmentation Tool. Available online: http://www.applied-statistics.de/lst.html (accessed on 21 September 2015).
- Schmidt, P.; Gaser, C.; Arsic, M.; Buck, D.; Förschler, A.; Berthele, A.; Hoshi, M.; Ilg, R.; Schmid, V.J.; Zimmer, C.; Hemmer, B.; Mühlau, M. An automated tool for detection of FLAIR-hyperintense white-matter lesions in Multiple Sclerosis. NeuroImage 2012, 59, 3774–3783. [Google Scholar] [CrossRef] [PubMed]
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Krämer, J.; Meuth, S.G.; Tenberge, J.-G.; Schiffler, P.; Wiendl, H.; Deppe, M. Early and Degressive Putamen Atrophy in Multiple Sclerosis. Int. J. Mol. Sci. 2015, 16, 23195-23209. https://doi.org/10.3390/ijms161023195
Krämer J, Meuth SG, Tenberge J-G, Schiffler P, Wiendl H, Deppe M. Early and Degressive Putamen Atrophy in Multiple Sclerosis. International Journal of Molecular Sciences. 2015; 16(10):23195-23209. https://doi.org/10.3390/ijms161023195
Chicago/Turabian StyleKrämer, Julia, Sven G. Meuth, Jan-Gerd Tenberge, Patrick Schiffler, Heinz Wiendl, and Michael Deppe. 2015. "Early and Degressive Putamen Atrophy in Multiple Sclerosis" International Journal of Molecular Sciences 16, no. 10: 23195-23209. https://doi.org/10.3390/ijms161023195
APA StyleKrämer, J., Meuth, S. G., Tenberge, J.-G., Schiffler, P., Wiendl, H., & Deppe, M. (2015). Early and Degressive Putamen Atrophy in Multiple Sclerosis. International Journal of Molecular Sciences, 16(10), 23195-23209. https://doi.org/10.3390/ijms161023195






