Pathogenesis of Target Organ Damage in Hypertension: Role of Mitochondrial Oxidative Stress
Abstract
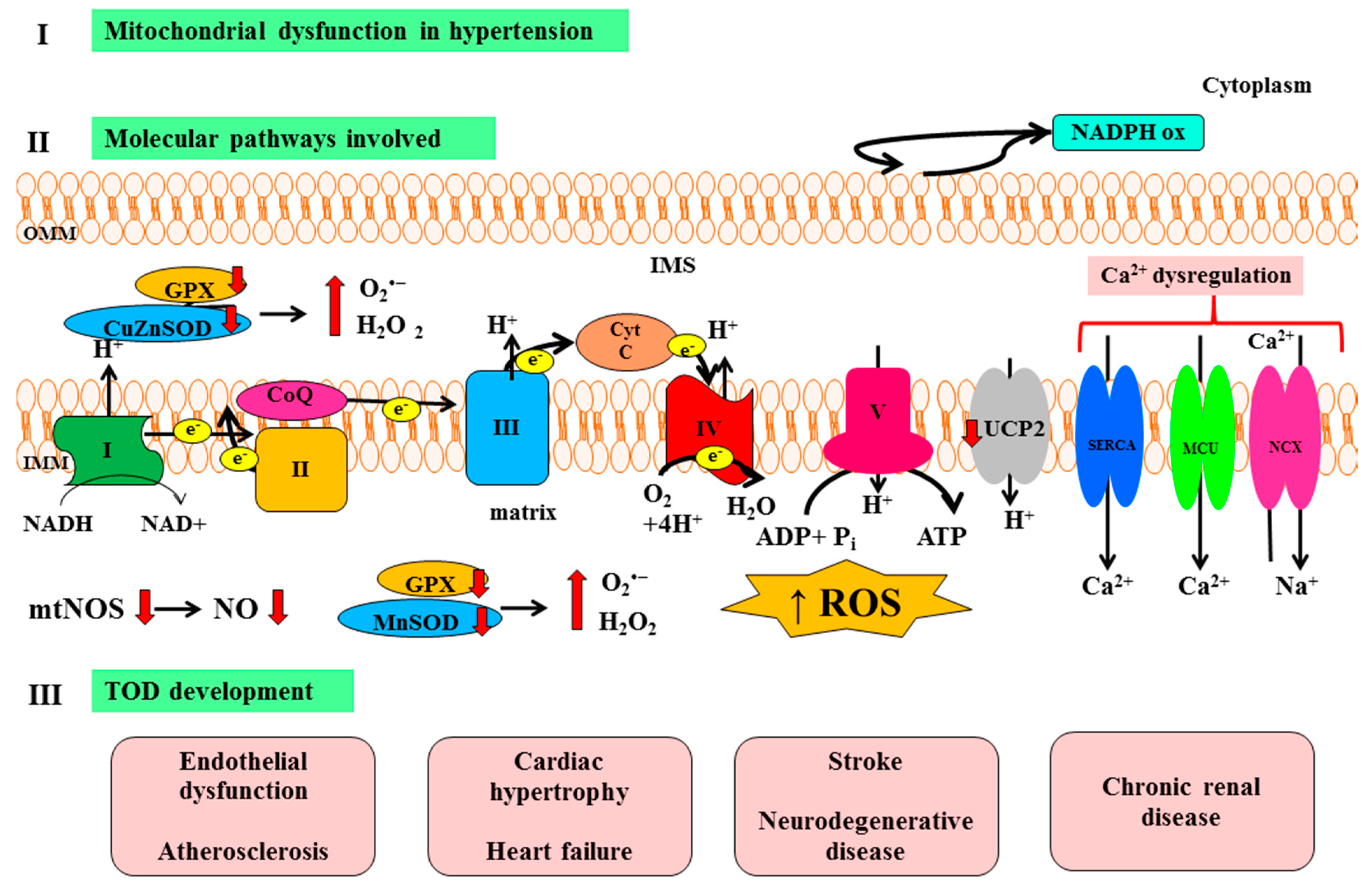
:1. Introduction
2. Molecular Mechanisms of Target Organ Damage (TOD): Focus on Oxidative Stress and Mitochondria
- Nicotinamide adenine dinucleotide phosphate (NADPH) oxidase;
- Uncoupled nitric oxide synthase (NOS);
- Xanthine oxidase;
- Mitochondria.
3. Mitochondrial Dysfunction and Vascular Hypertensive Damage
4. Mitochondrial Dysfunction and Cardiac Hypertensive Damage
5. Mitochondrial Dysfunction and Renal Hypertensive Damage
6. Mitochondrial Dysfunction and Cerebral Hypertensive Damage
7. Therapeutic Approaches
| Agents | Therapeutic Effects |
|---|---|
| l-Carnitine | ↓ Cardiac remodeling in experimental hypertension [80] |
| Conventional antioxidants (Vitamin C, Vitamin E | ↓ MPO activity; ↓ Lipids peroxidation [81,82,83,84,85,86]; ↓ Atherosclerosis process rate |
| ACEI, ARB, Statins | ↑ Vascular eNOS activity; ↓ Endothelin-1 expression; ↓ Ang II AT1 receptor expression [87,88,89,90,91,92]; ↓ NADPH oxidase activity |
| Mitochondria-targeted antioxidant therapies | |
| Mito Q10 | ↓ Lipid peroxidation and mitochondria damage; ↑ Endothelial NO bioavailability [93,94,95,96]; ↓ Cardiac hypertrophy in young SHRSP |
| SS-31 | ↓ Ang-II induced mitochondrial oxidative stress; ↓ Up-regulation of mitochondrial biogenesis; ↓ ROS mediated p38 MAPK signaling [97,98]; ↓ Caspase-3 activation [54] |
| Edaravone | ↓ Pressure overload-induced left ventricular hypertrophy (Ask1 inhibition); ↓ Perivascular and intermuscular fibrosis [99] |
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Chobanian, A.V.; Bakris, G.L.; Black, H.R.; Cushman, W.C.; Green, L.A.; Izzo, J.L., Jr.; Jones, D.W.; Materson, B.J.; Oparil, S.; Wright, J.T., Jr.; et al. Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure. National Heart, Lung, and Blood Institute; National High Blood Pressure Education Program Coordinating Committee: Seventh report of the Joint National Committeeon prevention, detection, evaluation, and treatment of high blood pressure. Hypertension 2003, 42, 1206–1252. [Google Scholar]
- Kearney, P.; Whelton, M.; Reynolds, K.; Muntner, P.; He, J. Global burden of hypertension analysis of worldwide data. Lancet 2005, 365, 217–223. [Google Scholar] [CrossRef] [PubMed]
- Karpha, M.; Lip, G.V. The pathophysiology of target organ damage in hypertension. Minerva Cardioangiol. 2006, 54, 417–429. [Google Scholar] [PubMed]
- Ezzati, M.; Lopez, A.D.; Rodgers, A.; VandernHoorn, S.; Murray, C.J. Selected major risk factors and global and regional burden of disease. Lancet 2002, 360, 1347–1360. [Google Scholar] [CrossRef] [PubMed]
- Dawood, T.; Schilaich, M.P. Mediators of target organ damage in hypertension: Focus on obesity associated factors and inflammation. Minerva Cardioangiol. 2009, 57, 687–703. [Google Scholar] [PubMed]
- Perlini, S.; Grassi, G. Hypertension-related target organ damage: Is it a continuum? J. Hypertens. 2013, 31, 1083–1085. [Google Scholar] [CrossRef]
- Nadar, S.K.; Tayebjee, M.H.; Messerli, F.; Lip, G.Y. Target organ damage in hypertension: Pathophysiology and implications for drug theraphy. Curr. Pharm. Des. 2006, 12, 1581–1592. [Google Scholar] [CrossRef] [PubMed]
- Laurent, S.; Alivon, M.; Beaussier, H.; Boutouyrie, P. Aortic stiffness as a tissue biomarker for predicting future cardiovascular events in asymptomatic hypertensive subjects. Ann. Med. 2012, 44, S93–S97. [Google Scholar] [CrossRef] [PubMed]
- Matsui, Y.; Ishikawa, J.; Shibasaki, S.; Shimada, K.; Kario, K. Association between home arterial stiffness index and target organ damage in hypertension: Comparison with pulse wave velocity and augmentation index. Atherosclerosis 2011, 219, 637–642. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Garcia, A.; Gomez-Marcos, M.A.; Recio-Rodriguez, J.I.; Patino-Alonso, M.C.; Rodriguez-Sanchez, E.; Agudo-Conde, C.; García-Ortiz, L. Office and 24-h heart rate and target organ damage in hypertensive patients. BMC Cardiovasc. Disord. 2012, 12, 19. [Google Scholar] [CrossRef] [PubMed]
- Grassi, G. Sympathetic neural activity in hypertension and related diseases. Am. J. Hypertens. 2010, 23, 1052–1060. [Google Scholar] [CrossRef] [PubMed]
- Falcao-Pires, I.; Palladini, G.; Goncalves, N.; van der Velden, J.; Moreira-Goncalves, D.; Miranda-Silva, D.; Salinaro, F.; Paulus, W.J.; Niessen, H.W.; Perlini, S.; et al. Distinct mechanisms for diastolic dysfunction in diabetes mellitus and chronic pressure-overload. Basic. Res. Cardiol. 2011, 106, 801–814. [Google Scholar]
- Tozzi, R.; Palladini, G.; Fallarini, S.; Nano, R.; Gatti, C.; Presotto, C.; Schiavone, A.; Micheletti, R.; Ferrari, P.; Fogari, R.; et al. Matrix metalloprotease activity is enhanced in the compensated but not in the decompensated phase of pressure overload hypertrophy. Am. J. Hypertens. 2007, 20, 663–669. [Google Scholar] [PubMed]
- Lee, M.Y.; Griendling, K.K. Redox signaling, vascular function, and hypertension. Antioxid. Redox Signal. 2008, 10, 1045–1059. [Google Scholar] [CrossRef] [PubMed]
- Schiffrin, E.L.; Touyz, R.M. From bedside to bench to bedside: Role of renin-angiotensin-aldosterone system in remodeling of resistance arteries in hypertension. Am. J. Physiol. Heart Circ. Physiol. 2004, 287, H435–H446. [Google Scholar] [CrossRef] [PubMed]
- Dikalov, S. Cross talk between mitochondria and NADPH oxidases. Free Radic. Biol. Med. 2011, 51, 1289–1301. [Google Scholar] [CrossRef] [PubMed]
- Schulz, E.; Wenzel, P.; Münzel, T.; Daiber, A. Mitochondrial redox signaling: Interaction of mitochondrial reactive oxygen species with other sources of oxidative stress. Antioxid. Redox Signal. 2014, 20, 308–324. [Google Scholar] [CrossRef] [PubMed]
- Yu, E.; Mercer, J.; Bennett, M. Mitochondria in vascular disease. Cardiovasc. Res. 2012, 95, 173–182. [Google Scholar] [CrossRef] [PubMed]
- Turrens, J.F. Mitochondrial formation of reactive oxygen species. J. Physiol. 2003, 552, 335–344. [Google Scholar] [CrossRef] [PubMed]
- Case, A.J.; Li, S.; Basu, U.; Tian, J.; Zimmerman, M.C. Mitochondrial-localized NADPH oxidase 4 is a source of superoxide in angiotensin II-stimulated neurons. Am. J. Physiol. Heart Circ. Physiol. 2013, 305, H19–H28. [Google Scholar] [CrossRef] [PubMed]
- Kuroda, J.; Ago, T.; Matsushima, S.; Zhai, P.; Schneider, M.D.; Sadoshima, J. NADPH oxidase 4 (Nox4) is a major source of oxidative stress in the failing heart. Proc. Natl. Acad. Sci. USA 2010, 107, 15565–15570. [Google Scholar] [CrossRef] [PubMed]
- Block, K.; Gorin, Y.; Abboud, H.E. Subcellular localization of Nox4 and regulation in diabetes. Proc. Natl. Acad. Sci. USA 2009, 106, 14385–14390. [Google Scholar] [CrossRef] [PubMed]
- Puddu, P.; Puddu, G.M.; Galletti, L.; Cravero, E.; Muscari, A. Mitochondrial dysfunction as an initiating event in atherogenesis: A plausible hypothesis. Cardiology 2005, 103, 137–141. [Google Scholar] [CrossRef] [PubMed]
- Dikalov, S.L.; Ungvari, Z. Role of mitochondrial oxidative stress in hypertension. Am. J. Physiol. Heart Circ. Physiol. 2013, 305, H1417–H1427. [Google Scholar] [CrossRef] [PubMed]
- De Cavanagh, E.M.; Toblli, J.E.; Ferder, L.; Piotrkowski, B.; Stella, I.; Fraga, C.G.; Inserra, F. Angiotensin II blockade improves mitochondrial function in spontaneously hypertensive rats. Cell. Mol. Biol. 2005, 51, 573–578. [Google Scholar] [PubMed]
- Ross, R. Cell biology of atherosclerosis. Annu. Rev. Physiol. 1995, 57, 791–804. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Mehta, J.L.; Haider, N.; Zhang, X.; Narula, J.; Li, D. Role of caspases in Ox-LDL-induced apoptotic cascade in human coronary artery endothelial cells. Circ. Res. 2004, 94, 370–376. [Google Scholar] [CrossRef] [PubMed]
- Ballinger, S.W.; Patterson, C.; Yan, C.N.; Doan, R.; Burow, D.L.; Young, C.G.; Yakes, F.M.; van Houten, B.; Ballinger, C.A.; Freeman, B.A.; et al. Hydrogen peroxide- and peroxynitrite-induced mitochondrial DNA damage and dysfunction in vascular endothelial and smooth muscle cells. Circ. Res. 2000, 86, 960–966. [Google Scholar]
- Choi, H.; Tostes, R.C.; Webb, R.C. Mitochondrial aldehyde dehydrogenase prevents ROS-induced vascular contraction in angiotensin-II hypertensive mice. J. Am. Soc. Hypertens. 2011, 5, 154–160. [Google Scholar] [CrossRef] [PubMed]
- Dikalova, A.E.; Bikineyeva, A.T.; Budzyn, K.; Nazarewicz, R.R.; McCann, L.; Lewis, W.; Harrison, D.G.; Dikalov, S.I. Therapeutic targeting of mitochondrial superoxide in hypertension. Circ. Res. 2010, 107, 106–116. [Google Scholar] [CrossRef] [PubMed]
- Doughan, A.K.; Harrison, D.G.; Dikalov, S.I. Molecular mechanisms of angiotensin II-mediated mitochondrial dysfunction: Linking mitochondrial oxidative damage and vascular endothelial dysfunction. Circ. Res. 2008, 102, 488–496. [Google Scholar] [CrossRef] [PubMed]
- Mehta, P.K.; Griendling, K.K. Angiotensin II cell signaling: Physiological and pathological effects in the cardiovascular system. Am. J. Physiol. Cell. Physiol. 2007, 292, C82–C97. [Google Scholar] [CrossRef] [PubMed]
- Dikalov, S.I.; Nazarewicz, R.R.; Bikineyeva, A.; Hilenski, L.; Lassègue, B.; Griendling, K.K.; Harrison, D.G.; Dikalova, A.E. Nox2 induced production of mitochondrial superoxide in Ang-II mediated endothelial oxidative stress and hypertension. Antioxid. Redox Signal. 2014, 20, 281–294. [Google Scholar] [CrossRef] [PubMed]
- Ma, S.; Wang, Q.; Zhang, Y.; Yang, D.; Li, D.; Tang, B.; Yang, Y. Transgenic overexpression of uncoupling protein-2 attenuates salt-induced vascular dysfunction by inhibition of oxidative stress. Am. J. Hypertens. 2014, 27, 345–354. [Google Scholar] [CrossRef] [PubMed]
- Tian, X.Y.; Wong, W.T.; Xu, A.; Lu, Y.; Zhang, Y.; Wang, L.; Cheang, W.S.; Wang, Y.; Yao, X.; Huang, Y. Uncoupling protein-2 protects endothelial function in diet-induced obese mice. Circ. Res. 2010, 110, 1211–1216. [Google Scholar] [CrossRef]
- Liu, L.; Liu, J.; Tian, X.Y.; Wong, W.T.; Lau, C.W.; Xu, G.; Ng, C.F.; Yao, X.; Gao, Y.; Huang, Y. Uncoupling protein-2 mediates DPP-4 inhibitor-induced restoration of endothelial function in hypertension through reducing oxidative stress. Antioxid. Redox Signal. 2014, 21, 1571–1581. [Google Scholar] [CrossRef] [PubMed]
- Langlois, M.; Duprez, D.; Delanghe, J.; de Buyzere, M.; Clement, D.L. Serum vitamin C concentration is low in peripheral arterial disease and is associated with inflammation and severity of atherosclerosis. Circulation 2001, 103, 1863–1868. [Google Scholar] [CrossRef] [PubMed]
- Cooper, G.T. Cardiocyte adaptation to chronically altered load. Annu. Rev. Physiol. 1987, 49, 501–518. [Google Scholar] [CrossRef] [PubMed]
- Bernardo, B.D.; Weeks, K.L.; Pretorious, L.; McMullen, J.R. Molecular distinction between physiological and pathological cardiac hypertrophy: Experimental findings and therapeutic strategies. Pharmacol. Ther. 2010, 128, 191–227. [Google Scholar] [CrossRef] [PubMed]
- Dale Abel, E.; Doenst, T. Mitochondrial adaptations to physiological vs. pathological cardiac hypertrophy. Cardiovasc. Res. 2011, 90, 234–242. [Google Scholar] [CrossRef]
- Takimoto, E.; Kass, D.A. Role of oxidative stress in cardiac hypertrophy and remodeling. Hypertension 2006, 49, 241–248. [Google Scholar] [CrossRef] [PubMed]
- Arany, Z.; Novikov, M.; Chin, S.; Ma, Y.; Rosenzweig, A.; Spiegelman, B.M. Transverse aortic constriction leads to accelerated heart failure in mice lacking PPAR-γ coactivator 1α. Proc. Natl. Acad. Sci. USA 2006, 103, 10086–10091. [Google Scholar] [CrossRef] [PubMed]
- Sirker, A.; Zhang, M.; Murdoch, C.; Shah, A.M. Involvement of NADPH oxidases in cardiac remodelling and heart failure. Am. J. Nephrol. 2007, 27, 649–660. [Google Scholar] [CrossRef] [PubMed]
- Xiao, L.; Pimentel, D.R.; Wang, J.; Singh, K.; Colucci, W.S.; Sawyer, D.B. Role of reactive oxygen species and NAD(P)H oxidase in α(1)-adrenoceptor signaling in adult rat cardiac myocytes. Am. J. Physiol. Cell. Physiol. 2002, 282, C926–C934. [Google Scholar] [CrossRef] [PubMed]
- Minhas, K.M.; Saraiva, R.M.; Schuleri, K.H.; Lehrke, S.; Zheng, M.; Saliaris, A.P.; Berry, C.E.; Barouch, L.A.; Vandegaer, K.M.; Li, D.; et al. Xanthine oxido reductase inhibition causes reverse remodeling in rats with dilated cardiomyopathy. Circ. Res. 2006, 98, 271–279. [Google Scholar]
- Takimoto, E.; Champion, H.C.; Li, M.; Ren, S.; Rodriguez, E.R.; Tavazzi, B.; Lazzarino, G.; Paolocci, N. Oxidant stress from nitric oxide synthase-3 uncoupling stimulates cardiac pathologic remodeling from chronic pressure load. J. Clin. Investig. 2005, 115, 1221–1231. [Google Scholar] [CrossRef] [PubMed]
- Aon, M.A.; Cortassa, S.; O’Rourke, B. ROS balance: A unifying hypothesis. Biochim. Biophys. Acta 2010, 1797, 865–877. [Google Scholar] [CrossRef] [PubMed]
- Murphy, M.P. How mitochondria produce reactive oxygen species. Biochem. J. 2009, 417, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Doenst, T.; Pytel, G.; Schrepper, A.; Amorim, P.; Färber, G.; Shingu, Y.; Mohr, F.W.; Schwarzer, M. Decreased rates of substrate oxidation ex vivo predict the onset of heart failure and contractile dysfunction in rats with pressure overload. Cardiovasc. Res. 2010, 86, 461–470. [Google Scholar] [CrossRef] [PubMed]
- Bugger, H.; Schwarzer, M.; Chen, D.; Schrepper, A.; Amorim, P.A.; Schoepe, M.; Nguyen, T.D.; Mohr, F.W. Proteomic remodelling of mitochondrial oxidative pathways in pressure overload-induced heart failure. Cardiovasc. Res. 2010, 85, 376–384. [Google Scholar] [CrossRef] [PubMed]
- Kohlhaas, M.; Liu, T.; Knopp, A.; Zeller, T.; Ong, M.F.; Böhm, M.; O’Rourke, B.; Maack, C. Elevated cytosolic Na+ increases mitochondrial formation of reactive oxygen species in failing cardiac myocytes. Circulation 2010, 121, 1606–1613. [Google Scholar] [CrossRef] [PubMed]
- Csonka, C.; Pataki, T.; Kovacs, P.; Müller, S.L.; Schroeter, M.L.; Tosaki, A.; Blasig, I.E. Effects of oxidative stress on the expression of antioxidative defense enzymes in spontaneously hypertensive rat hearts. Free Radic. Biol. Med. 2000, 29, 612–619. [Google Scholar] [CrossRef] [PubMed]
- Dai, F.; Johnson, S.C.; Villarin, J.J.; Chin, M.T.; Nieves-Cintrón, M.; Chen, T.; Marcinek, D.J.; Dorn, G.W. Mitochondrial oxidative stress mediates angiotensin II—Induced cardiac hypertrophy and Gαq overexpression—Induced heart failure. Circ. Res. 2011, 108, 837–846. [Google Scholar] [CrossRef] [PubMed]
- Dai, F.; Chen, T.; Szeto, H.; Nieves-Cintrón, M.; Kutyavin, V.; Santana, L.F.; Rabinovitch, P.S. Mitochondrial targeted antioxidant peptide ameliorates hypertensive cardiomyopathy. J. Am. Coll. Cardiol. 2011, 58, 73–82. [Google Scholar] [CrossRef] [PubMed]
- Graham, D.; Huynh, N.N.; Hamilton, C.A.; Beattie, E.; Smith, R.A.; Cochemé, H.M.; Murphy, M.P.; Dominiczak, A.F. Mitochondria-targeted antioxidant MitoQ10 improves endothelial function and attenuates cardiac hypertrophy. Hypertension 2009, 54, 322–328. [Google Scholar] [CrossRef] [PubMed]
- Udani, S.; Lazich, I.; Bakris, G.L. Epidemiology of hypertensive kidney disease. Nat. Rev. Nephrol. 2011, 7, 11–21. [Google Scholar] [CrossRef] [PubMed]
- Mennuni, S.; Rubattu, S.; Pierelli, G.; Tocci, G.; Fofi, C.; Volpe, M. Hypertension and kidneys: Unraveling complex molecular mechanisms underlying hypertensive renal damage. J. Hum. Hypertens. 2014, 28, 74–79. [Google Scholar] [CrossRef] [PubMed]
- Epstein, F.H. Oxygen and renal metabolism. Kidney Int. 1997, 51, 381–385. [Google Scholar] [CrossRef]
- Che, R.; Yuan, Y.; Huang, S.; Zhang, A. Mitochondrial dysfunction in the pathophysiology of renal diseases. Am. J. Physiol. Renal Physiol. 2014, 306, F367–F378. [Google Scholar] [CrossRef] [PubMed]
- Araujo, M.; Wilcox, C.S. Oxidative stress in hypertension: Role of the kidney. Antioxid. Redox Signal. 2014, 20, 74–101. [Google Scholar] [CrossRef] [PubMed]
- De Cavanagh, E.M.; Toblli, J.E.; Ferder, L.; Piotrkowski, B.; Stella, I.; Inserra, F. Renal mitochondrial dysfunction in spontaneously hypertensive rats is attenuated by losartan but not by amlodipine. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2006, 290, R1616–R1625. [Google Scholar]
- Laursen, J.B.; Rajagopalan, S.; Galis, Z.; Tarpey, M.; Freeman, B.A.; Harrison, D.G. Role of superoxide in angiotensin II-induced but not catecholamine-induced hypertension. Circulation 1997, 95, 588–593. [Google Scholar] [CrossRef] [PubMed]
- Ying, W.Z.; Sanders, P.W. Cytochrome c mediates apoptosis in hypertensive nephrosclerosis in Dahl/Rapp rats. Kidney Int. 2001, 59, 662–672. [Google Scholar] [CrossRef] [PubMed]
- Di Castro, S.; Scarpino, S.; Marchitti, S.; Bianchi, F.; Stanzione, R.; Cotugno, M.; Sironi, L.; Gelosa, P.; Duranti, E.; Ruco, L.; et al. Differential modulation of uncoupling protein-2 in kidneys of stroke-prone spontaneously hypertensive rats under high-salt/low-potassium diet. Hypertension 2013, 61, 534–541. [Google Scholar]
- Jin, K.; Vaziri, N.D. Salt-sensitive hypertension in mitochondrial superoxide dismutase deficiency is associated with intra-renal oxidative stress and inflammation. Clin. Exp. Nephrol. 2014, 18, 445–452. [Google Scholar] [CrossRef] [PubMed]
- Ohtsuki, T.; Matsumoto, M.; Suzuki, K.; Taniguchi, N.; Kamada, T. Mitochondrial lipid peroxidation and superoxide dismutase in rat hypertensive target organs. Am. J. Physiol. 1995, 268, H1418–H1421. [Google Scholar] [PubMed]
- Patten, D.A.; Germain, M.; Kelly, M.A.; Slack, R.S. Reactive oxygen species: Stuck in the middle of neurodegeneration. J. Alzheimers Dis. 2010, 20, S357–S367. [Google Scholar] [PubMed]
- Keane, P.C.; Kurzawa, M.; Blain, P.G.; Morris, C.M. Mitochondrial dysfunction in Parkinson’s disease. Parkinsons Dis. 2011, 2011, 716871. [Google Scholar] [PubMed]
- Paglieri, C.; Bisbocci, D.; Caserta, M.; Rabbia, F.; Bertello, C.; Canadè, A.; Veglio, F. Hypertension and cognitive function. Clin. Exp. Hypertens. 2008, 30, 701–710. [Google Scholar] [CrossRef] [PubMed]
- Lopez-Campistrous, A.; Hao, L.; Xiang, W.; Ton, D.; Semchuk, P.; Sander, J.; Ellison, M.J.; Fernandez-Patron, C. Mitochondrial dysfunction in the hypertensive rat brain: Respiratory complexes exhibit assembly defects in hypertension. Hypertension 2008, 51, 412–419. [Google Scholar] [CrossRef] [PubMed]
- Brandt, U. Energy converting NADH:quinone oxidoreductase (complex I). Annu. Rev. Biochem. 2006, 75, 69–92. [Google Scholar] [CrossRef] [PubMed]
- Galkin, A.; Dröse, S.; Brandt, U. The proton pumping stoichiometry of purified mitochondrial complex I reconstituted into proteoliposome. Biochim. Biophys. Acta 2006, 1757, 1575–1581. [Google Scholar] [CrossRef] [PubMed]
- Hunte, C.; Zickermann, V.; Brandt, U. Functional modules and structural basis of conformational coupling in mitochondrial complex I. Science 2010, 329, 448–451. [Google Scholar] [CrossRef] [PubMed]
- Nicholls, D.G. Mitochondrial membrane potential and aging. Aging Cell 2004, 3, 35–40. [Google Scholar] [CrossRef] [PubMed]
- Calderón-Cortés, E.; Cortés-Rojo, C.; Clemente-Guerrero, M.; Manzo-Avalos, S.; Villalobos-Molina, R.; Boldogh, I.; Saavedra-Molina, A. Changes in mitochondrial functionality and calcium uptake in hypertensive rats as a function of age. Mitochondrion 2008, 8, 262–272. [Google Scholar] [CrossRef] [PubMed]
- Aguilera-Aguirre, L.; González-Hernández, J.C.; Pérez-Vázquez, V.; Ramírez, J.; Clemente-Guerrero, M.; Villalobos-Molina, R.; Saavedra-Molina, A. Role of intramitochondrial nitric oxide in rat heart and kidney during hypertension. Mitochondrion 2002, 1, 413–423. [Google Scholar] [CrossRef] [PubMed]
- Saavedra-Molina, A.; Ramírez-Emiliano, J.; Clemente-Guerrero, M.; Pérez-Vázquez, V.; Aguilera-Aguirre, L.; González-Hernández, J.C. Mitochondrial nitric oxide inhibits ATP synthesis. Effect of free calcium in rat heart. Amino Acids 2003, 24, 95–102. [Google Scholar]
- Xu, X.; Zhao, L.; Hu, X.; Zhang, P.; Wessale, J.; Bache, R.; Chen, Y. Delayed treatment effects of xanthine oxidase inhibition on systolic overload-induced left ventricular hypertrophy and dysfunction. Nucleosides Nucleotides Nucleic Acids 2010, 29, 306–313. [Google Scholar] [CrossRef] [PubMed]
- Finckenberg, P.; Eriksson, O.; Baumann, M.; Merasto, S.; Lalowski, M.M.; Levijoki, J.; Haasio, K.; Kytö, V.; Muller, D.N.; Luft, F.C.; et al. Caloric restriction ameliorates angiotensin II-induced mitochondrial remodeling and cardiac hypertrophy. Hypertension 2012, 59, 76–84. [Google Scholar]
- O’Brien, D.; Chunduri, P.; Iyer, A.; Brown, L. l-Carnitine attenuates cardiac remodelling rather than vascular remodelling in deoxycorticosterone acetate-salt hypertensive rats. Basic Clin. Pharmacol. Toxicol. 2010, 106, 296–301. [Google Scholar] [PubMed]
- Frei, B.; Birlouez-Aragon, I.; Lykkesfeldt, J. Authors’ perspective: What is the optimum intake of vitamin C in humans? Crit. Rev. Food Sci. Nutr. 2012, 52, 815–829. [Google Scholar] [CrossRef] [PubMed]
- Cook, N.R.; Albert, C.M.; Gaziano, J.M.; Zaharris, E.; MacFadyen, J.; Danielson, E.; Buring, J.E.; Manson, J.E. A randomized factorial trial of vitamins C and E and β carotene in the secondary prevention of cardiovascular events in women: Results from the Women’s Antioxidant Cardiovascular Study. Arch. Intern. Med. 2007, 167, 1610–1618. [Google Scholar] [CrossRef] [PubMed]
- Sesso, H.D.; Buring, J.E.; Christen, W.G.; Kurth, T.; Belanger, C.; MacFadyen, J.; Bubes, V.; Manson, J.E.; Glynn, R.J.; Gaziano, J.M. Vitamins E and C in the prevention of cardiovascular disease in men: The Physicians’ Health Study II randomized controlled trial. JAMA 2008, 300, 2123–2133. [Google Scholar] [CrossRef] [PubMed]
- Podmore, I.D.; Griffiths, H.R.; Herbert, K.E.; Mistry, N.; Mistry, P.; Lunec, J. Vitamin C exhibits pro-oxidant properties. Nature 1998, 392, 559. [Google Scholar] [CrossRef] [PubMed]
- Michels, A.J.; Frei, B. Myths, artifacts, and fatal flaws: Identifying limitations and opportunities in vitamin C research. Nutrients 2013, 5, 5161–5192. [Google Scholar] [CrossRef] [PubMed]
- Michels, A.J.; Hagen, T.M.; Frei, B. Human genetic variation influences vitamin C homeostasis by altering vitamin C transport and antioxidant enzyme function. Annu. Rev. Nutr. 2013, 33, 45–70. [Google Scholar] [CrossRef] [PubMed]
- Wilson, T.W.; Alonso-Galicia, M.; Roman, R.J. Effects of lipid-lowering agents in the Dahl salt-sensitive rat. Hypertension 1998, 31, 225–231. [Google Scholar] [CrossRef] [PubMed]
- Wassmann, S.; Laufs, U.; Muller, K.; Konkol, C.; Ahlbory, K.; Baumer, A.T.; Linz, W.; Bohm, M.; Nickenig, G. Cellular antioxidant effects of atorvastatin in vitro and in vivo. Arterioscler. Thromb. Vasc. Biol. 2002, 22, 300–305. [Google Scholar] [CrossRef] [PubMed]
- Vecchione, C.; Brandes, R.P. Withdrawal of 3-hydroxy-3-methylglutaryl coenzyme A reductase inhibitors elicits oxidative stress and induces endothelial dysfunction in mice. Circ. Res. 2002, 91, 173–179. [Google Scholar] [CrossRef] [PubMed]
- Raij, L. Workshop: Hypertension and cardiovascular risk factors: Role of the angiotensin II-nitric oxide interaction. Hypertension 2001, 37, 767–773. [Google Scholar] [CrossRef] [PubMed]
- Opie, L.H. Renoprotection by angiotensin-receptor blockers and ACE inhibitors in hypertension. Lancet 2001, 358, 1829–1831. [Google Scholar] [CrossRef] [PubMed]
- De Cavanagh, E.M.; Inserra, F.; Ferder, L.; Fraga, C.G. Enalapril and captopril enhance glutathione-dependent antioxidant defenses in mouse tissues. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2000, 278, R572–R577. [Google Scholar] [PubMed]
- Murphy, M.P. Targeting lipophilic cations to mitochondria. Biochim. Biophys. Acta 2008, 1777, 1028–1031. [Google Scholar] [CrossRef] [PubMed]
- James, A.M.; Cocheme, H.M.; Smith, R.A.J.; Murphy, M.P. Interactions of mitochondrial-targeted and untargeted ubiquinones with the mitochondrial respiratory chain and reactive oxygen species: Implications for the use of exogenous ubiquinones as therapies and experimental tools. J. Biol. Chem. 2005, 280, 21295–21312. [Google Scholar] [CrossRef] [PubMed]
- Ross, M.F.; Prime, T.A.; Abakumova, I.; James, A.M.; Porteous, C.M.; Smith, R.A.; Murphy, M.P. Rapid and extensive uptake and activation of hydrophobic triphenylphosphonium cations within cells. Biochem. J. 2008, 411, 633–645. [Google Scholar] [CrossRef] [PubMed]
- McLachlan, J.; Beattie, E.; Murphy, M.P.; Koh-Tan, C.H.; Olson, E.; Beattie, W.; Dominiczak, A.F.; Nicklin, S.A.; Graham, D. Combined therapeutic benefit of mitochondria targeted antioxidant, MitoQ10, and angiotensin receptor blocker, losartan, on cardiovascular function. J. Hypertens. 2014, 32, 555–564. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Georgakopoulos, D.; Lu, G.; Hester, L.; Kass, D.A.; Hasday, J.; Wang, Y. p38 MAP kinase mediates inflammatory cytokine induction in cardiomyocytes and extracellular matrix remodeling in heart. Circulation 2005, 111, 2494–2502. [Google Scholar] [CrossRef] [PubMed]
- See, F.; Thomas, W.; Way, K.; Tzanidis, A.; Kompa, A.; Lewis, D.; Itescu, S.; Krum, H. p38 mitogen-activated protein kinase inhibition improves cardiac function and attenuates left ventricular remodeling following myocardial infarction in the rat. J. Am. Coll. Cardiol. 2004, 44, 1679–1689. [Google Scholar] [CrossRef] [PubMed]
- Date, M.; Morita, T.; Yamashita, N.; Nishida, K.; Yamaguchi, O.; Higuchi, Y.; Hirotani, S.; Matsumura, Y.; Hori, M.; Tada, M.; et al. The antioxidant N-2-mercaptopropionyl glycine attenuates left ventricular hypertrophy in in vivo murine pressure-overload model. J. Am. Coll. Cardiol. 2002, 39, 907–912. [Google Scholar]
- Subramanian, S.; Kalyanaraman, B.; Migrino, R.Q. Mitochondrially targeted antioxidants for the treatment of cardiovascular diseases. Recent Pat. Cardiovasc. Drug Discov. 2010, 5, 54–65. [Google Scholar] [CrossRef] [PubMed]
- Szeto, H.H. Mitochondria-targeted cytoprotective peptides for ischemia-reperfusion injury. Antioxid. Redox Signal. 2008, 10, 601–619. [Google Scholar] [CrossRef] [PubMed]
- Zhao, K.; Zhao, G.M.; Wu, D.; Soong, Y.; Birk, A.V.; Schiller, P.W.; Szeto, H.H. Cell-permeable peptide antioxidants targeted to inner mitochondrial membrane inhibit mitochondrial swelling, oxidative cell death, and reperfusion injury. J. Biol. Chem. 2004, 279, 34682–34690. [Google Scholar] [CrossRef] [PubMed]
- Watanabe, T.; Yuki, S.; Egawa, M.; Nishi, H. Protective effects of MCI-186 on cerebral ischemia: Possible involvement of free radical scavenging and antioxidant actions. J. Pharmacol. Exp. Ther. 1994, 268, 1597–1604. [Google Scholar] [PubMed]
- Tsujimoto, I.; Hikoso, S.; Yamaguchi, O.; Kashiwase, K.; Nakai, A.; Takeda, T.; Watanabe, T.; Taniike, M.; Matsumura, Y.; Nishida, K.; et al. The antioxidant Edaravone attenuates pressure overload induced left ventricular hypertrophy. Hypertension 2005, 45, 921–926. [Google Scholar]
© 2014 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rubattu, S.; Pagliaro, B.; Pierelli, G.; Santolamazza, C.; Di Castro, S.; Mennuni, S.; Volpe, M. Pathogenesis of Target Organ Damage in Hypertension: Role of Mitochondrial Oxidative Stress. Int. J. Mol. Sci. 2015, 16, 823-839. https://doi.org/10.3390/ijms16010823
Rubattu S, Pagliaro B, Pierelli G, Santolamazza C, Di Castro S, Mennuni S, Volpe M. Pathogenesis of Target Organ Damage in Hypertension: Role of Mitochondrial Oxidative Stress. International Journal of Molecular Sciences. 2015; 16(1):823-839. https://doi.org/10.3390/ijms16010823
Chicago/Turabian StyleRubattu, Speranza, Beniamino Pagliaro, Giorgia Pierelli, Caterina Santolamazza, Sara Di Castro, Silvia Mennuni, and Massimo Volpe. 2015. "Pathogenesis of Target Organ Damage in Hypertension: Role of Mitochondrial Oxidative Stress" International Journal of Molecular Sciences 16, no. 1: 823-839. https://doi.org/10.3390/ijms16010823
APA StyleRubattu, S., Pagliaro, B., Pierelli, G., Santolamazza, C., Di Castro, S., Mennuni, S., & Volpe, M. (2015). Pathogenesis of Target Organ Damage in Hypertension: Role of Mitochondrial Oxidative Stress. International Journal of Molecular Sciences, 16(1), 823-839. https://doi.org/10.3390/ijms16010823





