Hydrostatic Pressure Influences HIF-2 Alpha Expression in Chondrocytes
Abstract
:1. Introduction
2. Results
2.1. Influence of Inflammation to Chondrocytes
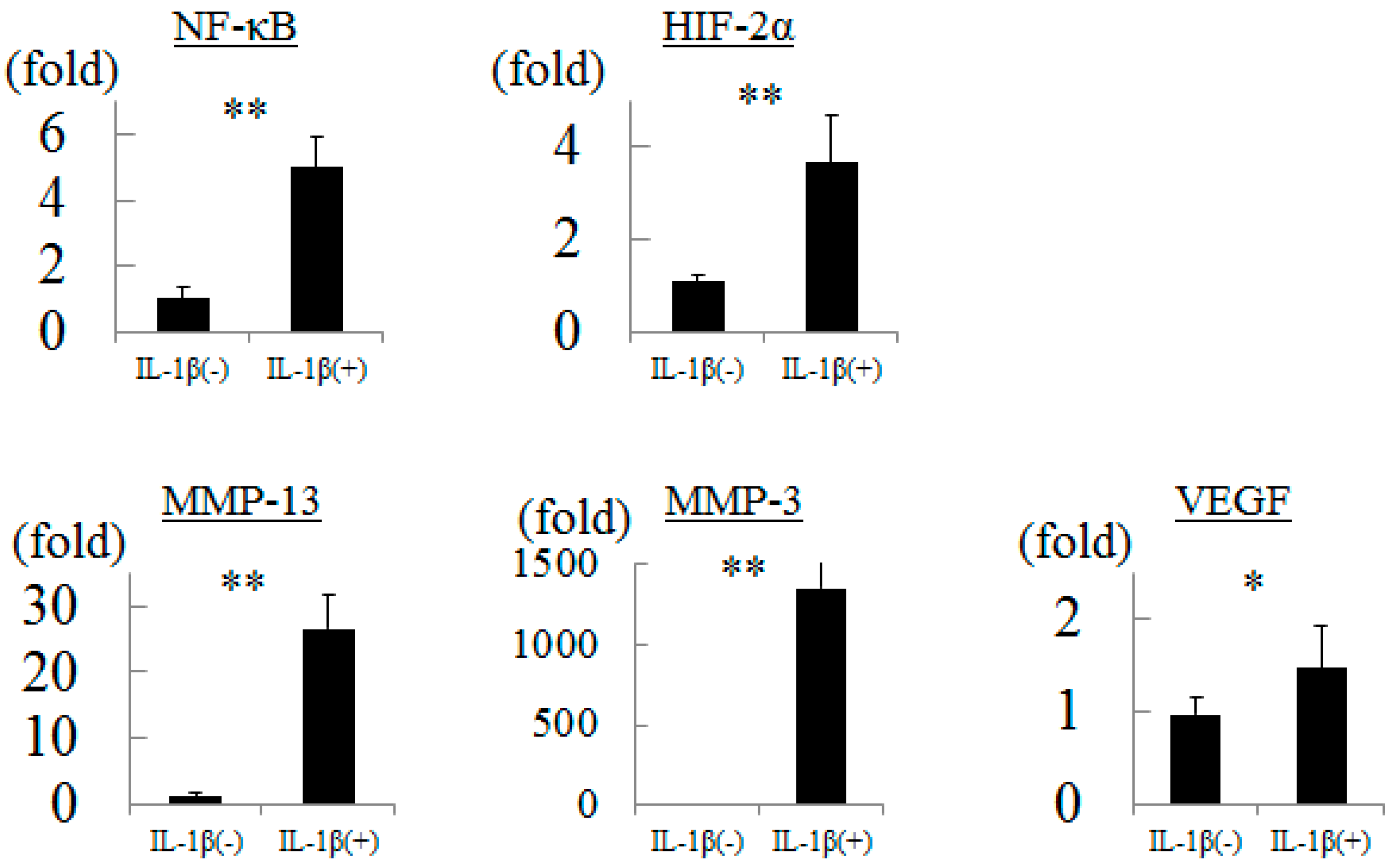
2.2. Influence of Hydrostatic Pressure to Chondrocytes

3. Discussion
4. Experimental Section
4.1. Preparation of Chondrocytes
4.2. IL-1β Treatment
4.3. Pressure Application
4.4. Quantitative Real-Time Reverse Transcription-Polymerase Chain Reaction
| Gene | Primer Sequence | Probe No. |
|---|---|---|
| HIF-2α | Forward: agcttcctgcggacacac Reverse: cctcggcttcagactcattt | 73 |
| NF-κB | Forward: aagaccaggtttccgaggac Reverse: tgcaccatgaggaaagaagtt | 133 |
| MMP-3 | Forward: tggacctggaaatgttttgg Reverse: atcaaagtgggcatctccat | 72 |
| MMP-13 | Forward: cctcttcttctccggaaacc Reverse: ggtagtcttggtccatggtatga | 50 |
| VEGF | Forward: ctacctccaccatgccaagt Reverse: ccacttcgtggggtttattg | 29 |
| 18S ribosomal RNA | Forward: atgagtccactttaaatcctttaacga Reverse: ctttaatatacgctattggagctggaa | - |
4.5. Statistical Analysis
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Takahashi, K.; Kubo, T.; Kobayashi, K.; Imanishi, J.; Takigawa, M.; Arai, Y.; Hirasawa, Y. Hydrostatic pressure influences mRNA expression of transforming growth factor-β1 and heat shock protein 70 in chondrocyte-like cell line. J. Orthop. Res. 1997, 15, 150–158. [Google Scholar] [CrossRef] [PubMed]
- Nakamura, S.; Arai, Y.; Takahashi, K.A.; Terauchi, R.; Ohashi, S.; Mazda, O.; Imanishi, J.; Inoue, A.; Tonomura, H.; Kubo, T. Hydrostatic pressure induces apoptosis of chondrocytes cultured in alginate beads. J. Orthop. Res. 2006, 24, 733–739. [Google Scholar] [CrossRef] [PubMed]
- Saito, T.; Fukai, A.; Mabuchi, A.; Ikeda, T.; Yano, F.; Ohba, S.; Nishida, N.; Akune, T.; Yoshimura, N.; Nakagawa, T.; et al. Transcriptional regulation of endochondral ossification by HIF-2α during skeletal growth and osteoarthritis development. Nat. Med. 2010, 16, 678–686. [Google Scholar] [CrossRef] [PubMed]
- Yang, S.; Kim, J.; Ryu, J.H.; Oh, H.; Chun, C.H.; Kim, B.J.; Min, B.H.; Chun, J.S. Hypoxia-inducible factor-2α is a catabolic regulator of osteoarthritic cartilage destruction. Nat. Med. 2010, 16, 687–693. [Google Scholar] [CrossRef] [PubMed]
- Ryu, J.H.; Yang, S.; Shin, Y.; Rhee, J.; Chun, C.H.; Chun, J.S. Interleukin-6 plays an essential role in hypoxia-inducible factor 2α-induced experimental osteoarthritic cartilage destruction in mice. Arthritis Rheumatol. 2011, 63, 2732–2743. [Google Scholar] [CrossRef]
- Lim, C.S.; Qiao, X.; Reslan, O.M.; Xia, Y.; Raffetto, J.D.; Paleolog, E.; Davies, A.H.; Khalil, R.A. Prolonged mechanical stretch is associated with upregulation of hypoxia-inducible factors and reduced contraction in rat inferior vena cava. J. Vasc. Surg. 2011, 53, 764–773. [Google Scholar] [CrossRef] [PubMed]
- Skuli, N.; Liu, L.; Runge, A.; Wang, T.; Yuan, L.; Patel, S.; Iruela-Arispe, L.; Simon, M.C.; Keith, B. Endothelial deletion of hypoxia-inducible factor-2α (HIF-2α) alters vascular function and tumor angiogenesis. Blood 2009, 114, 469–477. [Google Scholar] [CrossRef] [PubMed]
- Gong, W.; Wang, Z.Y.; Chen, G.X.; Liu, Y.Q.; Gu, X.Y.; Liu, W.W. Invasion potential of H22 hepatocarcinoma cells is increased by HMGB1-induced tumor NF-κB signaling via initiation of HSP70. Oncol. Rep. 2013, 30, 1249–1256. [Google Scholar] [PubMed]
- Yamashita, T.; Ohneda, O.; Nagano, M.; Iemitsu, M.; Makino, Y.; Tanaka, H.; Miyauchi, T.; Goto, K.; Ohneda, K.; Fujii-Kuriyama, Y.; et al. Abnormal heart development and lung remodeling in mice lacking the hypoxia-inducible factor-related basic helix-loop-helix PAS protein NEPAS. Mol. Cell Biol. 2008, 28, 1285–1297. [Google Scholar] [CrossRef] [PubMed]
- Hirata, M.; Kugimiya, F.; Fukai, A.; Saito, T.; Yano, F.; Ikeda, T.; Mabuchi, A.; Sapkota, B.R.; Akune, T.; Nishida, N.; et al. C/EBPβ and RUNX2 cooperate to degrade cartilage with MMP-13 as the target and HIF-2α as the inducer in chondrocytes. Hum. Mol. Genet. 2012, 21, 1111–1123. [Google Scholar] [CrossRef] [PubMed]
- Sun, H.; Breslin, J.W.; Zhu, J.; Yuan, S.Y.; Wu, M.H. Rho and ROCK signaling in VEGF-induced microvascular endothelial hyperpermeability. Microcirculation 2006, 13, 237–247. [Google Scholar] [CrossRef] [PubMed]
- Kondo, A.; Otsuka, T.; Kuroyanagi, G.; Yamamoto, N.; Matsushima-Nishiwaki, R.; Mizutani, J.; Kozawa, O.; Tokuda, H. Resveratrol inhibits BMP-4-stimulated VEGF synthesis in osteoblasts: suppression of S6 kinase. Int. J. Mol. Med. 2014, 33, 1013–1018. [Google Scholar] [PubMed]
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Inoue, H.; Arai, Y.; Kishida, T.; Terauchi, R.; Honjo, K.; Nakagawa, S.; Tsuchida, S.; Matsuki, T.; Ueshima, K.; Fujiwara, H.; et al. Hydrostatic Pressure Influences HIF-2 Alpha Expression in Chondrocytes. Int. J. Mol. Sci. 2015, 16, 1043-1050. https://doi.org/10.3390/ijms16011043
Inoue H, Arai Y, Kishida T, Terauchi R, Honjo K, Nakagawa S, Tsuchida S, Matsuki T, Ueshima K, Fujiwara H, et al. Hydrostatic Pressure Influences HIF-2 Alpha Expression in Chondrocytes. International Journal of Molecular Sciences. 2015; 16(1):1043-1050. https://doi.org/10.3390/ijms16011043
Chicago/Turabian StyleInoue, Hiroaki, Yuji Arai, Tsunao Kishida, Ryu Terauchi, Kuniaki Honjo, Shuji Nakagawa, Shinji Tsuchida, Tomohiro Matsuki, Keiichirou Ueshima, Hiroyoshi Fujiwara, and et al. 2015. "Hydrostatic Pressure Influences HIF-2 Alpha Expression in Chondrocytes" International Journal of Molecular Sciences 16, no. 1: 1043-1050. https://doi.org/10.3390/ijms16011043
APA StyleInoue, H., Arai, Y., Kishida, T., Terauchi, R., Honjo, K., Nakagawa, S., Tsuchida, S., Matsuki, T., Ueshima, K., Fujiwara, H., Mazda, O., & Kubo, T. (2015). Hydrostatic Pressure Influences HIF-2 Alpha Expression in Chondrocytes. International Journal of Molecular Sciences, 16(1), 1043-1050. https://doi.org/10.3390/ijms16011043





