SMG1 Acts as a Novel Potential Tumor Suppressor with Epigenetic Inactivation in Acute Myeloid Leukemia
Abstract
:1. Introduction
2. Results
2.1. SMG1 Was Down-Regulated in Acute Myeloid Leukemia (AML) Patient Samples
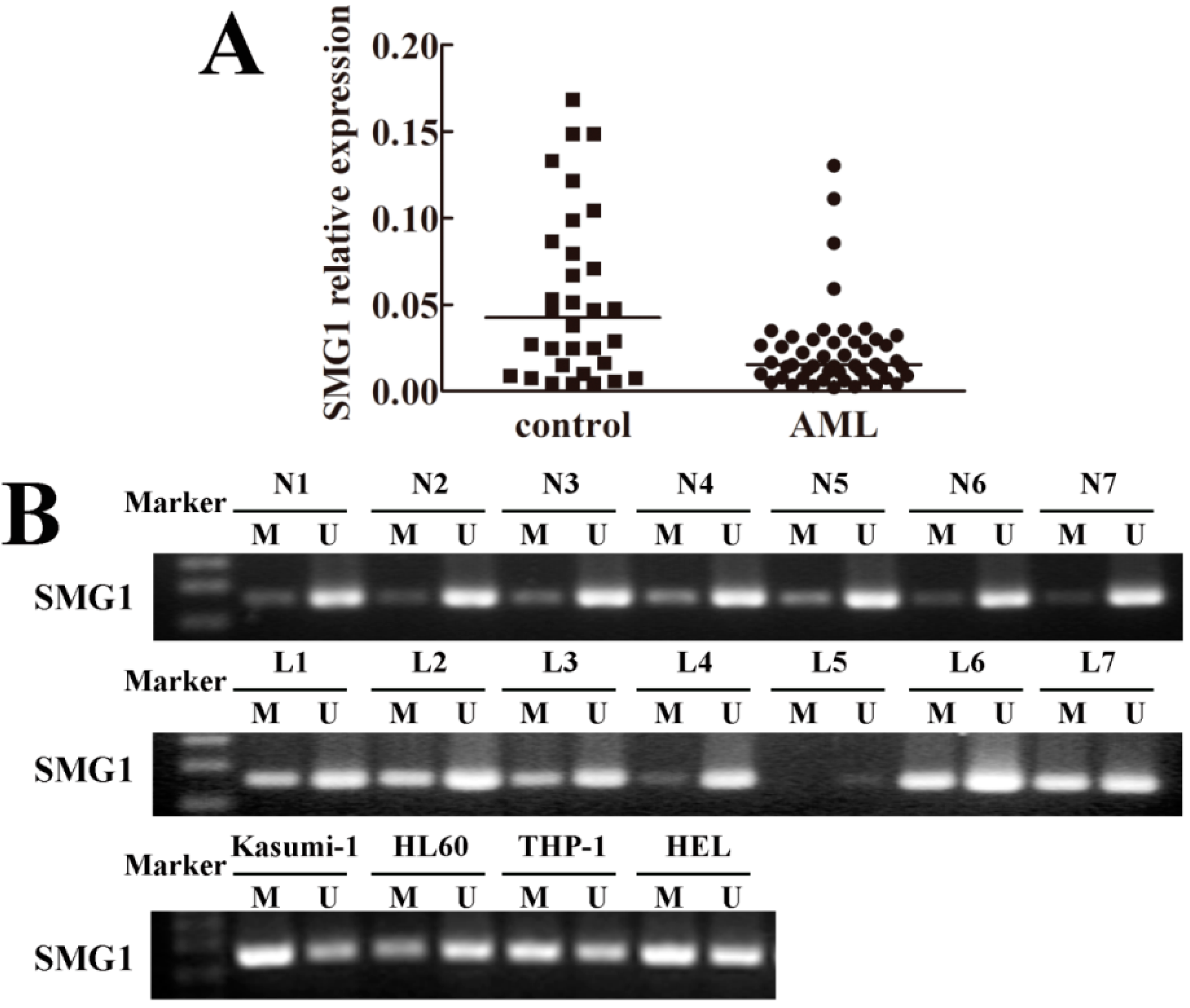
2.2. Hypermethylation Status of SMG1 Gene Was Associated with Transcriptional Down-Regulation
2.3. 5-Aza-2'-deoxycytidine (Decitabine, DAC) Decreased Methylation Degree of SMG1 and Restored SMG1 Expression
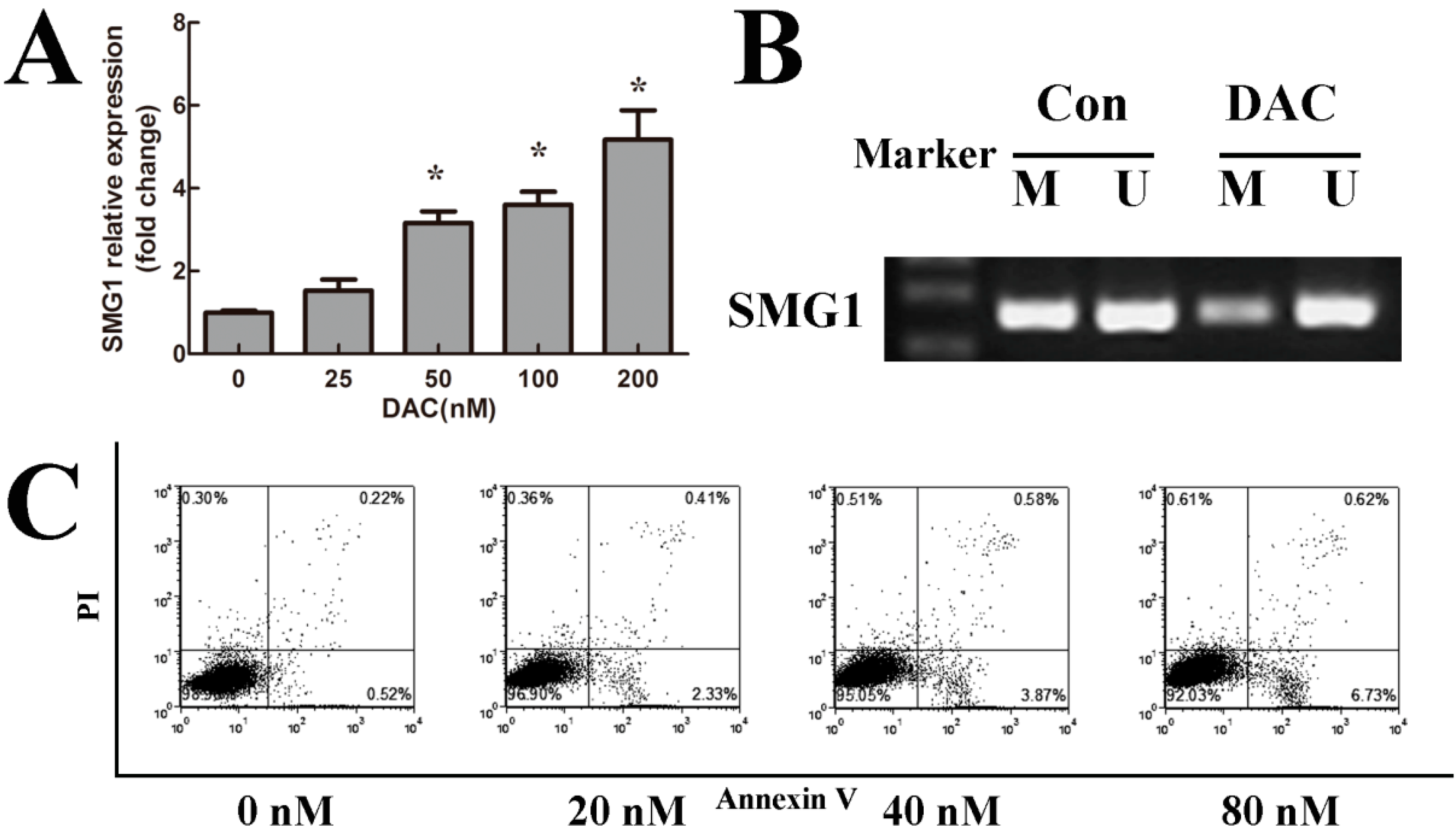
2.4. DAC Inhibited Cell Growth and Induced Apoptosis
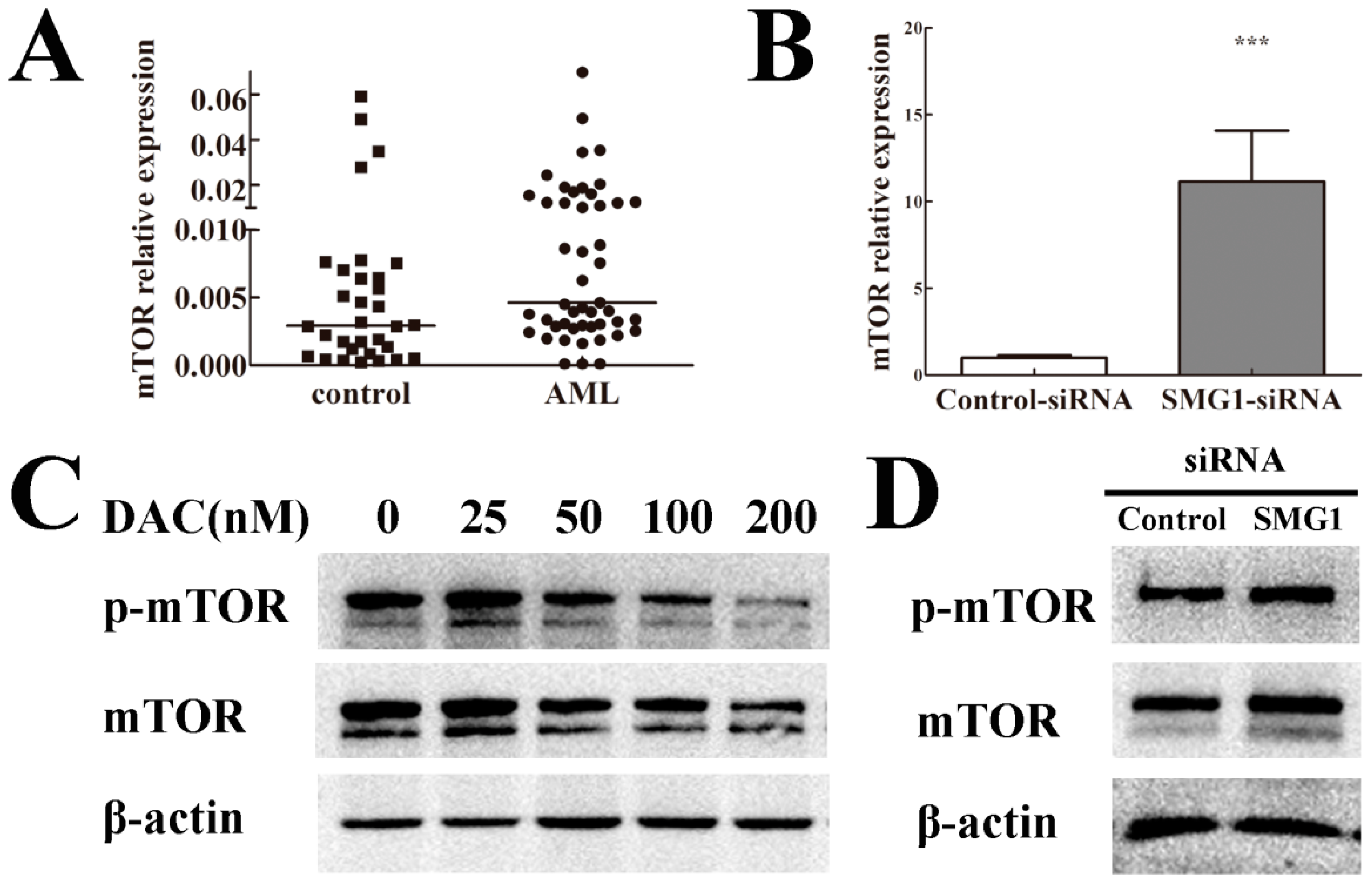
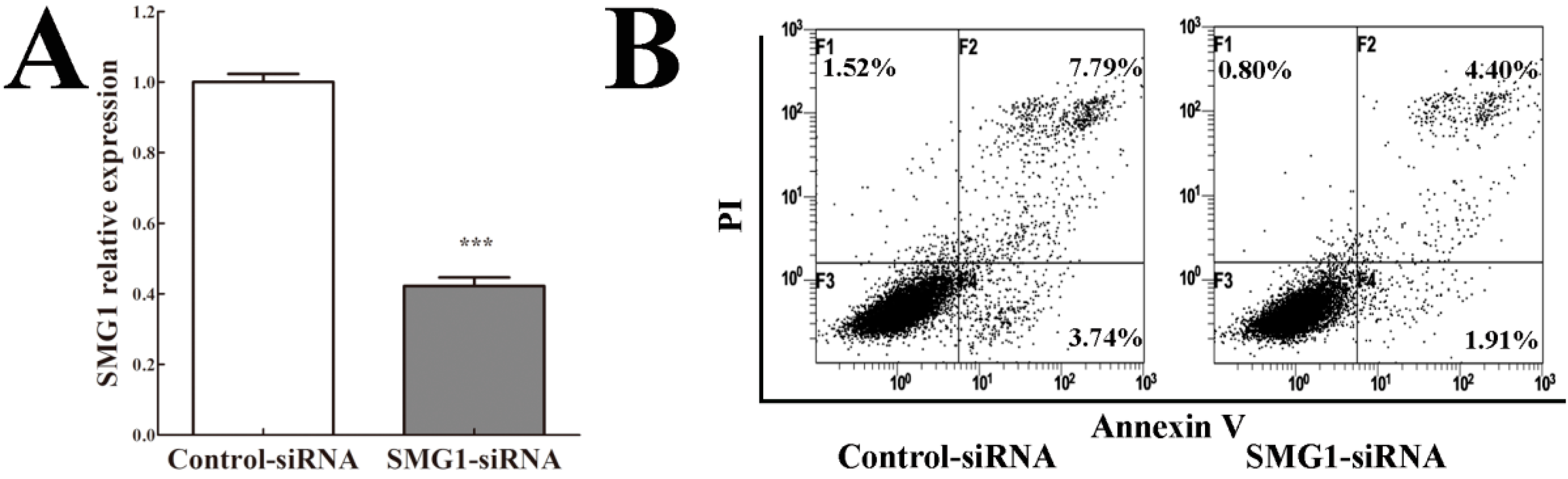
2.5. Knockdown of SMG1 Inhibited Apoptosis
3. Discussion
4. Experimental Section
4.1. Patients and Ethics Statement
| Variables | AML Patients |
|---|---|
| Sex (male/female) | 25/25 |
| Age: median years (range) | 45.50 (14–83) |
| WBC count (109/L) median: (range) | 37.28 (0.77–254) |
| Hemoglobin (g/dL) median: (range) | 74.60 (45–117) |
| Platelet count (109/L) median: (range) | 52.22 (4–263) |
| FAB Classification | Number of Patients |
| M0 | 0 |
| M1 | 0 |
| M2 | 6 |
| M3 | 14 |
| M4 | 6 |
| M5 | 24 |
| M6 | 0 |
4.2. Cell Culture
4.3. DNA Extraction and Methylation-Specific Polymerase Chain Reaction (MSP)
- SMG1 M-forward primer sequence: 5'-GCGTACGTGAATTTAAGGGTAC-3';
- SMG1 M-reverse primer sequence: 5'-AACAAAAAATCTCCACTACTACGAC-3';
- SMG1 U-forward primer sequence: 5'-GGTGTATGTGAATTTAAGGGTATGT-3';
- SMG1 U-reverse primer sequence: 5'-AACAAAAAATCTCCACTACTACAAC-3'.
4.4. Demethylating Agent DAC Treatment
4.5. SMG1 Knockdown and Transfection
4.6. Quantitative Real-Time Polymerase Chain Reaction (RT-PCR) Analysis
4.7. Apoptosis Assay
4.8. Western Blot Analysis
4.9. Statistical Analysis
5. Conclusions
Supplementary Materials
Supplementary Files
Supplementary File 1Acknowledgments
Author Contributions
Conflicts of Interest
References
- Marcucci, G.; Yan, P.; Maharry, K.; Frankhouser, D.; Nicolet, D.; Metzeler, K.H.; Kohlschmidt, J.; Mrozek, K.; Wu, Y.Z.; Bucci, D.; et al. Epigenetics meets genetics in acute myeloid leukemia: clinical impact of a novel seven-gene score. J. Clin. Oncol. 2014, 32, 548–556. [Google Scholar]
- Ganetsky, A. The role of decitabine for the treatment of acute myeloid leukemia. Ann. Pharmacother. 2012, 46, 1511–1517. [Google Scholar]
- Tawfik, B.; Sliesoraitis, S.; Lyerly, S.; Klepin, H.D.; Lawrence, J.; Isom, S.; Ellis, L.R.; Manuel, M.; Dralle, S.; Berenzon, D.; et al. Efficacy of the hypomethylating agents as frontline, salvage, or consolidation therapy in adults with acute myeloid leukemia (AML). Ann. Hematol. 2014, 93, 47–55. [Google Scholar]
- Lloyd, J.P.; Davies, B. SMG1 is an ancient nonsense-mediated mRNA decay effector. Plant J. 2013, 76, 800–810. [Google Scholar]
- Gonzalez-Estevez, C.; Felix, D.A.; Smith, M.D.; Paps, J.; Morley, S.J.; James, V.; Sharp, T.V.; Aboobaker, A.A. SMG-1 and mTORC1 act antagonistically to regulate response to injury and growth in planarians. PLoS Genet. 2012, 8, e1002619. [Google Scholar]
- Gubanova, E.; Brown, B.; Ivanov, S.V.; Helleday, T.; Mills, G.B.; Yarbrough, W.G.; Issaeva, N. Down-regulation of SMG-1 in HPV-positive head and neck squamous cell carcinoma due to promoter hypermethylation correlates with improved survival. Clin. Cancer Res. 2012, 18, 1257–1267. [Google Scholar]
- Mohamed, A.; El-Rayes, B.; Khuri, F.R.; Saba, N.F. Targeted therapies in metastatic esophageal cancer: Advances over the past decade. Crit. Rev. Oncol. Hematol. 2014, 91, 186–196. [Google Scholar]
- Virani, S.; Colacino, J.A.; Kim, J.H.; Rozek, L.S. Cancer epigenetics: A brief review. ILAR J. 2012, 53, 359–369. [Google Scholar]
- Takahashi, S. Epigenetic aberrations in myeloid malignancies. Int. J. Mol. Med. 2013, 32, 532–538. [Google Scholar]
- Bodoor, K.; Haddad, Y.; Alkhateeb, A.; Al-Abbadi, A.; Dowairi, M.; Magableh, A.; Bsoul, N.; Ghabkari, A. DNA hypermethylation of cell cycle (p15 and p16) and apoptotic (p14, p53, DAPK and TMS1) genes in peripheral blood of leukemia patients. Asian Pac. J. Cancer Pre. 2014, 15, 75–84. [Google Scholar]
- Marcucci, G.; Metzeler, K.H.; Schwind, S.; Becker, H.; Maharry, K.; Mrozek, K.; Radmacher, M.D.; Kohlschmidt, J.; Nicolet, D.; Whitman, S.P.; et al. Age-related prognostic impact of different types of DNMT3A mutations in adults with primary cytogenetically normal acute myeloid leukemia. J. Clin. Oncol. 2012, 30, 742–750. [Google Scholar]
- Shivarov, V.; Gueorguieva, R.; Stoimenov, A.; Tiu, R. DNMT3A mutation is a poor prognosis biomarker in AML: Results of a meta-analysis of 4500 AML patients. Leuk. Res. 2013, 37, 1445–1450. [Google Scholar]
- Ley, T.J.; Ding, L.; Walter, M.J.; McLellan, M.D.; Lamprecht, T.; Larson, D.E.; Kandoth, C.; Payton, J.E.; Baty, J.; Welch, J.; et al. DNMT3A mutations in acute myeloid leukemia. N. Engl. J. Med. 2010, 363, 2424–2433. [Google Scholar]
- Oliveira, V.; Romanow, W.J.; Geisen, C.; Otterness, D.M.; Mercurio, F.; Wang, H.G.; Dalton, W.S.; Abraham, R.T. A protective role for the human SMG-1 kinase against tumor necrosis factor-alpha-induced apoptosis. J. Biol. Chem. 2008, 283, 13174–13184. [Google Scholar]
- Masse, I.; Molin, L.; Mouchiroud, L.; Vanhems, P.; Palladino, F.; Billaud, M.; Solari, F. A novel role for the SMG-1 kinase in lifespan and oxidative stress resistance in Caenorhabditis elegans. PLoS One 2008, 3, e3354. [Google Scholar]
- McIlwain, D.R.; Pan, Q.; Reilly, P.T.; Elia, A.J.; McCracken, S.; Wakeham, A.C.; Itie-Youten, A.; Blencowe, B.J.; Mak, T.W. SMG1 is required for embryogenesis and regulates diverse genes via alternative splicing coupled to nonsense-mediated mRNA decay. Proc. Natl. Acad. Sci. USA 2010, 107, 12186–12191. [Google Scholar]
- Gewandter, J.S.; Bambara, R.A.; O’Reilly, M.A. The RNA surveillance protein SMG1 activates p53 in response to DNA double-strand breaks but not exogenously oxidized mRNA. Cell Cycle 2011, 10, 2561–2567. [Google Scholar]
- Martelli, A.M.; Chiarini, F.; Evangelisti, C.; Grimaldi, C.; Ognibene, A.; Manzoli, L.; Billi, A.M.; McCubrey, J.A. The phosphatidylinositol 3-kinase/AKT/mammalian target of rapamycin signaling network and the control of normal myelopoiesis. Histol. Histopathol. 2010, 25, 669–680. [Google Scholar]
- Barrett, D.; Brown, V.I.; Grupp, S.A.; Teachey, D.T. Targeting the PI3K/AKT/mTOR signaling axis in children with hematologic malignancies. Paediatr. Drugs 2012, 14, 299–316. [Google Scholar]
- Altman, J.K.; Sassano, A.; Platanias, L.C. Targeting mTOR for the treatment of AML. New agents and new directions. Oncotarget 2011, 2, 510–517. [Google Scholar]
© 2014 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Du, Y.; Lu, F.; Li, P.; Ye, J.; Ji, M.; Ma, D.; Ji, C. SMG1 Acts as a Novel Potential Tumor Suppressor with Epigenetic Inactivation in Acute Myeloid Leukemia. Int. J. Mol. Sci. 2014, 15, 17065-17076. https://doi.org/10.3390/ijms150917065
Du Y, Lu F, Li P, Ye J, Ji M, Ma D, Ji C. SMG1 Acts as a Novel Potential Tumor Suppressor with Epigenetic Inactivation in Acute Myeloid Leukemia. International Journal of Molecular Sciences. 2014; 15(9):17065-17076. https://doi.org/10.3390/ijms150917065
Chicago/Turabian StyleDu, Yahui, Fei Lu, Peng Li, Jingjing Ye, Min Ji, Daoxin Ma, and Chunyan Ji. 2014. "SMG1 Acts as a Novel Potential Tumor Suppressor with Epigenetic Inactivation in Acute Myeloid Leukemia" International Journal of Molecular Sciences 15, no. 9: 17065-17076. https://doi.org/10.3390/ijms150917065
APA StyleDu, Y., Lu, F., Li, P., Ye, J., Ji, M., Ma, D., & Ji, C. (2014). SMG1 Acts as a Novel Potential Tumor Suppressor with Epigenetic Inactivation in Acute Myeloid Leukemia. International Journal of Molecular Sciences, 15(9), 17065-17076. https://doi.org/10.3390/ijms150917065