Flavonoids from Machilus japonica Stems and Their Inhibitory Effects on LDL Oxidation
Abstract
:1. Introduction
2. Results and Discussion
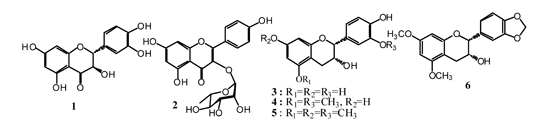
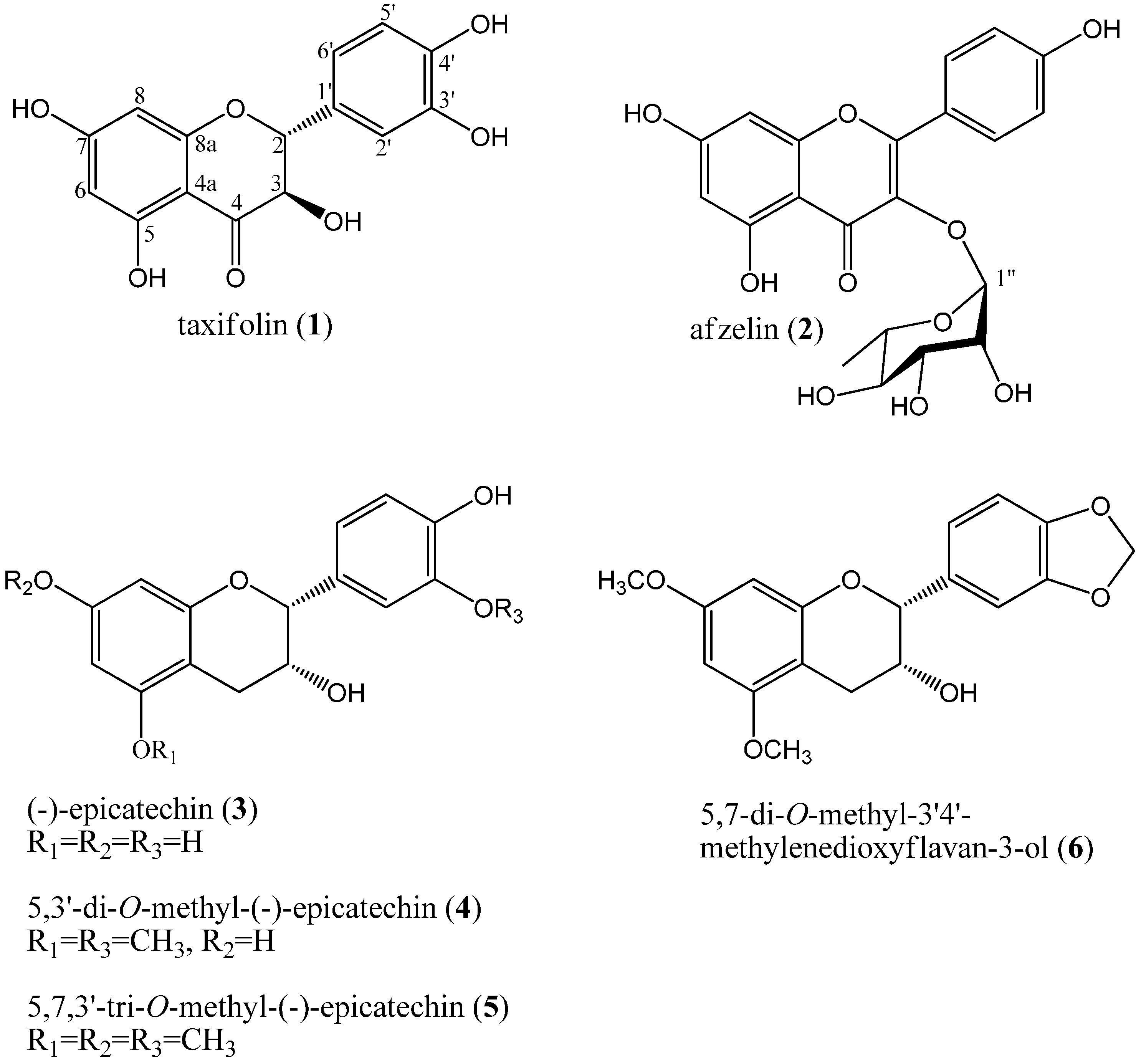
2.1. Isolation and Structure Elucidation
2.2. Evaluation of Radical Scavenging Activity and Inhibitory Effect on LDL Oxidation
| Compound | 1 | 2 | 3 | 4 | 5 | 6 | Ascorbic Acid |
|---|---|---|---|---|---|---|---|
| IC50 (mM) a | 0.16 | – | 0.21 | 0.17 | 0.15 | 0.07 | 0.18 |
| Compound | Inhibition Effect (%) | IC50 (µM) a | |||
|---|---|---|---|---|---|
| 5 µM | 10 µM | 40 µM | 80 µM | ||
| 1 | 90.1 ± 0.0 | 95.8 ± 0.6 | 100.4 ± 0.0 | 104.2 ±1.5 | 2.8 ± 0.5 |
| 2 | – | – | – | 3.0 ± 1.8 | >50 |
| 3 | 23.1 ± 2.6 | 93.6 ± 1.6 | 99.0 ± 0.2 | 104.0 ± 0.0 | 7.1 ± 1.1 |
| 4 | – | – | – | 51.8 ± 0.2 | >50 |
| 5 | 58.9 ± 0.7 | 65.3 ± 1.4 | 72.9 ± 0.5 | 84.2 ± 0.7 | 4.6 ± 0.8 |
| 6 | – | – | – | 13.7 ± 0.2 | >50 |
| BHT | – | – | – | – | 1.9 ± 0.4 |
3. Experimental Section
3.1. Plant Materials
3.2. General Experimental Procedures
3.3. Isolation of Flavonoids from the EtOAc Fraction Obtained from M. japonica Stems
3.4. DPPH Radical Scavenging Activity
3.5. LDL Isolation and Oxidation Assay
4. Conclusions
Supplementary Materials
Supplementary Files
Supplementary File 1Acknowledgments
Author Contributions
Conflicts of Interest
References
- Heywood, V.H. Flowering Plants of the World, 1st ed.; Academy Publishing Co.: Seoul, Korea, 1991; p. 11. [Google Scholar]
- Takaoka, D.; Watanabe, K.; Hiroi, M. Studies on lignoids in Lauraceae. II. Studies on lignans in the leaves of Machilus japonica Siebold & Zucc. Bull. Chem. Soc. Jpn. 1976, 49, 3564–3566. [Google Scholar] [CrossRef]
- Khien, P.V.; Huy, D.Q.; Huong, D.T.V. Chemical composition of essential oil of Machilus japonica Sieb. & Zucc. (Lauraceae) from Vietnam. VNU J. Sci. Nat. Sci. Technol. 2009, 25, 81–83. [Google Scholar]
- Gonzalez-Coloma, A.; Escoubas, P.; Mizutani, J.; Lajide, L. Insect growth inhibitors from Machilus japonica. Phytochemistry 1994, 3, 607–610. [Google Scholar] [CrossRef]
- Moon, J.Y.; Yim, E.Y.; Song, G.; Lee, N.H.; Hyun, C.G. Screening of elastase and tyrosinase inhibitory activity from Jeju Island plants. Eur. Asia. J. BioSci. 2010, 4, 41–53. [Google Scholar]
- Lee, Y.L.; Lee, M.H.; Chang, P.Y.; Huang, I.J.; Cheng, K.T.; Leu, S.J. Taiwanese native plants inhibit matrix metalloproteinase-9 activity after ultraviolet B irradiation. Molecules 2009, 14, 1062–1071. [Google Scholar] [CrossRef]
- Re, R.; Pellegrini, N.; Proteggente, A.; Pannala, A.; Yang, M.; Rice-Evans, C. Antioxidant activity applying an improved ABTS ridical cation decolorization assay. Free Radic. Biol. Med. 1999, 26, 1231–1237. [Google Scholar] [CrossRef]
- Steinberg, D. Oxidative modification of LDL and atherogenesis. Circulation 1997, 95, 1062–1071. [Google Scholar] [CrossRef]
- Glass, C.K. Atherosclerosis: A the road ahead. Cell 2001, 23, 503–516. [Google Scholar] [CrossRef]
- Kuspradinl, H.; Mitsunaga, T.; Ohashi, H. Antimicrobial activity against Streptococcus sobrinus and glucosyltransferase inhibitory activity of taxifolin and some flavanonol rhamnosides from kempas (Koompassia malaccensis) extracts. J. Wood Sci. 2009, 55, 308–313. [Google Scholar] [CrossRef]
- Imai, T.; Inoue, S.; Ohdaira, N.; Matsushita, Y.; Suzuki, R.; Sakurai, M.; Henriques de Jesus, J.M.; Ozaki, S.K.; Finger, Z.; Fukushima, K. Heartwood extractives from the Amazonian trees Dipteryx odorata, Hymenaea courbaril, and Astonium lecointei and their antioxidant activities. J. Wood Sci. 2008, 54, 470–475. [Google Scholar] [CrossRef]
- Kim, Y.K.; Kim, Y.S.; Choi, S.U.; Ryu, S.Y. Isolation of flavonol rhamnosides from Loranthus tanakae and cytotoxic effect of them on human tumor cell lines. Arch. Pham. Res. 2004, 27, 44–47. [Google Scholar] [CrossRef]
- Kim, D.K. Antioxidative constituents from the whole plant of Actinostemma lobatum Maxim. J. Korean Soc. Appl. Biol. Chem. 2010, 53, 746–751. [Google Scholar]
- Masika, P.J.; Sultana, N.; Afolayan, A.J. Antibacterial activity of two flavonoids isolated from Schotia latifolia. Pharm. Biol. 2004, 42, 105–108. [Google Scholar] [CrossRef]
- Morimoto, S.; Nonak, G.I.; Nishioka, I.; Ezaki, N.; Takizawa, N. Tannins and related compounds. XXIX.1) Seven new methyl derivatives of flavan-3-ols and a 1,3-diarylpropan-2-ol from Cinnamomum cassia, C. obtusifolium and Lindera umbellata var. membranacea. Chem. Pharm. Bull. 1985, 33, 2281–2286. [Google Scholar] [CrossRef]
- Ku, Y.L.; Chen, C.H.; Lee, S.S. Chemical constituents from Phoebe minutiflora II. Nat. Prod. Res. 2006, 13, 1199–1206. [Google Scholar]
- Yokozawa, T.; Chen, C.P.; Dong, E.; Tanaka, T.; Nonaka, G.I.; Nishioka, I. Study on the inhibitory effect of tannins and flavonoids against the 1,1-diphenyl-2-picrylhydrazyl radical. Biochem. Pharmacol. 1998, 56, 213–222. [Google Scholar] [CrossRef]
- Lawrence, J.; Machlin, L.J.; Bendich, A. Free radical tissue damage: Protective role of antioxidant nutrients. FASEB J. 1987, 6, 441–445. [Google Scholar]
- Bondet, V.; Brand-Williams, W.; Berset, C. Kinetics and mechanisms of antioxidant activity using the DPPH free radical method. Lebensm. Wiss. Technol. 1997, 30, 609–615. [Google Scholar] [CrossRef]
- Steinberg, D.; Parthasarathy, S.; Carew, T.E.; Khoo, J.C.; Witztum, J.L. Beyond cholesterol.Modification of low density lipoprotein that increases its atherogenicity. N. Engl. J. Med. 1989, 320, 915–924. [Google Scholar] [CrossRef]
- Diaz, M.N.; Frei, B.; Vita, J.A.; Keaney, J.F. Antioxidants and atherosclerotic heart disease. N. Engl. J. Med. 1997, 337, 408–416. [Google Scholar] [CrossRef]
- Regnstrom, J.; Nilsson, J.; Tornvall, P.; Hamsten, A.; Landou, C. Susceptibility to low-density lipoprotein oxidation and coronary atherosclerosis in man. Lancet 1992, 339, 1183–1186. [Google Scholar] [CrossRef]
- Salonen, J.T.; Yla-Herttuala, S.; Yamamoto, R.; Butler, S.; Korpela, H.; Salonen, R.; Nyyssonen, K.; Palinski, W.; Witztum, J.L. Auto antibody against oxidised LDL and progression of carotid atherosclerosis. Lancet 1992, 339, 883–887. [Google Scholar] [CrossRef]
- Vinson, J.A.; Jang, J.H.; Dabbagh, Y.A.; Serry, M.M.; Cai, S.H. Plant polyphenols exhibit lipoprotein-bound antioxidant activity using an in vitro oxidation model for heart disease. J. Agric. Food Chem. 1995, 43, 2798–2799. [Google Scholar] [CrossRef]
- Xu, X.; Gu, L.; Holden, J.; Haytowitz, D.B.; Gebhardt, S.E.; Beecher, G.; Prior, R.L. Development of a database for total antioxidant capacity in foods: A preliminary study. J. Food Compos. Anal. 2004, 17, 407–422. [Google Scholar] [CrossRef]
- Shrestha, S.; Park, J.H.; Lee, D.Y.; Cho, J.G.; Lee, D.G.; Cho, M.H.; Jeong, T.S.; Kang, H.C.; Baek, N.I. Inhibition of low density lipoprotein-oxidation, ACAT-1, and ACAT-2 by lignans from the bark of Machilus thunbergii. J. Appl. Biol. Chem. 2011, 54, 63–66. [Google Scholar] [CrossRef]
- Lee, D.Y.; Lee, M.H.; Jung, T.S.; Kwon, B.M.; Baek, N.I.; Rho, Y.D. Triterpenoid and lignan from the fruits of Cornus kousa inhibit the activities of PRL-3 and LDL oxidation. J. Korean Soc. Appl. Biol. Chem. 2010, 53, 97–100. [Google Scholar]
- Bueqe, J.A.; Aust, S.D. Microsomal lipid peroxidation. Methods Enzymol. 1978, 52, 302–310. [Google Scholar]
© 2014 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Joo, S.-J.; Park, H.-J.; Park, J.-H.; Cho, J.-G.; Kang, J.-H.; Jeong, T.-S.; Kang, H.C.; Lee, D.-Y.; Kim, H.-S.; Byun, S.-Y.; et al. Flavonoids from Machilus japonica Stems and Their Inhibitory Effects on LDL Oxidation. Int. J. Mol. Sci. 2014, 15, 16418-16429. https://doi.org/10.3390/ijms150916418
Joo S-J, Park H-J, Park J-H, Cho J-G, Kang J-H, Jeong T-S, Kang HC, Lee D-Y, Kim H-S, Byun S-Y, et al. Flavonoids from Machilus japonica Stems and Their Inhibitory Effects on LDL Oxidation. International Journal of Molecular Sciences. 2014; 15(9):16418-16429. https://doi.org/10.3390/ijms150916418
Chicago/Turabian StyleJoo, Se-Jin, Hee-Jung Park, Ji-Hae Park, Jin-Gyeong Cho, Ji-Hyun Kang, Tae-Sook Jeong, Hee Cheol Kang, Dae-Young Lee, Hack-Soo Kim, Sang-Yo Byun, and et al. 2014. "Flavonoids from Machilus japonica Stems and Their Inhibitory Effects on LDL Oxidation" International Journal of Molecular Sciences 15, no. 9: 16418-16429. https://doi.org/10.3390/ijms150916418
APA StyleJoo, S.-J., Park, H.-J., Park, J.-H., Cho, J.-G., Kang, J.-H., Jeong, T.-S., Kang, H. C., Lee, D.-Y., Kim, H.-S., Byun, S.-Y., & Baek, N.-I. (2014). Flavonoids from Machilus japonica Stems and Their Inhibitory Effects on LDL Oxidation. International Journal of Molecular Sciences, 15(9), 16418-16429. https://doi.org/10.3390/ijms150916418