Application of Ionic Liquids in Hydrometallurgy
Abstract
:1. Introduction

2. Results and Discussion
2.1. Ionic Liquids
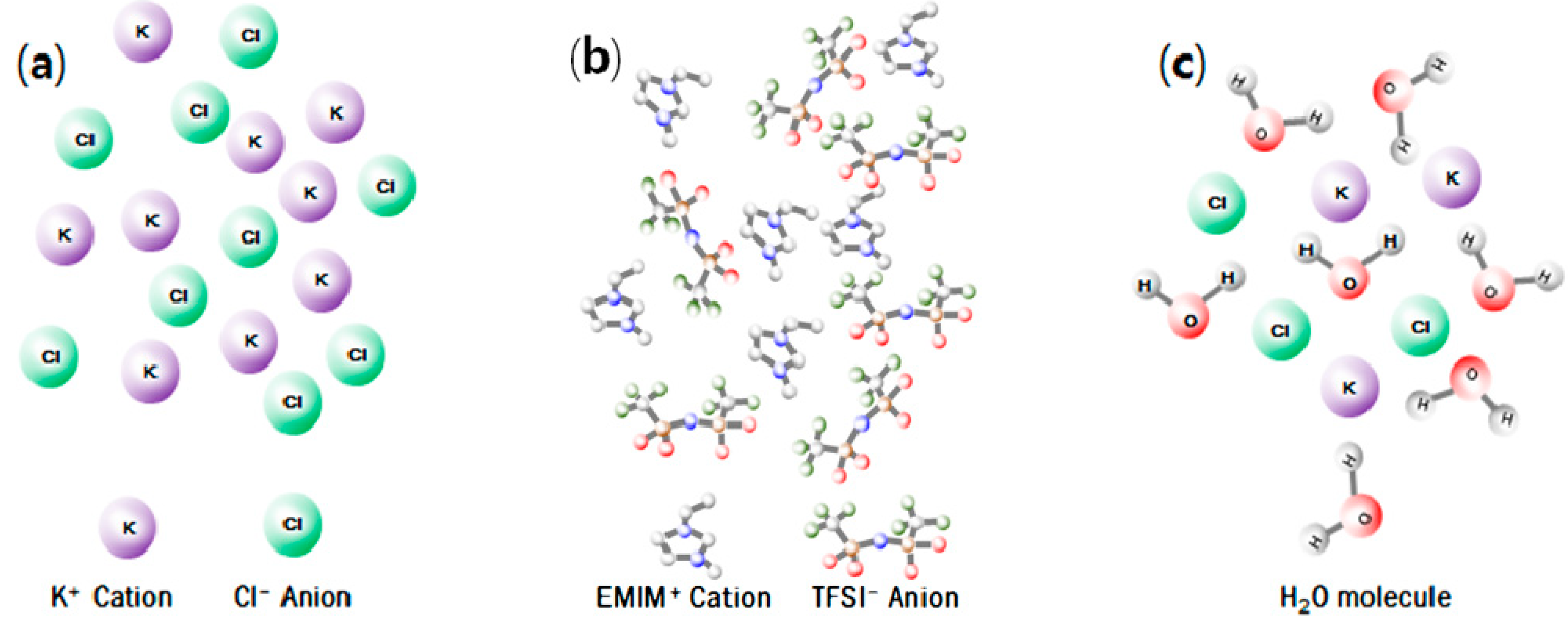
| Properties | Values |
|---|---|
| Melting point | Preferably below 100 °C |
| Liquidus range | Often > 200 °C |
| Thermal stability | Usually high |
| Viscosity | Normally < 100 cP, workable |
| Dielectric constant | Implied < 30 |
| Polarity | Moderate |
| Ionic conductivity | Usually < 10 mS/cm |
| Molar conductivity | <10 Scm2/mol |
| Electrochemical window | Often > 4 V |
| Vapor pressure | Usually negligible |
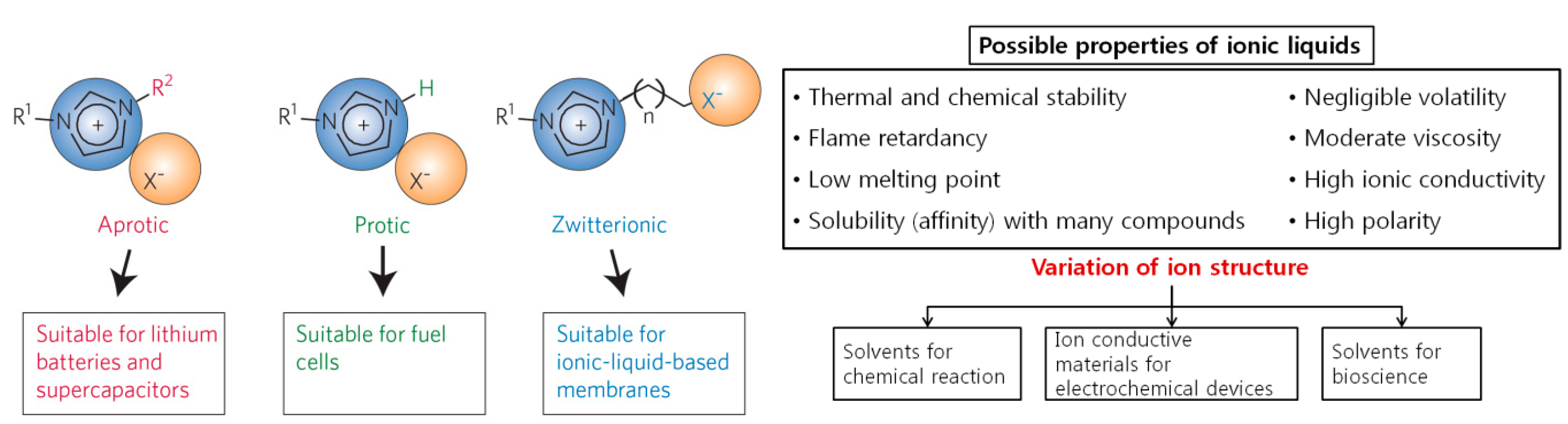

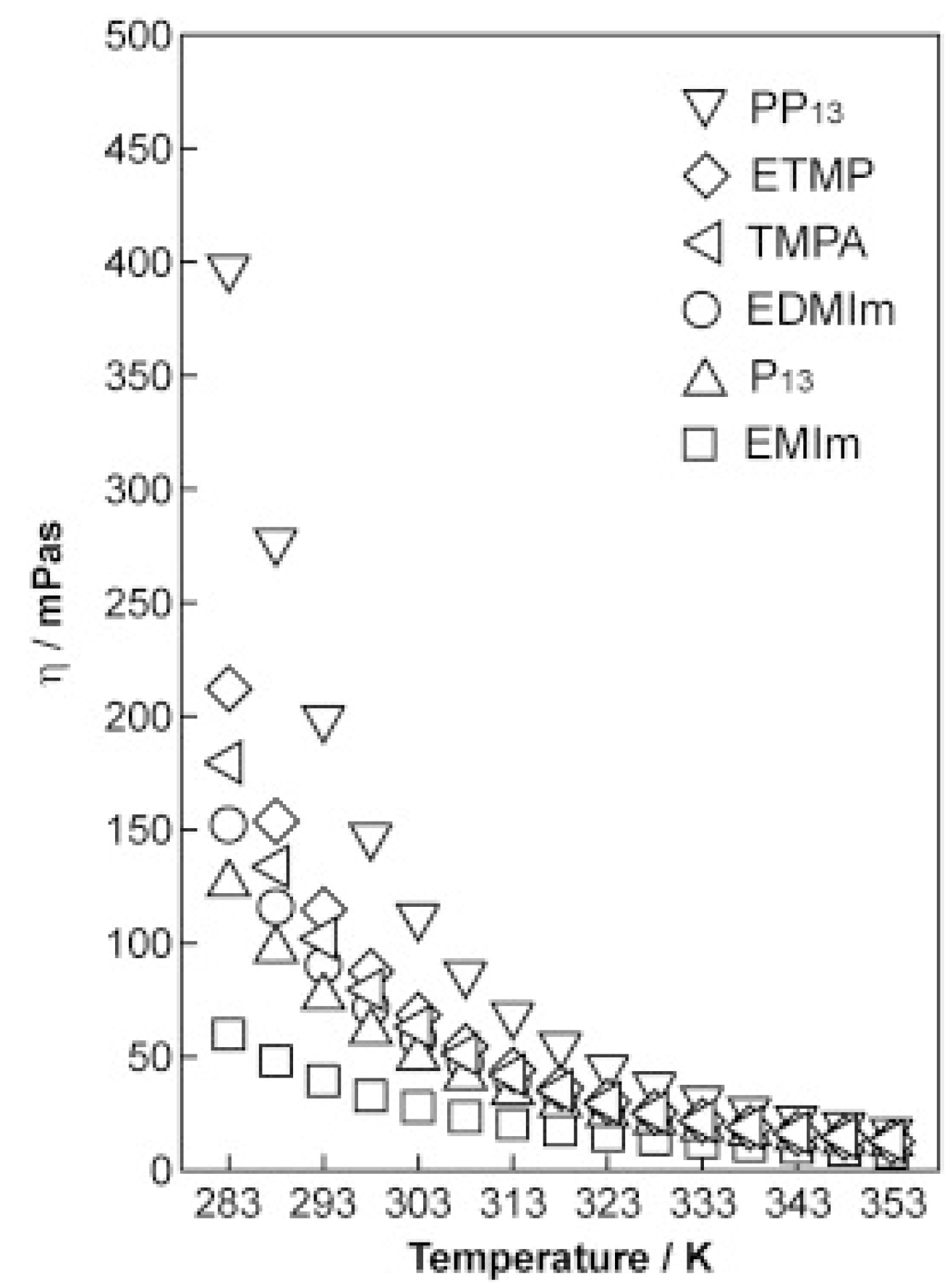
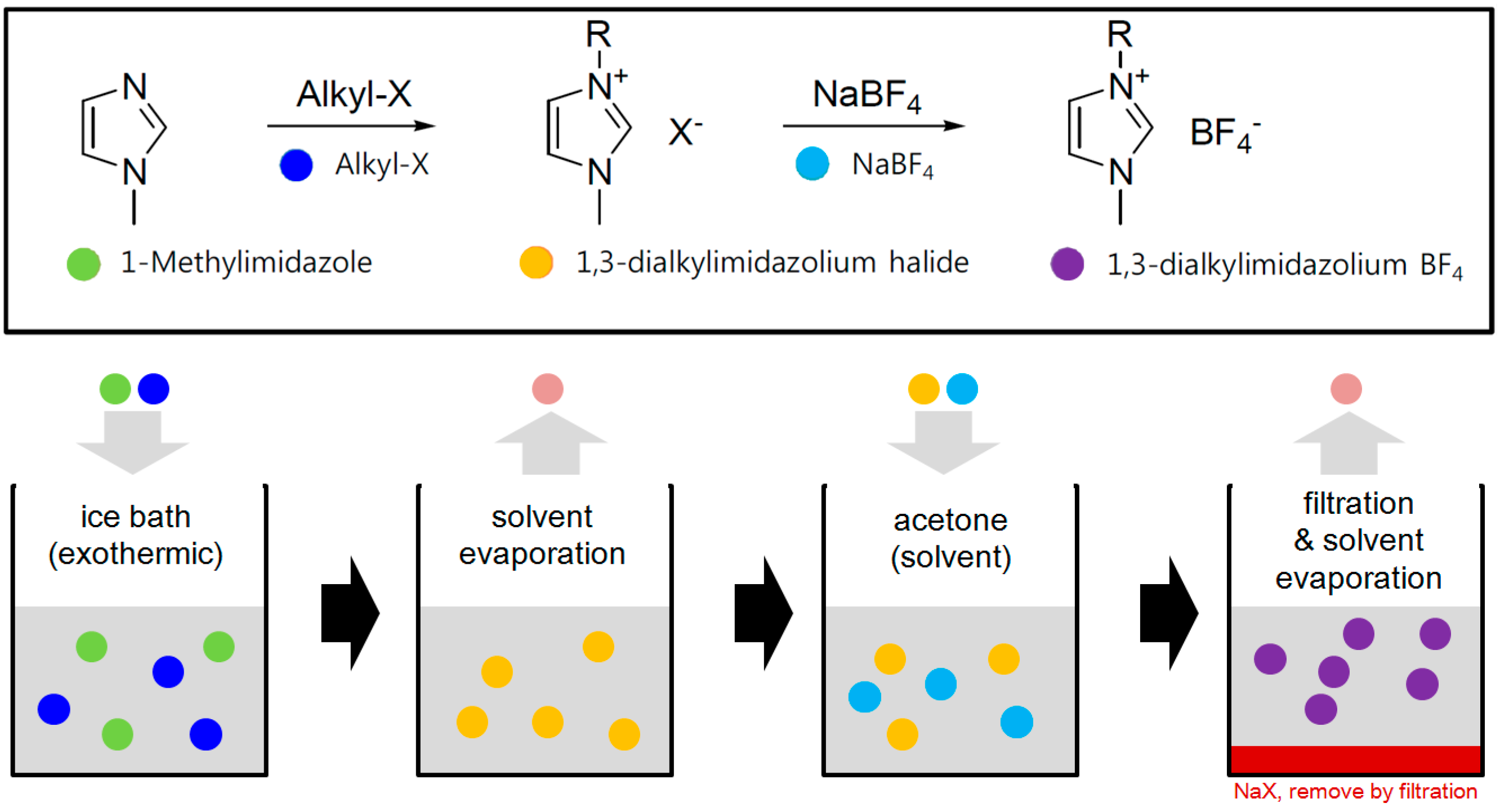

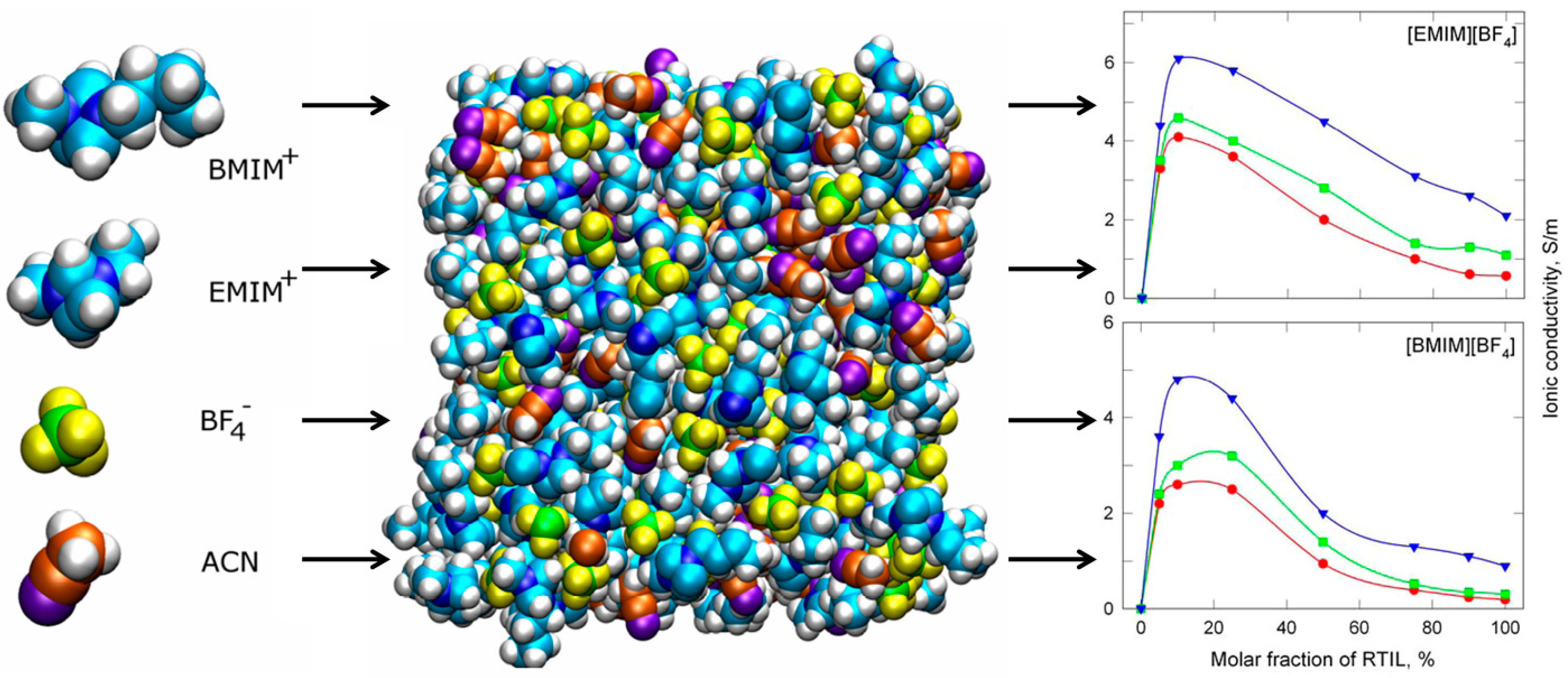
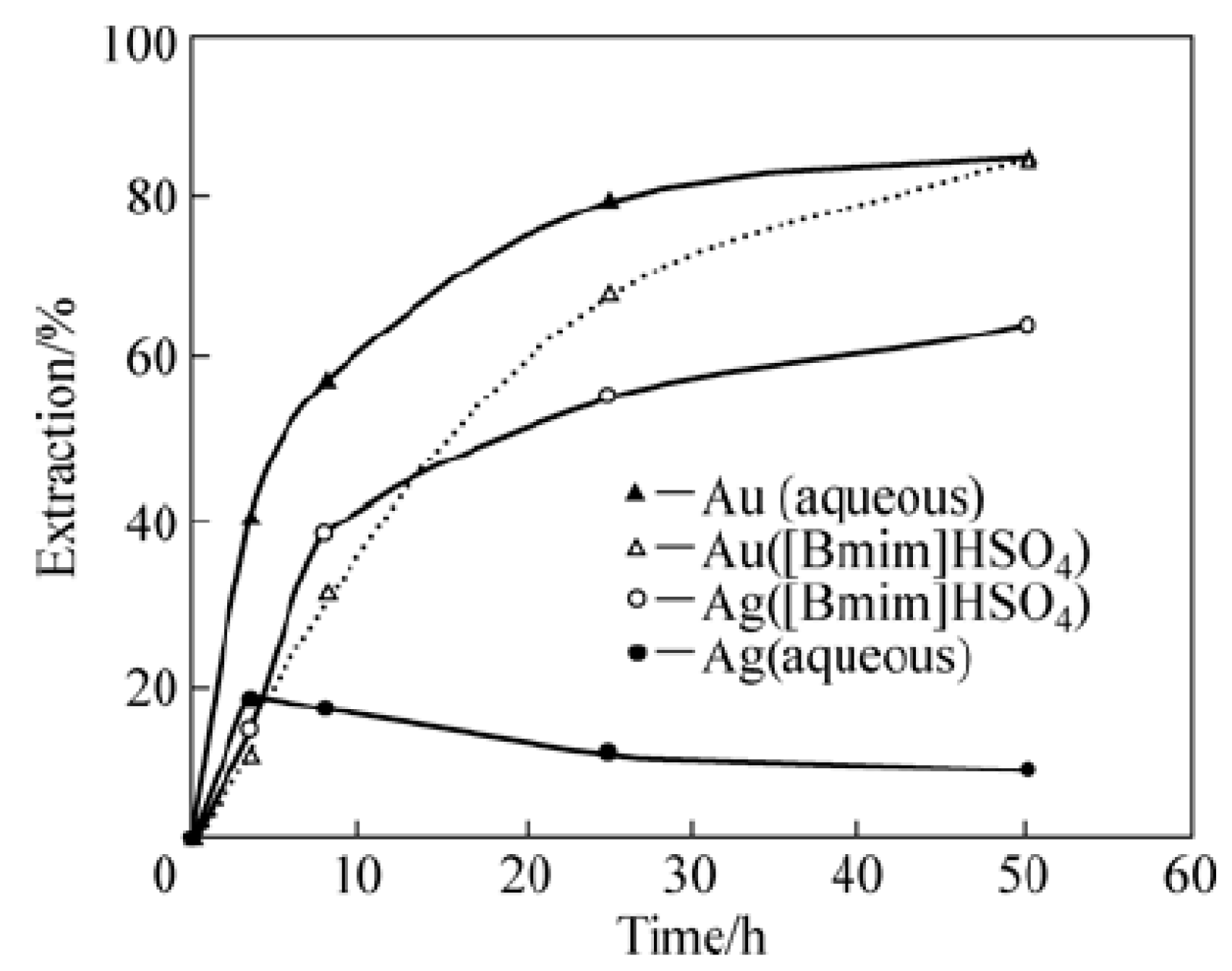
2.2. Extraction of Metals
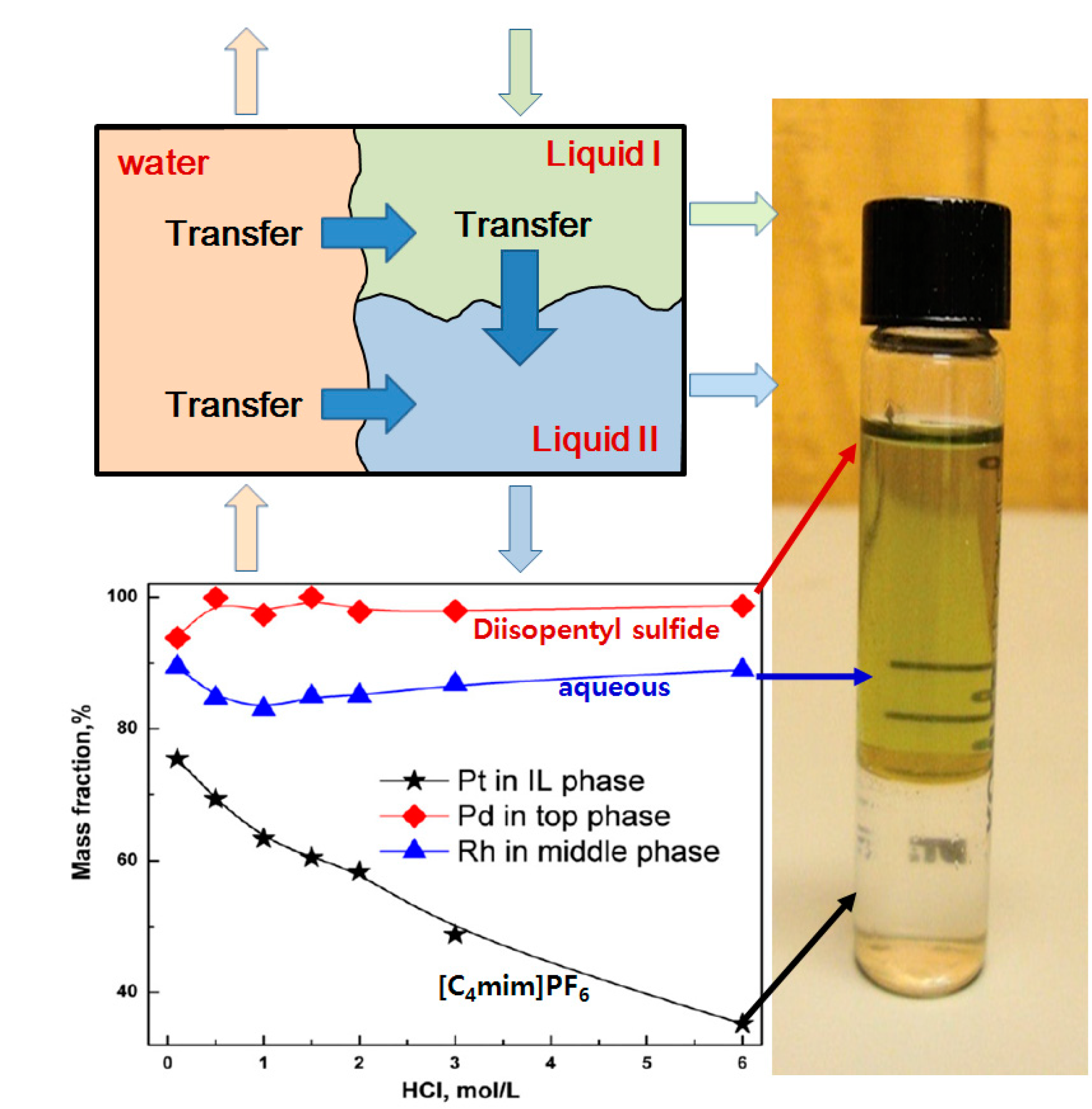

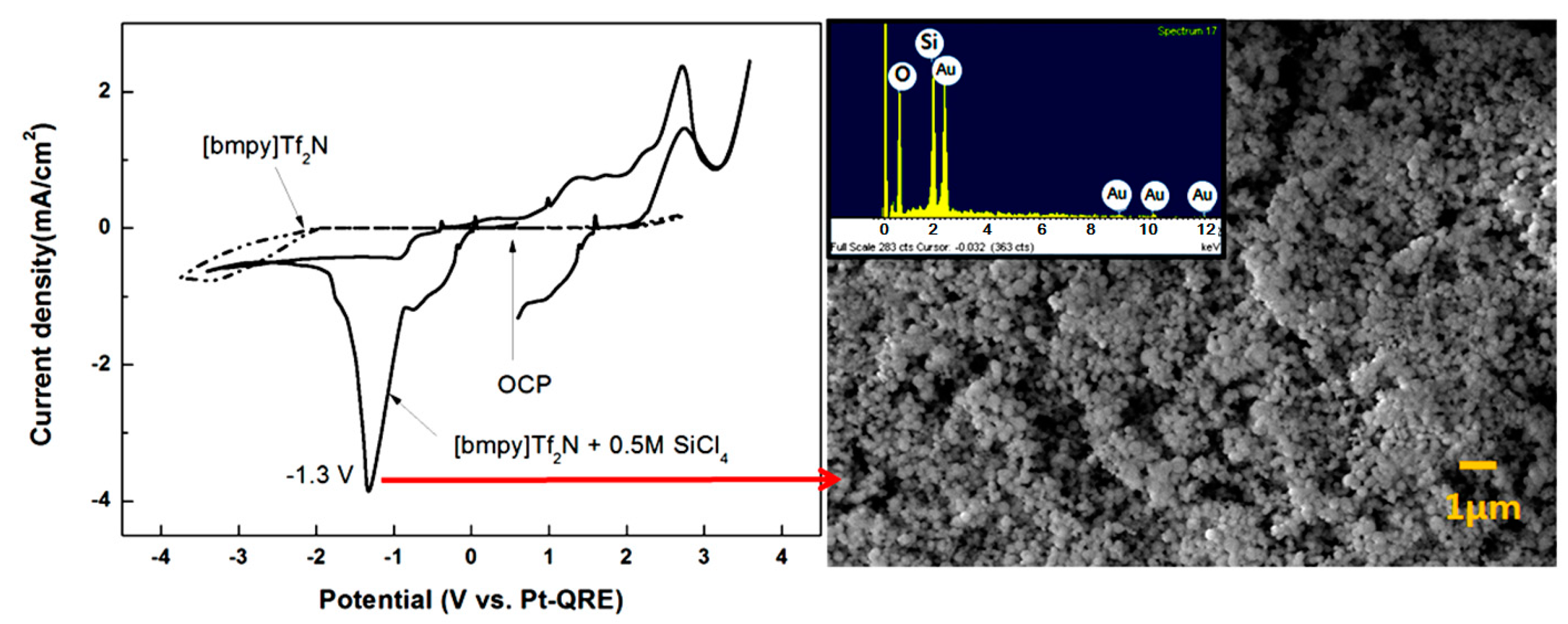
2.3. Electrolysis of Metals
| Metal | Li | Mg | Au | Pt | Pd | |
|---|---|---|---|---|---|---|
| Ionic Liquids | ||||||
| BMI[TFSI] | [42] | |||||
| BMP[TFSI] | [43] | [44] | ||||
| Bu3HexP+[TFSI]− | [45] | |||||
| Bu3HexN+[TFSI]− | [45] | |||||
| C3mpyr[TFSI] | [46] | |||||
| DEME[TFSI] | [47,48] | |||||
| EMI[TFSI] | [49] | |||||
| EMIm[TFSI] | [50] | |||||
| N1113[TFSI] | [49] | |||||
| N1114[TFSI] | [51] | |||||
| Metal | Li | Mg | Au | Ta | Si | Nd | |
|---|---|---|---|---|---|---|---|
| Ionic Liquids | |||||||
| PP13[TFSI] | [52] | ||||||
| Pyr14[TFSI] | [53] | ||||||
| TMHA[TFSI] | [54,55] | ||||||
| C3mpyr[FSI] | [56] | ||||||
| BMP[TFSA] | [57] | ||||||
| EMIm[TFSA] | [58] | ||||||
| PMIm[TFSA] | [59] | ||||||
| PP13[TFSA] | [60,61] | ||||||
| P2225[TFSA] | [62] | ||||||
| Py14[TFSA] | [60,63] | [64] | |||||
| Metal | Li | Mg | Au | Pt | Ti | Ta | Si | In | |
|---|---|---|---|---|---|---|---|---|---|
| Ionic Liquids | |||||||||
| TMHA[TFSA] | [60] | ||||||||
| EMI[FSA] | [61] | ||||||||
| EMI[TSAC] | [49] | ||||||||
| N2222[TSAC] | [49] | ||||||||
| ABN1n[Tf2N] | [65] | ||||||||
| BEPip[Tf2N] | [66] | ||||||||
| BMIm[Tf2N] | [67] | ||||||||
| BMP[Tf2N] | [68] | [69] | [67,70,71,72,73] | [67,69,74,75] | [76] | ||||
| C3(OH)2mim[Tf2N] | [77] | ||||||||
| C4Mim[Tf2N] | [78] | [78] | |||||||
| Metal | Mg | Au | Pt | Pd | Ti | Si | |
|---|---|---|---|---|---|---|---|
| Ionic Liquids | |||||||
| C10MIm[Tf2N] | [79] | ||||||
| EMIm[Tf2N] | [80] | ||||||
| P66614[Tf2N] | [81] | [81,82] | |||||
| P(C6)3C14[Tf2N] | [83] | [83,84,85] | |||||
| Py14[Tf2N] | [86,87,88] | ||||||
| TBMA[Tf2N] | [89] | ||||||
| BMP[TfO] | [68] | ||||||
| BMIm[Cl] | [90] | [90] | [91,92,93,94,95,96,97,98] | [99,100] | |||
| C3COOH[Cl] | [77] | ||||||
| C3OHMIm[Cl] | [77] | ||||||
| Metal | Li | Au | Pt | Ti | |
|---|---|---|---|---|---|
| Ionic Liquids | |||||
| C3CNmim[Cl] | [77] | ||||
| C3(OH)2mim[Cl] | [77] | ||||
| C3(OCOCH2SH)2mim | [77] | ||||
| BMECl[AlCl3] | [101] | ||||
| BMIC[AlCl3] | [102] | ||||
| BTMAC[AlCl3] | [103] | ||||
| EMICl[AlCl3] | [51,104] | ||||
| EMImCl[AlCl3] | [105] | ||||
| EtMelmCl[AlCl3] | [106,107] | ||||
| BMI[BF4] | [108] | ||||
| Metal | Mg | Au | Pt | Pd | Ti | In | |
|---|---|---|---|---|---|---|---|
| Ionic Liquids | |||||||
| BMIm[BF4] | [52,109,110,111,112] | [113] | |||||
| BMMIm[BF4] | [114] | ||||||
| C3CNMIm[BF4] | [77] | ||||||
| C3(OH)2mim[BF4] | [77] | ||||||
| DEME[BF4] | [115] | ||||||
| EMI[BF4] | [108] | ||||||
| EMIm[BF4] | [68] | [67] | [67] | [67] | |||
| BMIm[BR] | [116] | ||||||
| BMIm[BTA] | [117,118] | ||||||
| BMP[DCA] | [119] | [43] | [120,121] | ||||
| Metal | Au | Pt | Pd | In | |
|---|---|---|---|---|---|
| Ionic Liquids | |||||
| BMIm[PF6] | [122] | [113,123] | |||
| C3(OH)2mim[PF6] | [77] | ||||
| C8Py[PF6] | [124] | ||||
| ZnCl2[EMIC] | [125,126,127] | ||||
| ZnCl2[EMIm-Cl] | [67] | ||||
| EMI[Cl-BF4] | [42] | [42] | [128] | ||
| mercapto IL[1-methyl-3(2'-mercaptoacetoxyethyl) imidazoliumhexafluorophosphate] | [129] | [129] | [129] | ||
| TOMAC(Aliquat 336 chloride-IL) | [98,130] | ||||
| TOMAN(Aliquat 336 nitrate-IL) | [130] | ||||
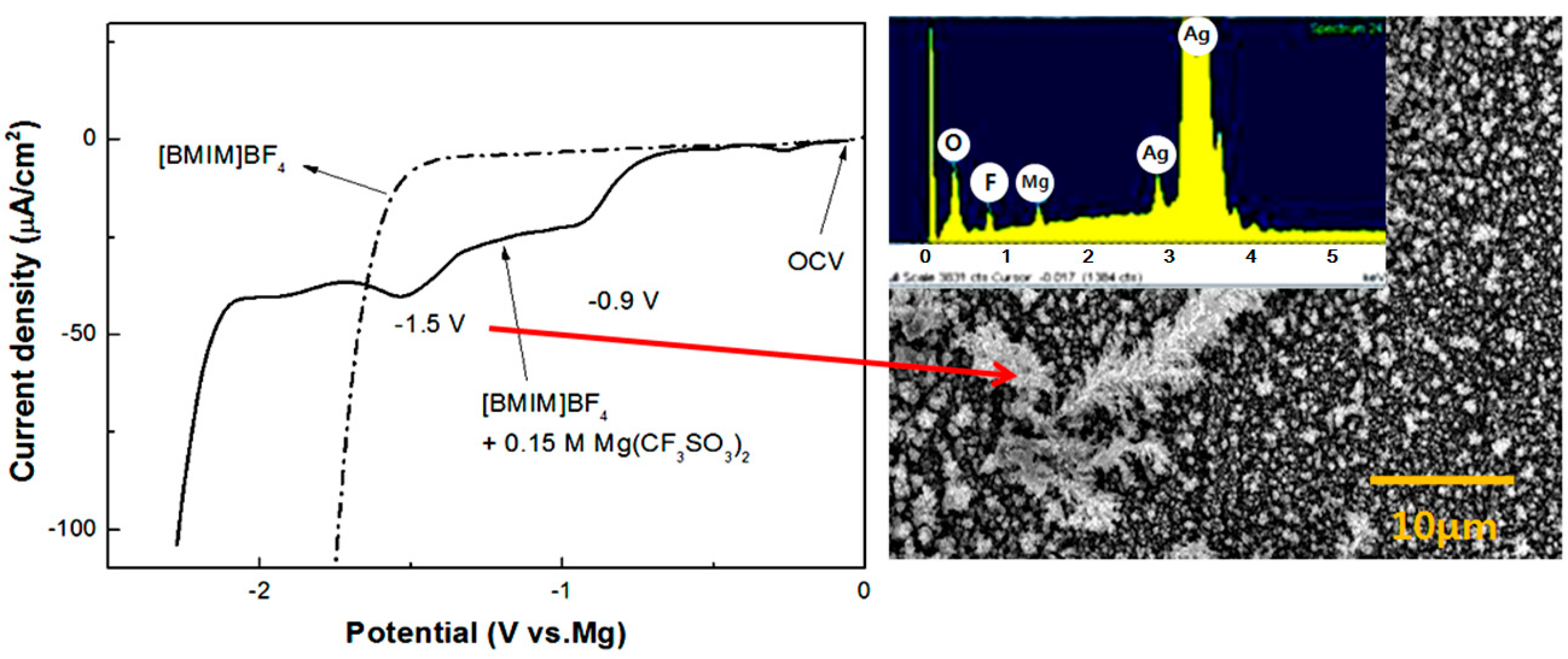
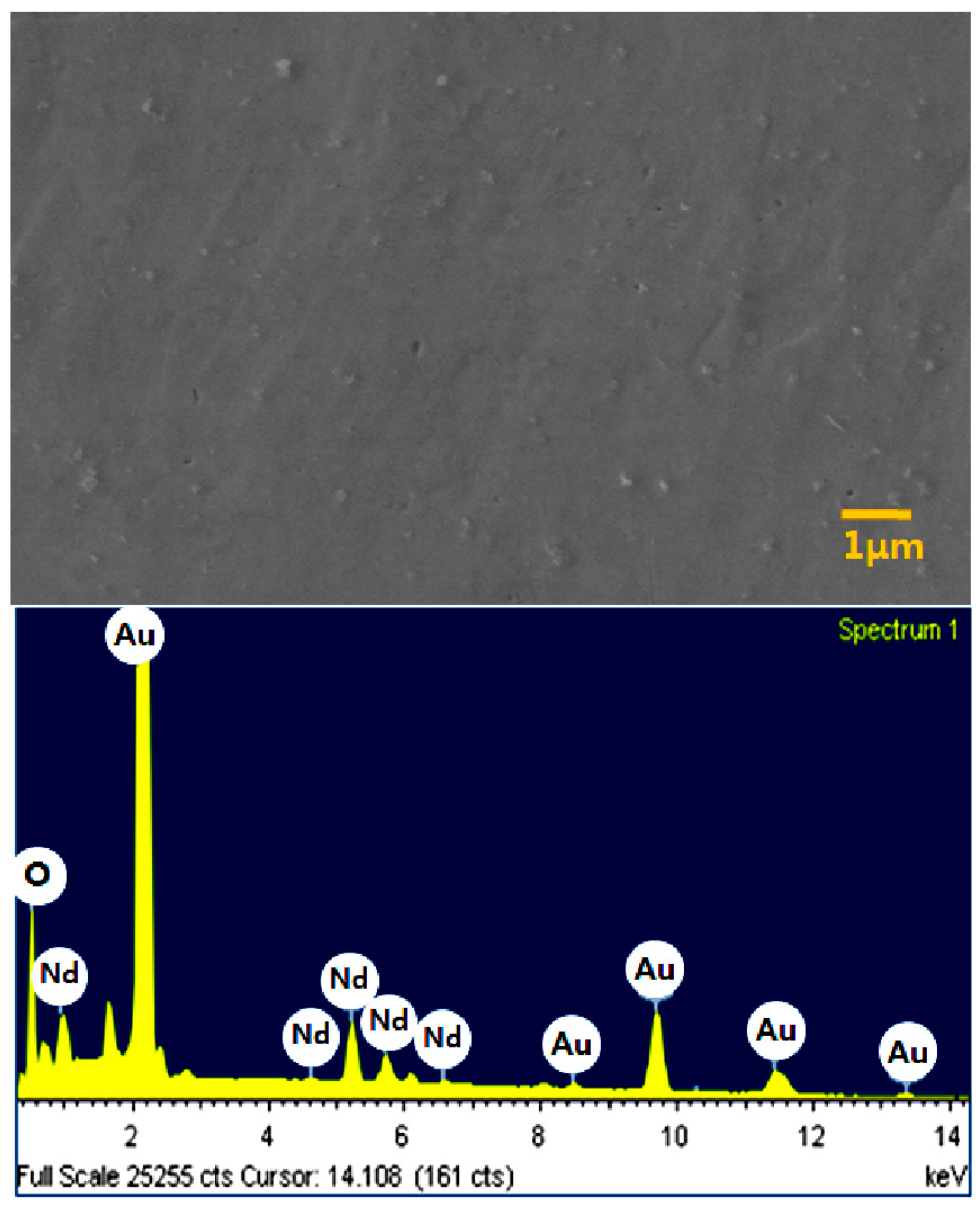
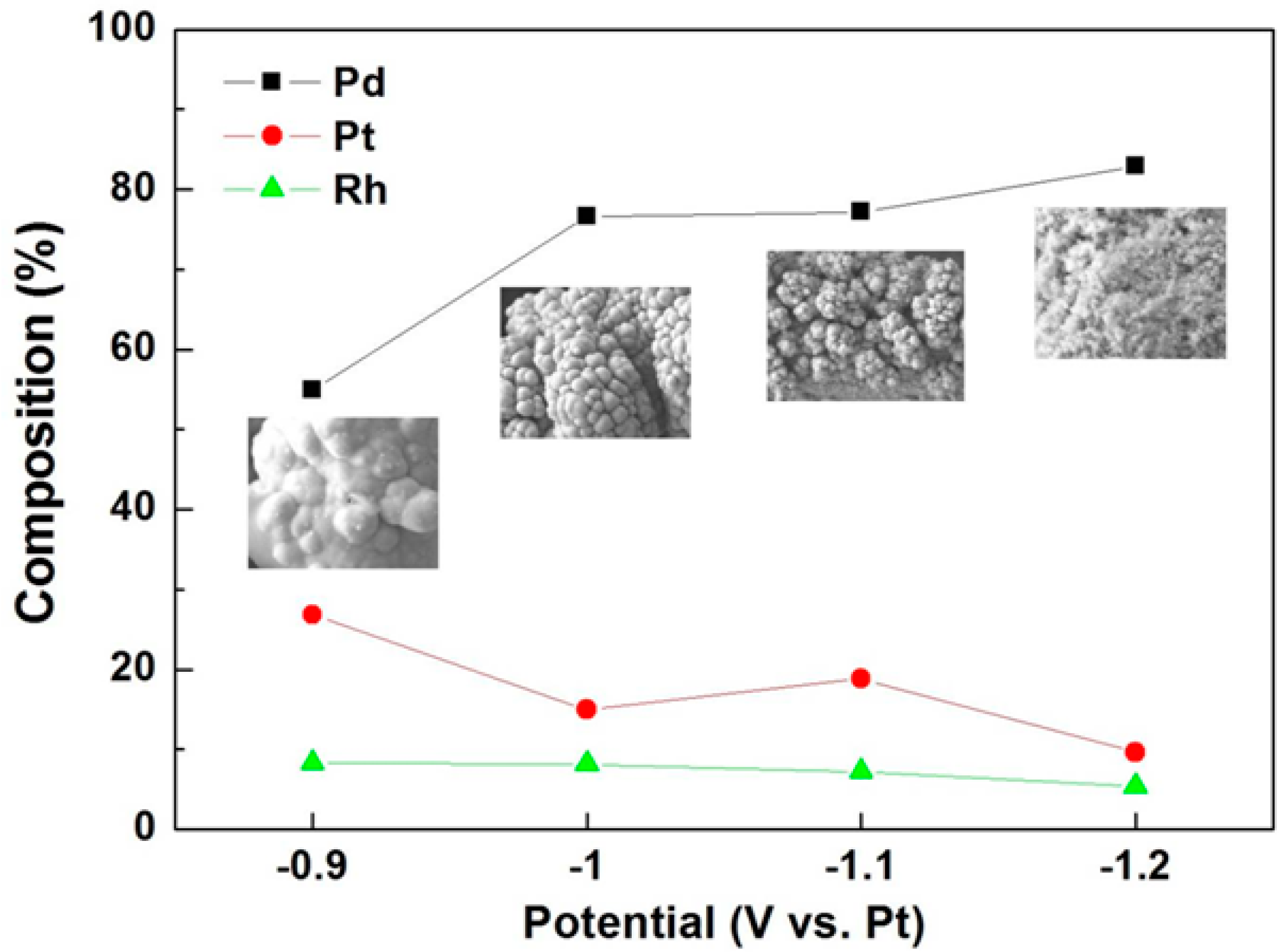
3. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Anastas, P.T. Handbook of Green Chemistry—Green Solvents, 1st ed.; Willey-VCH: Weinheim, Germany, 2010; pp. 1–1344. [Google Scholar]
- SciFinder. Available online: http://scifinder.cas.org/ (accessed on 12 April 2014).
- Johnson, K.E. What’s an ionic liquid? Electrochem. Soc. Interface 2007, 16, 38–41. [Google Scholar]
- Turner, E.A.; Pye, C.C.; Singer, D.J. Use of ab initio calculations toward the rational design of room temperature ionic liquids. Phys. Chem. 2003, 107, 2277–2288. [Google Scholar] [CrossRef]
- Wasserscheid, P.; Welton, T. Ionic Liquids in Synthesis, 2nd ed.; Willey-VCH: Weinheim, Germany, 2008; pp. 1–724. [Google Scholar]
- Marsh, K.N.; Boxall, J.A.; Lichtenthaler, R. Room temperature ionic liquids and their mixtures—A review. Fluid Phase Equilib. 2004, 219, 93–98. [Google Scholar] [CrossRef]
- Sutto, T.E.; de long, H.C.; Trulove, P.C. Ionic liquid, graphite and gel polymer electrolytes and electrodes using 1,2-dimethyl-3-propyl-imidazolium tetrafluoroborate. Proc. Electrochem. Soc. 1999, 99, 32–42. [Google Scholar]
- Wilkes, J.S.; Zaworotko, M.J. Air and water stable 1-ethyl-3-methylimidazolium based ionic liquids. Chem. Commun. 1992, 13, 965–967. [Google Scholar]
- Guerfi, A.; Dontigny, M.; Charest, P.; Petitclerc, M.; Lagacě, M.; Vijh, A.; Zaghib, K. Improved electrolytes for Li-ion batteries: Mixtures of ionic liquid and organic electrolyte with enhanced safety and electrochemical performance. J. Power Sources 2010, 195, 845–852. [Google Scholar]
- Chiappe, C.; Capraro, D.; Conte, V.; Pieraccini, D. Stereoselective halogenations of alkenes and alkynes in ionic liquids. Org. Lett. 2001, 3, 1061–1063. [Google Scholar] [CrossRef]
- Stark, A.; MacLean, B.L.; Singer, R.D. 1-Ethyl-3-methylimidazolium halogenoaluminate ionic liquids as solvents for Friedel–Crafts acylation reactions of ferrocene. J. Chem. Soc. 1999, 63, 63–66. [Google Scholar]
- Sheldon, R. Catalytic reactions in ionic liquids. Chem. Commun. 2001, 23, 2399–2407. [Google Scholar] [CrossRef]
- Kim, H.S.; Kim, Y.J.; Lee, H.; Park, K.Y.; Lee, C.; Chin, C.S. Ionic liquids containing anionic selenium species: Applications for the oxidative carbonylation of aniline. Angew. Chem. Int. Ed. 2002, 41, 4300–4303. [Google Scholar]
- Lewandowski, A.; Świderska, A. Electrochemical capacitors with polymer electrolytes based on ionic liquids. Solid State Ion. 2003, 161, 243–249. [Google Scholar] [CrossRef]
- Wang, P.; Zakeeruddin, S.M.; Exnar, I.; Grätzel, M. High efficiency dye-sensitized nanocrystalline solar cells based on ionic liquid polymer gel electrolyte. Chem. Commun. 2002, 24, 2972–2973. [Google Scholar]
- Shin, J.H.; Henderson, W.A.; Passerini, S. Ionic liquids to the rescue? Overcoming the ionic conductivity limitations of polymer electrolytes. Electrochem. Commun. 2003, 5, 1016–1020. [Google Scholar]
- Armand, M.; Endres, F.; MacFarlane, D.R.; Ohno, H.; Scrosati, B. Ionic liquid materials for the electrochemical challenges of the future. Nat. Mater. 2009, 8, 621–629. [Google Scholar]
- Seki, S.; Kobayashi, T.; Kobayashi, Y.; Takei, K.; Miyashiro, H.; Hayamizu, K.; Tsuzuki, S.; Mitsugi, T.; Umebayashi, Y. Effects of cation and anion on physical properties of room-temperature ionic liquids. J. Mol. Liq. 2010, 152, 9–13. [Google Scholar] [CrossRef]
- Chaban, V.V.; Voroshylova, I.V.; Kalugin, O.N.; Prezhdo, O.V. Acetonitrile boosts conductivity of imidazolium ionic liquids. J. Phys. Chem. 2012, 116, 7719–7727. [Google Scholar] [CrossRef]
- Fannin, A.A., Jr.; Floreani, D.A.; King, L.A.; Landers, J.S.; Piersma, B.J.; Stech, D.J.; Vaughn, R.L.; Wilkers, J.S. Properties of 1,3-dialkylimidazollum chloride-aluminum chloride ionic liquids. 2. Phase transitions, densities, electrical conductivities, and viscosities. J. Phys. Chem. 1984, 88, 2614–2621. [Google Scholar] [CrossRef]
- Fannin, A.A., Jr.; Levisky, J.A.; Wilkers, J.S. Properties of 1,3-dialkylimidazolium chloride-aluminum chloride ionic liquids. 1. Ion interactions by nuclear magnetic resonance spectroscopy. J. Phys. Chem. 1984, 88, 2609–2614. [Google Scholar] [CrossRef]
- McCluscey, A.; Lawrance, G.A.; Leitch, S.K.; Owen, M.P.; Hamilton, I.C. Ionic liquids industrial applications for green chemistry. Am. Chem. Soc. 2002, 818, 199–212. [Google Scholar]
- Whitehead, J.A.; Lawrance, G.A.; McCluskey, A. Green leaching: Recyclable and selective leaching of gold-bearing ore in an ionic liquid. Green Chem. 2004, 6, 313–315. [Google Scholar] [CrossRef]
- Tian, G.; Li, J.; Hua, Y. Application of ionic liquids in hydrometallurgy of nonferrous metals. Trans. Nonferrous Met. Soc. China 2010, 20, 513–520. [Google Scholar] [CrossRef]
- Zhang, C.; Huang, K.; Yu, P.; Liu, H. Ionic liquid based three-liquid-phase partitioning and one-step separation of Pt(IV), Pd(II) and Rh(III). Sep. Purif. Technol. 2013, 108, 166–173. [Google Scholar]
- Cieszynska, A.; Wisniewski, M. Selective extraction of palladium(II) from hydrochloric acid solutions with phosphonium extractants. Sep. Purif. Technol. 2011, 80, 385–389. [Google Scholar] [CrossRef]
- Mehdi, H.; Binnemans, K.; Hecke, K.V.; Meervelt, L.V.; Nockemann, P. Hydrophobic ionic liquids with strongly coordinating anions. Chem. Commun. 2010, 46, 234–236. [Google Scholar] [CrossRef]
- Wellens, S.; Thijs, B.; Möller, C.; Binnemans, K. Separation of cobalt and nickel by solvent extraction with two mutually immiscible ionic liquids. Phys. Chem. Chem. Phys. 2013, 15, 9663–9669. [Google Scholar] [CrossRef]
- Wellens, S.; Goovaerts, R.; Möller, C.; Luyten, J.; Thijs, B.; Binnemans, K. A continuous ionic liquid extraction process for the separation of cobalt from nickel. Green Chem. 2013, 15, 3160–3164. [Google Scholar] [CrossRef]
- Hoogerstraete, T.V.; Onghena, B.; Binnemans, K. Homogeneous liquid–liquid extraction of metal ions with a functionalized ionic liquid. Phys. Chem. Lett. 2013, 4, 1659–1663. [Google Scholar] [CrossRef]
- Hoogerstraete, T.V.; Onghena, B.; Binnemans, K. Homogeneous liquid–liquid extraction of rare earths with the betaine—betainium bis(trifluoromethylsulfonyl)imide ionic liquid system. Int. J. Mol. Sci. 2013, 14, 21353–21377. [Google Scholar] [CrossRef]
- Hoogerstraete, T.V.; Binnemans, K. Highly efficient separation of rare earths from nickel and cobalt by solvent extraction with the ionic liquid trihexyl(tetradecyl)phosphonium nitrate: A process relevant to the recycling of rare earths from permanent magnets and nickel metal hydride batteries. Green Chem. 2014, 16, 1594–1606. [Google Scholar] [CrossRef]
- Rout, A.; Binnemans, K. Liquid–liquid extraction of europium(III) and other trivalent rare-earth ions using a non-fluorinated functionalized ionic liquid. Dalton Trans. 2014, 43, 1862–1872. [Google Scholar] [CrossRef]
- Rout, A.; Binnemans, K. Separation of rare earths from transition metals by liquid–liquid extraction from a molten salt hydrate to an ionic liquid phase. Dalton Trans. 2014, 43, 3186–3195. [Google Scholar] [CrossRef]
- Rout, A.; Wellens, S.; Binnemans, K. Separation of rare earths and nickel by solvent extraction with two mutually immiscible ionic liquids. RSC Adv. 2014, 4, 5753–5758. [Google Scholar] [CrossRef]
- Rout, A.; Souza, E.R.; Binnemans, K. Solvent extraction of europium(III) to a fluorine-free ionic liquid phase with a diglycolamic acid extractant. RSC Adv. 2014, 4, 11899–11906. [Google Scholar]
- Cascon, H.R.; Choudhari, S.K. 1-Butanol pervaporation performance and intrinsic stability of phosphonium and ammonium ionic liquid-based supported liquid membranes. J. Membr. Sci. 2013, 429, 214–224. [Google Scholar] [CrossRef]
- Hoshino, T. Preliminary studies of lithium recovery technology from seawater by electrodialysis using ionic liquid membrane. Desalination 2013, 317, 11–16. [Google Scholar] [CrossRef]
- Andres, F. Ionic liquids: Solvents for the electrodeposition of metals and semiconductors. Chem. Phys. Chem. 2002, 3, 144–154. [Google Scholar]
- Abbott, A.P.; McKenzie, K.J. Application of ionic liquids to the electrodeposition of metals. Phys. Chem. Chem. Phys. 2006, 8, 4265–4279. [Google Scholar]
- Simka, W.; Puszczyk, D.; Nawrat, G. Electrodeposition of metals from non-aqueous solutions. Electrochim. Acta 2009, 54, 5307–5319. [Google Scholar] [CrossRef]
- Su, F.Y.; Huang, J.F.; Sun, I.W. Galvanostatic deposition of palladium gold alloys in a Lewis basic EMI-Cl-BF4 ionic liquid. J. Electrochem. Soc. 2004, 151, C811–C815. [Google Scholar] [CrossRef]
- Huang, H.Y.; Su, C.J.; Kao, C.L.; Chen, P.Y. Electrochemical study of Pt and Fe and electrodeposition of PtFe alloys. J. Electroanal. Chem. 2010, 650, 1–9. [Google Scholar] [CrossRef]
- Bando, Y.; Katayama, Y.; Miura, T. Electrodeposition of palladium in a hydrophobic 1-n-butyl-1-methylpyrrolidinium bis(trifluoromethylsulfonyl)imide room temperature ionic liquid. Electrochim. Acta 2009, 53, 87–91. [Google Scholar] [CrossRef]
- Vega, J.A.; Zhou, J.; Kohl, P.A. Electrochemical comparison and deposition of lithium and potassium from phosphonium and ammonium TFSI ionic liquids. J. Electrochem. Soc. 2009, 156, A253–A259. [Google Scholar] [CrossRef]
- Lanea, G.H.; Best, A.S.; MacFarlane, D.R.; Forsyth, M.; Bayley, P.M.; Hollenkamp, A.F. The electrochemistry of lithium in ionic liquid organic diluent mixtures. Electrochim. Acta 2010, 55, 8947–8952. [Google Scholar] [CrossRef]
- Yoshimoto, N.; Hotta, K.; Egashira, M.; Morita, M. Electrochemical behavior of magnesium in mixed solutions consisting of ionic liquid and alkylmagnesiumbromides with different alkyl-chains. Electrochemistry 2012, 80, 774–776. [Google Scholar] [CrossRef]
- Shimamura, O.; Yoshimoto, N.; Matsumoto, M.; Egashia, E.; Morita, M. Electrochemical co-deposition of magnesium with lithium from quaternary ammonium-based ionic liquid. J. Power Sources 2011, 196, 1586–1588. [Google Scholar] [CrossRef]
- Matsumoto, H.; Sakaebe, H.; Tatsumi, K. Preparation of room temperature ionic liquids based on aliphatic onium cations and asymmetric amide anions and their electrochemical properties as a lithium battery electrolyte. J. Power Sources 2005, 146, 45–50. [Google Scholar]
- Yan, B.; Yang, P.; Zhao, Y.; Zhang, J.; An, M. Electrocodeposition of lithium and copper from room temperature ionic liquid 1-ethyl-3-methyllimidazolium bis(trifluoromethylsulfonyl)imide. RSC Adv. 2012, 2, 12926–12931. [Google Scholar] [CrossRef]
- Stark, J.K.; Ding, Y.; Kohl, P.A. Dendrite free electrodeposition and reoxidation of lithium sodium alloy for metal anode battery. J. Electrochem. Soc. 2011, 158, A1100–A1105. [Google Scholar] [CrossRef]
- Wang, P.; NuLi, Y.; Yang, J.; Feng, Z. Mixed ionic liquids as electrolyte for reversible deposition and dissolution of magnesium. Surf. Coat. Technol. 2006, 201, 3783–3787. [Google Scholar] [CrossRef]
- Schmuck, M.; Balducci, A.; Rupp, B.; Kern, W.; Passerini, S.; Winter, M. Alloying of electrodeposited silicon with lithium a principal study of applicability as anode material for lithium ion batteries. J. Solid-State Electrochem. 2010, 14, 2203–2207. [Google Scholar] [CrossRef]
- Komadina, J.; Akiyoshi, T.; Ishibashi, Y.; Fukunaka, Y.; Homma, T. Electrochemical quartz crystal microbalance study of Si electrodeposition in ionic liquid. Electrochim. Acta 2012, 100, 236–241. [Google Scholar]
- Nishimura, Y.; Fukunaka, Y.; Miranda, C.R.; Nishida, T.; Nohira, T.; Hagiwara, R. In-situ Raman spectroscopy studies of the electrolyte substrate interface during electrodeposition of silicon in a room temperature ionic liquid. ECS Trans. 2009, 16, 1–6. [Google Scholar]
- Bhatt, A.I.; Best, A.S.; Huang, J.; Hollenkamp, A.F. Application of the N-propyl-N-methyl-pyrrolidinium bis(fluorosulfonyl)imide RTIL containing lithium bis(fluorosulfonyl)imide in ionic liquid based lithium. J. Electrochem. Soc. 2010, 157, A66–A74. [Google Scholar]
- Bozzini, B.; Busson, B.; Humbert, C.; Mele, C.; Raffa, P.; Tadjeddine, A. Investigation of Au electrodeposition from [BMP][TFSA] room-temperature ionic liquid containing K[Au(CN)2] by in situ two-dimensional sum frequency generation spectroscopy. J. Electroanal. Chem. 2011, 661, 20–24. [Google Scholar] [CrossRef]
- Bozzini, B.; Tondo, E.; Bund, A.; Ispas, A.; Mele, C. Electrodeposition of Au from [EMIm][TFSA] room-temperature ionic liquid: An electrochemical and surface-enhanced Raman spectroscopy study. J. Electroanal. Chem. 2011, 651, 1–11. [Google Scholar] [CrossRef]
- Ispas, A.; Adolphi, B.; Bund, A.; Endres, F. On the electrodeposition of tantalum from three different ionic liquids with the bis(trifluoromethyl sulfonyl) amide anion. Phys. Chem. Chem. Phys. 2010, 12, 1793–1803. [Google Scholar] [CrossRef]
- Sano, H.; Sakaebe, H.; Matsumoto, H. In-situ optical microscope morphology observation of lithium electrodeposited in room temperature ionic liquids containing aliphatic quaternary ammonium cation. Electrochemistry 2012, 80, 777–779. [Google Scholar] [CrossRef]
- Sanoa, H.; Sakaebe, H.; Matsumoto, H. Observation of electrodeposited lithium by optical microscope in room temperature ionic liquid-based electrolyte. J. Power Sources 2011, 196, 6663–6669. [Google Scholar] [CrossRef]
- Kondo, H.; Matsumiya, M.; Tsunashima, K.; Kodama, S. Attempts to the electrodeposition of Nd from ionic liquids at elevated temperatures. Electrochim. Acta 2012, 66, 313–319. [Google Scholar] [CrossRef]
- Gasparotto, L.H.S.; Borisenko, N.; Bocchi, N.; el Abedin, S.Z.; Endres, F. In situ STM investigation of the lithium underpotential deposition on Au(111) in the air- and water-stable ionic liquid 1-butyl-1-methylpyrrolidinium bis(trifluoromethylsulfonyl)amide. Phys. Chem. Chem. Phys. 2009, 11, 11140–11145. [Google Scholar]
- Borisenkoa, N.; Ispas, A.; Zschippang, E.; Liu, Q.; Zein el Abedin, S.; Bund, A.; Endres, F. In situ STM and EQCM studies of tantalum electrodeposition from TaF5 in the air- and water-stable ionic liquid 1-butyl-1-methylpyrrolidinium. Electrochim. Acta 2009, 54, 1519–1528. [Google Scholar] [CrossRef]
- Ruther, T.; Huang, J.; Hollenkamp, A.F. A new family of ionic liquids based on N,N-dialkyl-3-azabicyclo[3.2.2]nonanium cations organic plastic crystal behaviour and highly reversible lithium metal electrodeposition. Chem. Commun. 2007, 48, 5226–5228. [Google Scholar] [CrossRef]
- Traore, Y.; Legeai, S.; Dilibertob, S.; Arrachart, G.; Pellet-Rostaing, S.; Draye, M. New insight into indium electrochemistry in a Tf2N-based room-temperature ionic liquid. Electrochim. Acta 2011, 58, 532–540. [Google Scholar]
- Zein el Abedin, S.; Endres, F. Electrodeposition of metals and semiconductors in air-and water-stable ionic liquids. Chem. Phys. Chem. 2006, 7, 58–61. [Google Scholar]
- Cheek, G.T.; O’Grady, W.E.; Zein el Abedin, S.; Moustafa, E.M.; Endres, F. Studies on the electrodeposition of magnesium in ionic liquids. J. Electrochem. Soc. 2008, 155, D91–D95. [Google Scholar] [CrossRef]
- Fournier, C.; Favier, F. Zn, Ti and Si nanowires by electrodeposition in ionic liquid. Electrochem. Commun. 2011, 13, 1252–1255. [Google Scholar] [CrossRef]
- Zein el Abedin, S.; Welz-Biermann, U.; Endres, F. A study on the electrodeposition of tantalum on NiTi alloy in an ionic liquid and corrosion behaviour of the coated alloy. Electrochem. Commun. 2005, 7, 941–946. [Google Scholar] [CrossRef]
- Arnould, C.; Delhalle, J.; Mekhalif, Z. Electrodeposition from ionic liquid of 2D ordered Ta2O5on titanium substrate through a polystyrene template. J. Electrochem. Soc. 2009, 156, K186–K190. [Google Scholar] [CrossRef]
- Zein el Abedin, S.; Farag, H.K.; Moustafa, E.M.; Welz-Biermanny, U.; Endres, F. Electroreduction of tantalum fluoride in a room temperature ionic liquid at variable temperatures. Phys. Chem. Chem. Phys. 2005, 7, 2333–2339. [Google Scholar] [CrossRef]
- Arnould, C.; Delhalle, J.; Mekhalif, Z. Multifunctional hybrid coating on titanium towards hydroxyapatite growth electrodeposition of tantalum and its molecular functionalization with organophosphonic acids films. Electrochim. Acta 2008, 53, 5632–5638. [Google Scholar] [CrossRef]
- Zein el Abedin, S.; Borissenko, N.; Endres, F. Electrodeposition of nanoscale silicon in a room temperature ionic liquid. Electrochem. Commun. 2004, 6, 510–514. [Google Scholar] [CrossRef]
- Borisenko, N.; Zein el Abedin, S.; Endres, F. In-situ STM investigation of gold reconstruction and of silicon electrodeposition on Au(111) in the room temperature ionic liquid 1-butyl-1-methylpyrrolidinium bis(trifluoromethylsulfonyl)imide. J. Phys. Chem. B 2006, 110, 6250–6256. [Google Scholar] [CrossRef]
- Zein el Abedin, S.; Saad, A.Y.; Farag, H.K.; Borisenko, N.; Liu, Q.X.; Endres, F. Electrodeposition of selenium, indium and copper in an air- and water-stable ionic liquid at variable temperatures. Electrochim. Acta 2007, 52, 2746–2754. [Google Scholar]
- Yu, Y.; Sun, Q.; Liu, X.; Wu, H.; Zhou, T.; Shi, G. Size controllable gold platinum alloy nanoparticles on nine functionalized ionic liquid surfaces and their application as electrocatalysts for hydrogen peroxide reduction. Chem. Eur. J. 2011, 17, 11314–11323. [Google Scholar] [CrossRef]
- Aldous, L.; Silvester, D.S.; Pitner, W.R.; Compton, R.G.; Lagunas, M.C.; Hardacre, C. Voltammetric studies of gold, protons and [HCl2]− in ionic liquids. J. Phys. Chem. C 2007, 111, 8496–8503. [Google Scholar]
- Kaminska, I.; Niedziolka-Jonsson, J.; Roguska, A.; Opallo, M. Electrodeposition of gold nanoparticles at a solidionic liquidaqueous electrolyte three phase junction. Electrochem. Commun. 2010, 12, 1742–1745. [Google Scholar] [CrossRef]
- Ding, J.; Wu, J.; MacFarlane, D.; Price, W.E.; Wallace, G. Induction of titanium reduction using pyrrole and polypyrrole in the ionic liquid ethyl-methyl-imidazolium. Electrochem. Commun. 2008, 10, 217–221. [Google Scholar] [CrossRef]
- Xiao, F.; Mo, Z.; Zhao, F.; Zeng, B. Ultrasonic electrodeposition of gold platinum alloy nanoparticles on multi walled carbon nanotubes ionic liquid composite film and their electrocatalysis towards the oxidation of nitrite. Electrochem. Commun. 2008, 10, 1740–1743. [Google Scholar] [CrossRef]
- Mo, Z.; Zhao, F.; Xiao, F.; Zeng, B. Preparation and characterization of AuPt alloy nanoparticle–multi-walled carbon nanotube–ionic liquid composite film for electrocatalytic oxidation of cysteine. J. Solid-State Electrochem. 2010, 14, 1615–1620. [Google Scholar] [CrossRef]
- Xiao, F.; Zhao, F.; Zhang, Y.; Guo, G.; Zeng, B. Ultrasonic electrodeposition of gold platinum alloy nanoparticles on ionic liquid chitosan composite film and their application in fabricating nonenzyme hydrogen peroxide sensors. J. Phys. Chem. C 2009, 113, 849–855. [Google Scholar]
- Zhao, F.; Xiao, F.; Zeng, B. Electrodeposition of PtCo alloy nanoparticles on inclusion complex film of functionalized cyclodextrin-ionic liquid and their application in glucose sensing. Electrochem. Commun. 2010, 12, 168–171. [Google Scholar] [CrossRef]
- Xiao, F.; Zhao, F.; Mei, D.; Mo, Z.; Zeng, B. Nonenzymatic glucose sensor based on ultrasonic-electrodeposition of bimetallic PtM (M = Ru, Pd and Au) nanoparticles on carbon nanotubes–ionic liquid composite film. Biosens. Bioelectron. 2009, 24, 3481–3486. [Google Scholar] [CrossRef]
- Al-Salman, R.; Zein el Abedinw, S.; Endres, F. Electrodeposition of Ge, Si and SixGe1-x from an air- and water-stable ionic liquid. Phys. Chem. Chem. Phys. 2008, 10, 4650–4657. [Google Scholar] [CrossRef]
- Al-Salman, R.; Mallet, J.; Molinari, M.; Fricoteaux, P.; Martineau, F.; Troyon, M.; Zein el Abedinw, S.; Endres, F. Template assisted electrodeposition of germanium and silicon nanowires in an ionic liquid. Phys. Chem. Chem. Phys. 2008, 10, 6233–6237. [Google Scholar]
- Liu, X.; Zhang, Y.; Ge, D.; Zhao, J.; Li, Y.; Endres, F. Three-dimensionally ordered macroporous silicon films made by electrodeposition from an ionic liquid. Phys. Chem. Chem. Phys. 2012, 14, 5100–5105. [Google Scholar] [CrossRef]
- Monzon, L.M.A.; Byrne, F.; Coey, J.M.D. Gold electrodeposition in organic media. J. Electroanal. Chem. 2011, 657, 54–60. [Google Scholar] [CrossRef]
- Safavi, A.; Farjami, F. Electrodeposition of gold platinum alloy nanoparticles on ionic liquid chitosan. Biosens. Bioelectron. 2011, 26, 2547–2552. [Google Scholar]
- Jayakumar, M.; Venkatesan, K.A.; Srinivasan, T.G. Electrochemical behavior of fission palladium in 1-butyl-3-methylimidazolium chloride. Electrochim. Acta 2007, 52, 7121–7127. [Google Scholar] [CrossRef]
- Jayakumar, M.; Venkatesan, K.A.; Srinivasan, T.G.; Vasudeva Rao, P.R. Electrochemical behavior of ruthenium(III), rhodium(III) and palladium(II) in 1-butyl-3-methylimidazolium chloride ionic liquid. Electrochim. Acta 2009, 54, 6747–6755. [Google Scholar] [CrossRef]
- Jou, L.S.; Chang, J.K.; Twhang, T.J.; Sun, I.W. Electrodeposition of palladium-copper films from 1-ethyl-3-methylimidazolium chloride-tetrafluoroborate ionic liquid on indium-tin oxide electrodes. J. Electrochem. Soc. 2009, 156, D193–D197. [Google Scholar] [CrossRef]
- Hsiu, S.I.; Tai, C.C.; Sun, I.W. Electrodeposition of palladium-indium from 1-ethyl-3-methylimidazolium chloride tetrafluoroborate ionic liquid. Electrochim. Acta 2006, 51, 2607–2613. [Google Scholar] [CrossRef]
- Tai, C.C.; Su, F.Y.; Sun, I.W. Electrodeposition of palladium-silver in a Lewis basic 1-ethyl-3-methylimidazolium chloride-tetrafluoroborate ionic liquid. Electrochim. Acta 2005, 50, 5504–5509. [Google Scholar] [CrossRef]
- Jou, L.H.; Chang, J.K.; Whang, T.J.; Sun, I.W. Electrodeposition of palladium-tin alloys from 1-ethyl-3-methylimidazolium chloride-tetrafluoroborate ionic liquid for ethanol electro oxidation. J. Electrochem. Soc. 2010, 157, D443–D449. [Google Scholar] [CrossRef]
- Jayakumar, M.; Venkatesan, K.A.; Sudha, R.; Srinivasan, T.G.; Vasudeva Rao, P.R. Electrodeposition of ruthenium, rhodium and palladium from nitric acid and ionic liquid media Recovery and surface morphology of the deposits. Mater. Chem. Phys. 2011, 128, 141–150. [Google Scholar] [CrossRef]
- Vasudeva Rao, P.R.; Venkatesan, K.A.; Srinivasan, T.G. Studies on applications of room temperature ionic liquids. Prog. Nucl. Energy 2008, 50, 449–455. [Google Scholar] [CrossRef]
- Pradhan, D.; Reddy, R.G. Electrochemical production of Ti–Al alloys using TiCl4–AlCl3–1-butyl-3-methyl imidazolium chloride (BMImCl) electrolytes. Electrochim. Acta 2009, 54, 1874–1880. [Google Scholar] [CrossRef]
- Pradhan, D.; Reddy, R.G.; Lahiri, A. Low temperature production of Ti-Al alloys using ionic liquid electrolytes effect of process variables on current density, current efficiency and deposit morphology. Metall. Mater. Trans. B 2009, 40B, 114–122. [Google Scholar] [CrossRef]
- Doyle, K.P.; Lang, C.M.; Kim, K.; Kohl, P.A. Dentrite free electrochemical deposition of Li–Na alloys from an ionic liquid electrolyte. J. Electrochem. Soc. 2006, 153, A1353–A1357. [Google Scholar] [CrossRef]
- Zhang, X.Y.; Hua, Y.X.; Xu, C.Y.; Zhang, Q.B.; Cong, X.B.; Xu, N. Direct electrochemical reduction of titanium dioxide in Lewis basic AlCl3-1-butyl-3-methylimidizolium ionic liquid. Electrochim. Acta 2011, 56, 8530–8533. [Google Scholar] [CrossRef]
- Abbott, A.P.; Eardley, C.A.; Farley, N.R.S.; Griffith, G.A.; Pratt, A. Electrodeposition of aluminium and aluminium-platinum alloys from AlCl-3-benzyltrimethylammonium chloride room temperature ionic liquids. J. Appl. Electrochem. 2001, 31, 1345–1350. [Google Scholar] [CrossRef]
- Piersma, B.J.; Ryan, D.B.; Schumacher, E.R.; Rieche, T.L. Electrodeposition and stripping of lithium and sodium on inert electrodes in room temperature chloroaluminate molten salts. J. Electrochem. Soc. 1996, 143, 908–913. [Google Scholar] [CrossRef]
- Gasparotto, L.H.S.; Prowald, A.; Borisenko, N.; Zein el Abedin, S.; Garsuch, A.; Endres, F. Electrochemical synthesis of macroporous alumunium films and their behaviour towards lithium deposition-stripping. J. Power Sources 2011, 196, 2879–2883. [Google Scholar] [CrossRef]
- Tsuda, T.; Hussey, C.L.; Stafford, G.R.; Bonevich, J.E. Electrochemistry of titanium and the electrodeposition of Al–Ti alloys in the lewis acidic aluminum chloride1-ethyl-3-methylimidazolium chloride melt. J. Electrochem. Soc. 2003, 150, C234–C243. [Google Scholar]
- Tsuda, T.; Arimoto, S.; Kuwabata, S.; Hussey, C.L. Electrodeposition of Al–Mo–Ti ternary alloys in the lewis acidic alumunium chloride-1-ethyl-3-methylimidazolium chloride room temperature ionic liquid. J. Electrochem. Soc. 2008, 155, D256–D262. [Google Scholar] [CrossRef]
- Oyama, T.; Okajima, T.; Ohsaka, T. Electrodeposition of gold at glassy carbon electrodes in room temperature ionic liquids. J. Electrochem. Soc. 2007, 154, D322–D327. [Google Scholar] [CrossRef]
- NuLi, Y.; Yang, J.; Wang, J.; Xu, J.; Wang, P. Electrochemical magnesium deposition and dissolution with high efficiency in ionic liquid. Electrochem. Solid-State Lett. 2005, 8, C166–C169. [Google Scholar] [CrossRef]
- NuLi, Y.; Yang, J.; Wang, P. Electrodeposition of magnesium film from BMIMBF4 ionic liquid. Appl. Surf. Sci. 2006, 252, 8086–8090. [Google Scholar] [CrossRef]
- NuLi, Y.; Yang, J.; Wu, R. Reversible deposition and dissolution of magnesium from BMIMBF4 ionic liquid. Electrochem. Commun. 2005, 7, 1105–1110. [Google Scholar] [CrossRef]
- Feng, Z.; NuLi, Y.; Wang, J.; Yang, J. Study of key factors influencing electrochemical reversibility of magnesium deposition and dissolution. J. Electrochem. Soc. 2006, 153, C689–C693. [Google Scholar] [CrossRef]
- He, P.; Liu, H.; Li, Z.; Li, J. Electrodeposition of platinum in room-temperature ionic liquids and electrocatalytic effect on electro-oxidation of methanol. J. Electrochem. Soc. 2005, 152, E146–E153. [Google Scholar] [CrossRef]
- Andriyko, Y.; Andriiko, A.; Babushkina, O.B.; Nauer, G.E. Electrochemistry of TiF4 in 1-butyl-2,3-dimethylimidazolium tetrafluoroborate. Electrochim. Acta 2010, 55, 1081–1089. [Google Scholar] [CrossRef]
- Zhang, D.; Chang, W.C.; Okajima, T.; Ohsaka, T. Electrodeposition of platinum nanoparticles in a room temperature ionic liquid. Langmuir 2011, 27, 14662–14668. [Google Scholar] [CrossRef]
- Dong, S.; Li, N.; Zhang, P.; Li, Y.; Chen, Z.; Huang, T. Fabrication of hemoglobin ionic liquid modified carbon paste electrode based on the electrodeposition of gold nanoparticles CdS quantum dots and its electrochemical application. Electroanalysis 2012, 24, 1554–1560. [Google Scholar]
- Mukhopadhyay, I.; Aravinda, C.L.; Borissov, D.; Freyland, W. Electrodeposition of Ti from TiCl4 in the ionic liquid 1-methyl-3-butyl-imidazolium bis(trifluoro methyl sulfone) imide at room temperature Study on phase formation by in-situ electrochemical scan. Electrochim. Acta 2005, 50, 1275–1281. [Google Scholar] [CrossRef]
- Mukhopadhyay, I.; Freyland, W. Electrodeposition of Ti nanowires on highly oriented pyrolytic graphite from an ionic liquid at room temperature. Langmuir 2003, 19, 1951–1953. [Google Scholar]
- De Sa, A.I.; Eugénio, S.; Quaresma, S.; Rangel, C.M.; Vilar, R. Electrodeposition of gold thin films from 1-butyl-1-methylpyrrolidinium. Thin Solid Films 2011, 519, 6278–6283. [Google Scholar] [CrossRef]
- Huang, H.Y.; Chen, P.Y. PdNi and Pd coated electrodes prepared by electrodeposition from ionic liquid for nonenzymatic electrochemical determination of ethanol and glucose in alkaline media. Talanta 2010, 83, 379–385. [Google Scholar] [CrossRef]
- Huang, H.Y.; Chen, P.Y. Voltammetric behavior of Pd(II) and Ni(II) ions and electrodeposition of PdNi bimetal in N-butyl-N-methylpyrrolidinium dicyanamide ionic liquid. Electrochim. Acta 2011, 56, 2336–2343. [Google Scholar] [CrossRef]
- Fu, C.; Zhou, H.; Xie, D.; Sun, L.; Yin, Y.; Chen, J.; Kuang, Y. Electrodeposition of gold nanoparticles from ionic liquid microemulsion. Colloid Polym. Sci. 2010, 288, 1097–1103. [Google Scholar] [CrossRef]
- Yu, P.; Yan, J.; Zhang, J.; Mao, L. Cost effective electrodeposition of platinum nanoparticles with ionic liquid droplet confined onto electrode surface as micro media. Electrochem. Commun. 2007, 9, 1139–1144. [Google Scholar] [CrossRef]
- Safavi, A.; Maleki, N.; Tajabadi, F.; Farjami, E. High electrocatalytic effect of palladium nanoparticle arrays electrodeposited on carbon ionic liquid electrode. Electrochem. Commun. 2007, 9, 1963–1968. [Google Scholar] [CrossRef]
- Huang, J.F.; Sun, I.W. Electrodeposition of PtZn in a lewis acidic ZnCl2–1-ethyl-3-methylimidazolium chloride ionic liquid. Electrochim. Acta 2004, 49, 3251–3258. [Google Scholar]
- Huang, J.F.; Sun, I.W. Formation of nanoporous platinum by selectiv anodic dissolution of PtZn surface alloy in a Lewis acidic zinc chloride-1-ethyl-3-methylimidazolium chloride ionic liquid. Chem. Mater. 2004, 16, 1829–1831. [Google Scholar] [CrossRef]
- Huan, J.F.; Chen, H.Y. Heat-assisted electrodissolution of platinum in an ionic liquid. Angew. Chem. Int. Ed. 2012, 51, 1684–1688. [Google Scholar] [CrossRef]
- Yang, M.H.; Yang, M.C.; Sun, I.W. Electrodeposition of indium antimonide from the water-stable 1-ethyl-3-methylimidazolium chloride-tetrafluoroborate ionic liquid. J. Electrochem. Soc. 2003, 150, C544–C548. [Google Scholar] [CrossRef]
- Xiao, F.; Zhao, F.; Deng, L.; Zeng, B. High electrocatalytic effect of PtAuPd ternary alloy nanoparticles electrodeposited on mercapto ionic liquid film. Electrochem. Commun. 2010, 12, 620–623. [Google Scholar] [CrossRef]
- Giridhar, P.; Venkatesan, K.A.; Srinivasan, T.G.; Vasudeva Rao, P.R. Extraction of fission palladium by Aliquat 336 and electrochemical studies on direct recovery from ionic liquid phase. Hydrometallurgy 2006, 81, 30–39. [Google Scholar]
- Park, J.; Kwon, K.; Kim, H.; Lee, C.K. Electrodeposition of silicon from 1-butyl-3-methyl-pyridinium bis(trifluromethylsulfonyl) imide ionic liquid. Int. J. Electrochem. Sci. 2013, 8, 4206–4214. [Google Scholar]
- Lee, C.K.; Park, J.; Park, J. Recovery Method of Elemental Silicon by Electrolysis in Non-Aqueous Electrolyte from Silicon Sludge. KR Patent 1340601, 5 December 2013. [Google Scholar]
- Park, J.; Lee, C.K. Preparation of magnesium thin film by non-aqueous electrolysis at room temperature. Int. J. Precis. Eng. Manuf. 2014, 15, 553–556. [Google Scholar] [CrossRef]
- Kim, M.A.; Lee, J.C.; Kim, C.K.; Kim, M.S.; Kim, B.S.; Yoo, K. Solvent extraction of platinum group metals from the leach liquor of spent automotive catalyst. J. Korean Inst. Resour. Recycl. 2006, 15, 3–10. [Google Scholar]
© 2014 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Park, J.; Jung, Y.; Kusumah, P.; Lee, J.; Kwon, K.; Lee, C.K. Application of Ionic Liquids in Hydrometallurgy. Int. J. Mol. Sci. 2014, 15, 15320-15343. https://doi.org/10.3390/ijms150915320
Park J, Jung Y, Kusumah P, Lee J, Kwon K, Lee CK. Application of Ionic Liquids in Hydrometallurgy. International Journal of Molecular Sciences. 2014; 15(9):15320-15343. https://doi.org/10.3390/ijms150915320
Chicago/Turabian StylePark, Jesik, Yeojin Jung, Priyandi Kusumah, Jinyoung Lee, Kyungjung Kwon, and Churl Kyoung Lee. 2014. "Application of Ionic Liquids in Hydrometallurgy" International Journal of Molecular Sciences 15, no. 9: 15320-15343. https://doi.org/10.3390/ijms150915320
APA StylePark, J., Jung, Y., Kusumah, P., Lee, J., Kwon, K., & Lee, C. K. (2014). Application of Ionic Liquids in Hydrometallurgy. International Journal of Molecular Sciences, 15(9), 15320-15343. https://doi.org/10.3390/ijms150915320






