Separators for Li-Ion and Li-Metal Battery Including Ionic Liquid Based Electrolytes Based on the TFSI− and FSI− Anions
Abstract
:1. Introduction
2. Results and Discussion
2.1. Separator Characterization
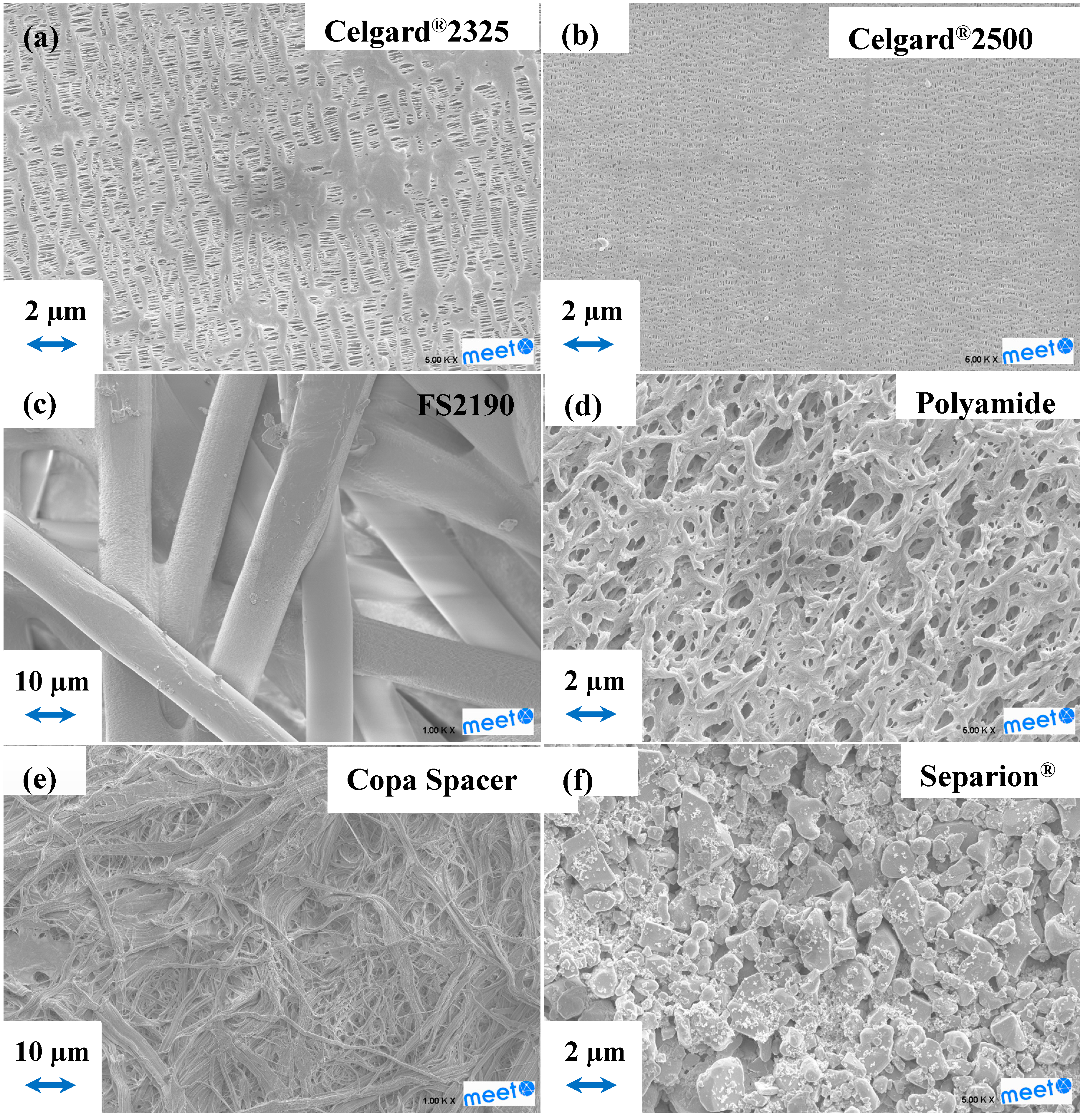
| Separator | Composition | Thickness (µm) | Gurley Number (s) |
|---|---|---|---|
| Celgard®2325 (Polypore) | PP/PE/PP | 27 | 570.0 |
| Celgard®2500 (Polypore) | PP | 27 | 180.1 |
| FS2190 (Freudenberg and Co.KG) | PP | 176 | – |
| Polyamide 0.2 µm (Sartotius Stedium Biotech GmbH) | Polyamide | 116 | 31.9 |
| Copa Spacer (Spez. Papierfabrik Oberschmitt GmbH) | Cellulose | 50 | 6.5 |
| Separion® (Evonik) | Ceramic on PET | 28 | 22.8 |
| GF/C (Whatman) | Glass fiber | 283 | 1.0 |
| GF/F (Whatman) | Glass fiber | 359 | 2.3 |
2.2. Wetting of the Separators
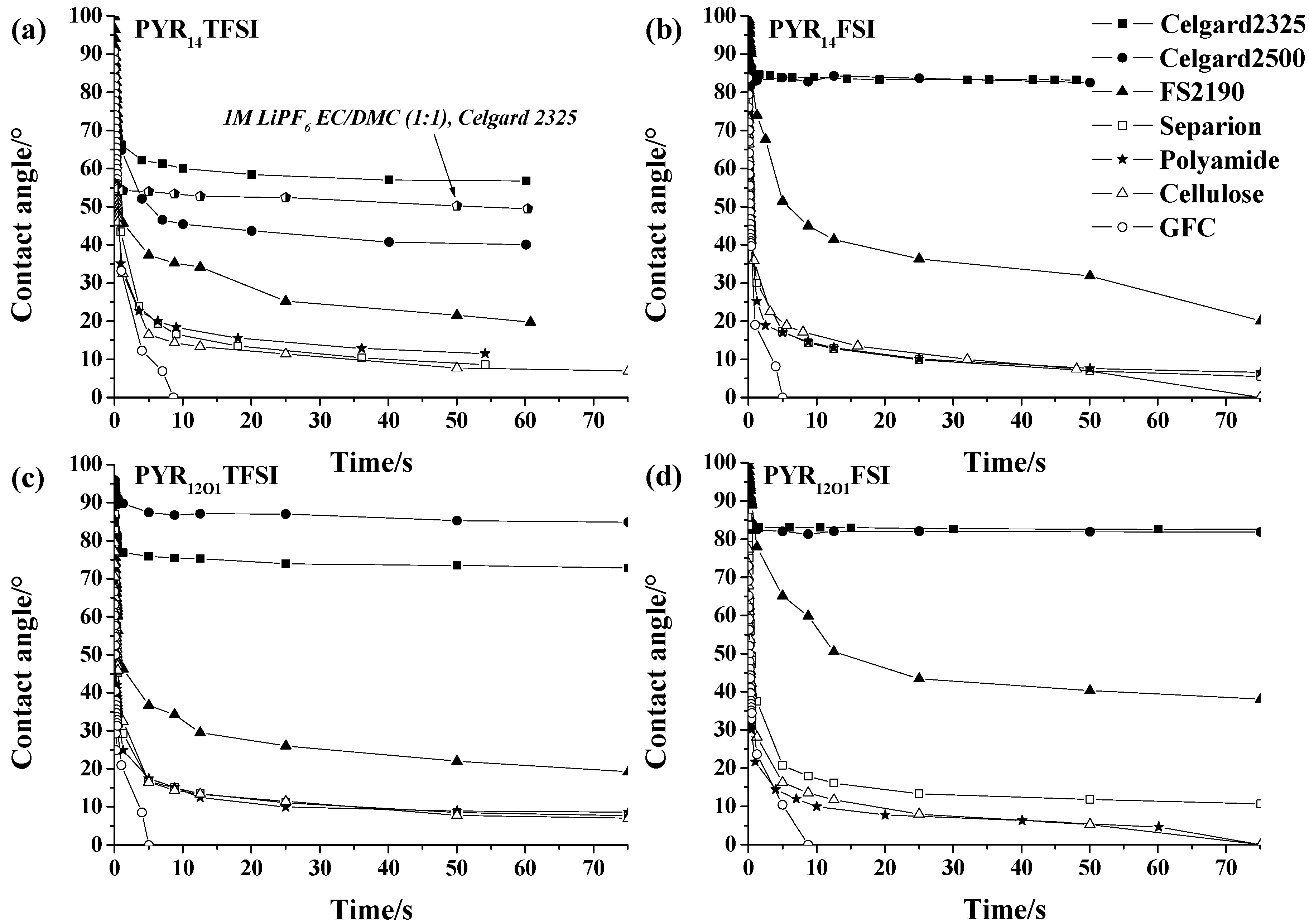
2.3. Influence of the Separator on the Conductivity
| Separator | PYR14TFSI | PYR1(2O1)TFSI | PYR14FSI | PYR1(2O1)FSI |
|---|---|---|---|---|
| Celgard®2325 | 11 | 27 * | / | / |
| Celgard®2500 | 5 | 36 * | / | / |
| Freudenberg FS2190 | 3 | 4 | 2 | 3 |
| Polyamide | 6 | 3 | 2 | 3 |
| Copa Spacer | 3 | 4 | 3 | 4 |
| Separion® | 9 | 10 | 7 | 11 |
| Whatman®GF/C | 1 | 1 | 1 | 1 |
| Whatman®GF/F | 1 | 1 | 1 | 1 |
2.4. Static Evolution of the Solid Electrolyte Interphase (SEI) Resistance
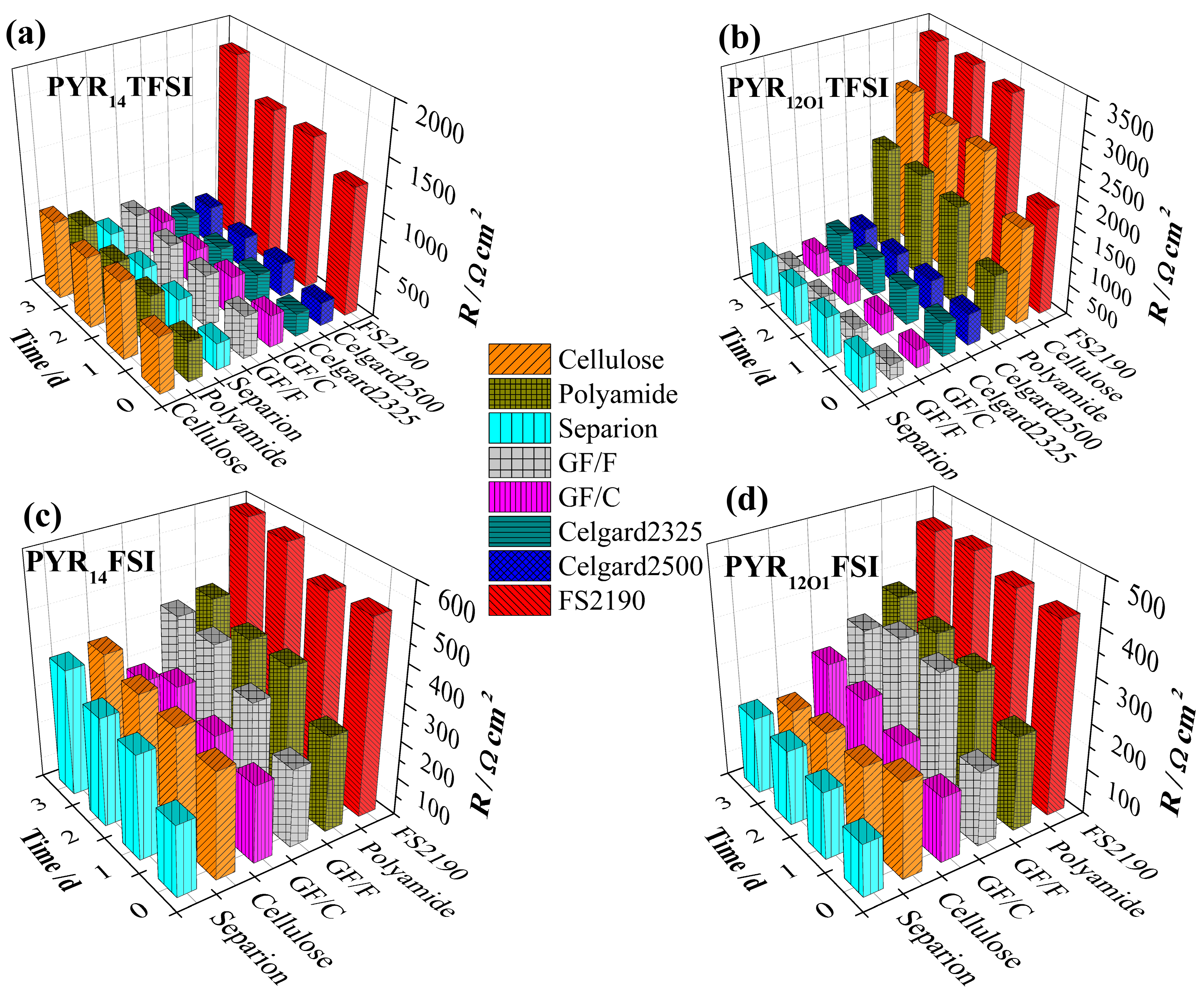
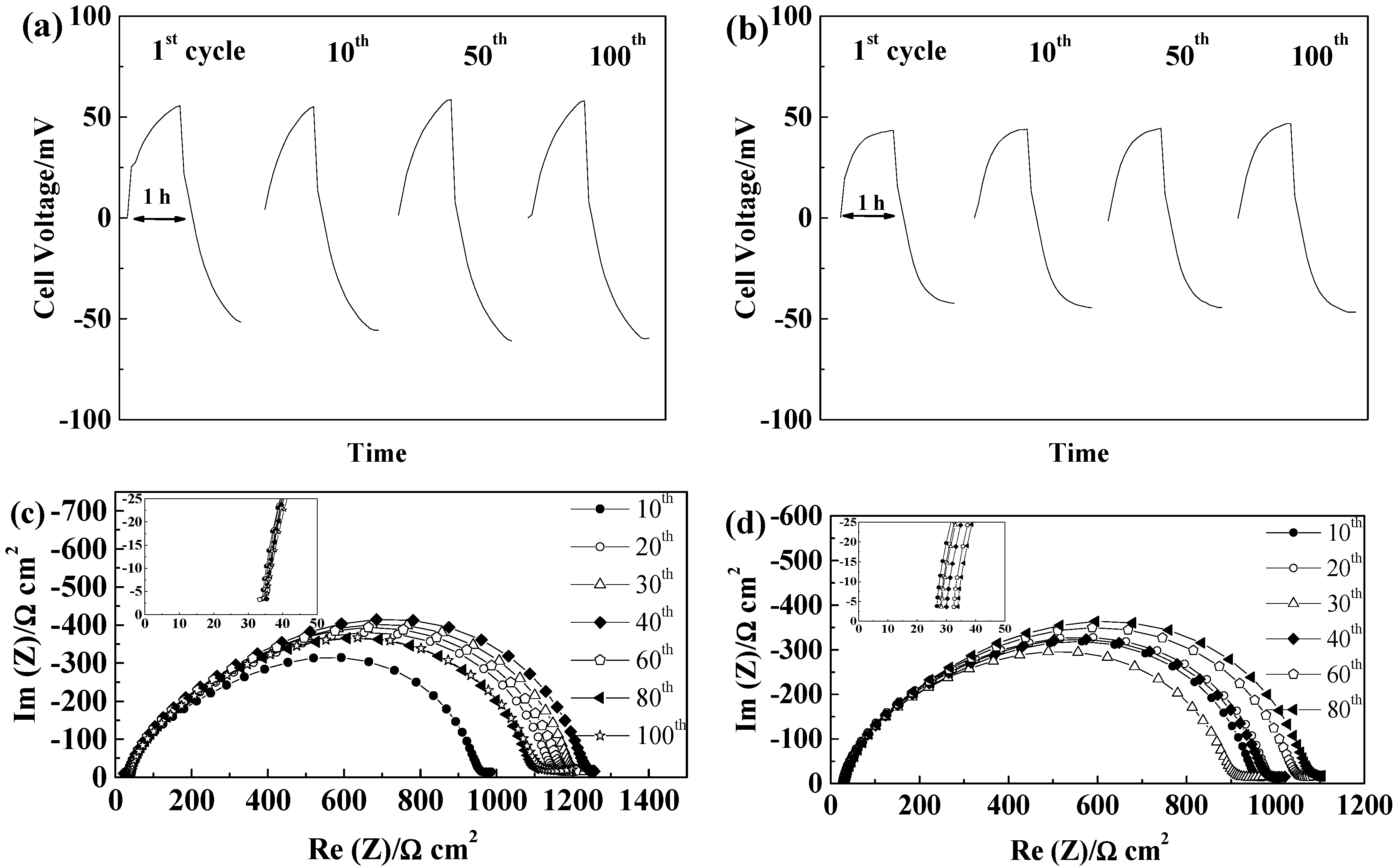
2.5. Lithium Plating/Stripping Tests
2.5.1. Glass Fiber Separators
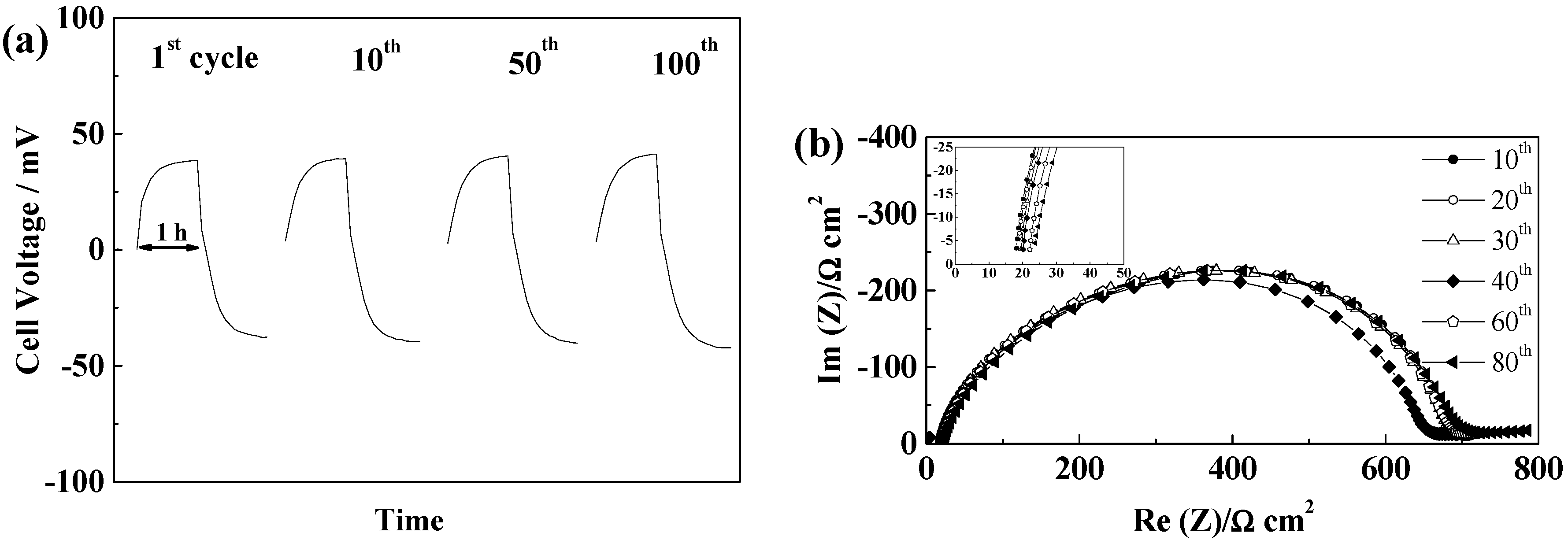
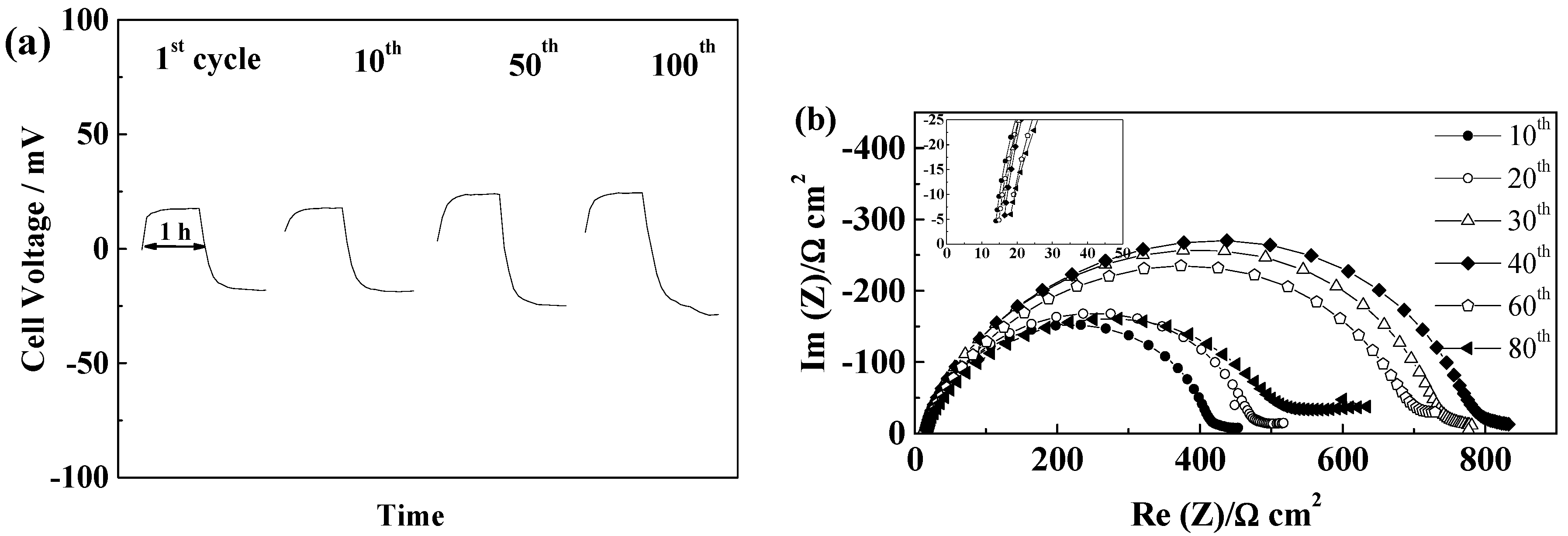
2.5.2. Celgard®2500 and Celgard®2325
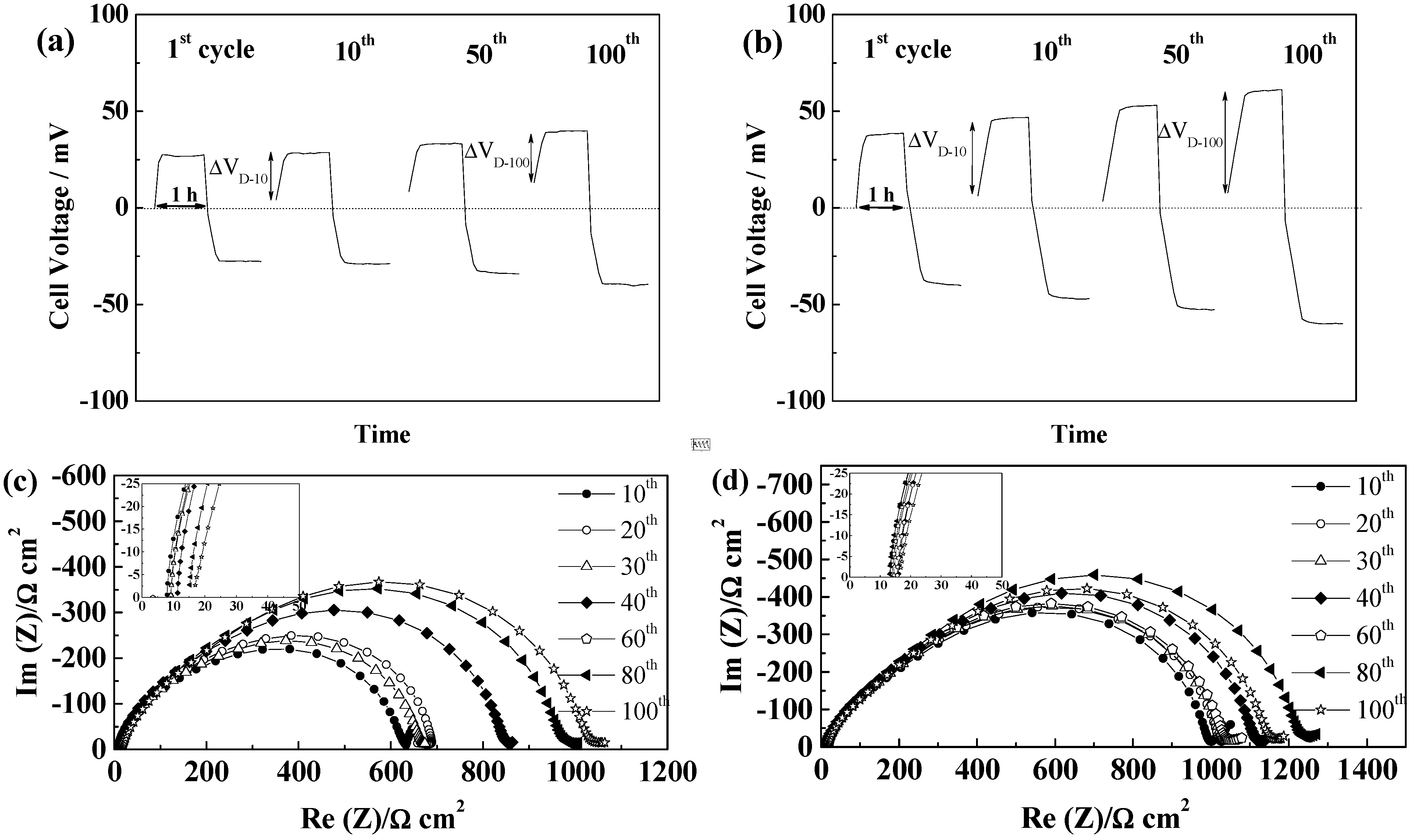
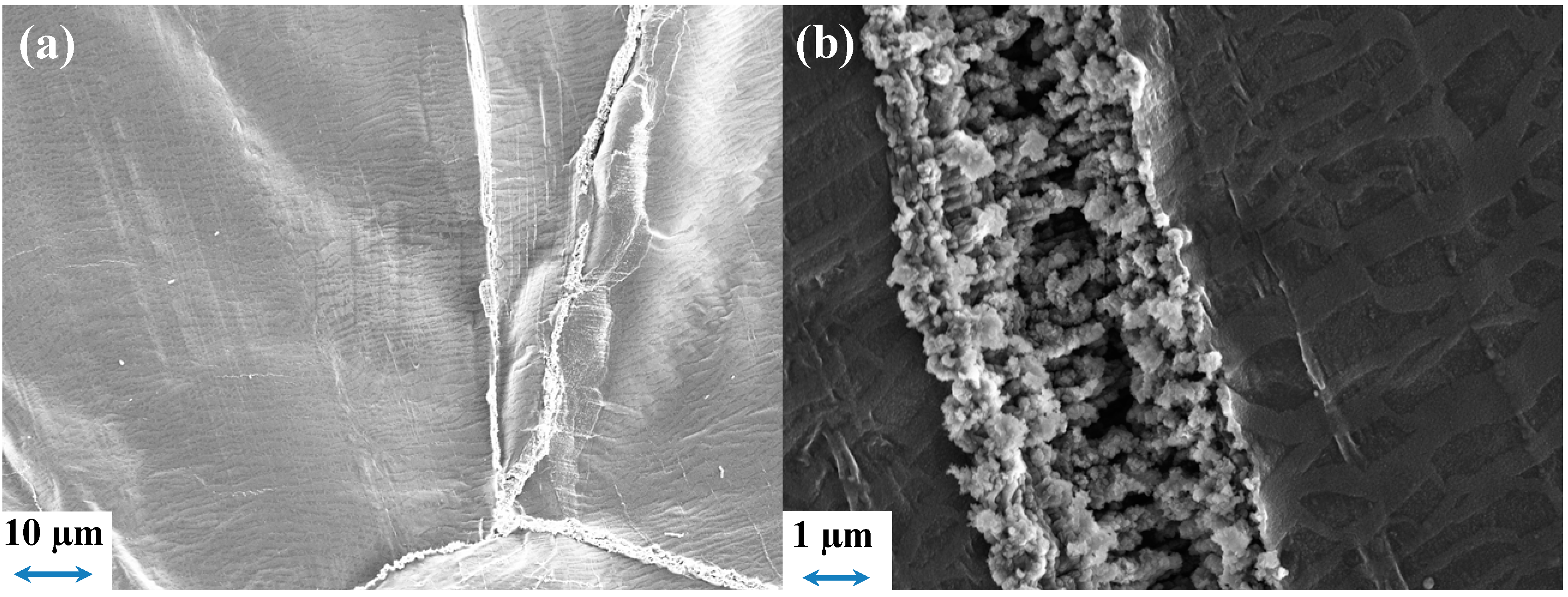
2.5.3. Freudenberg FS2190
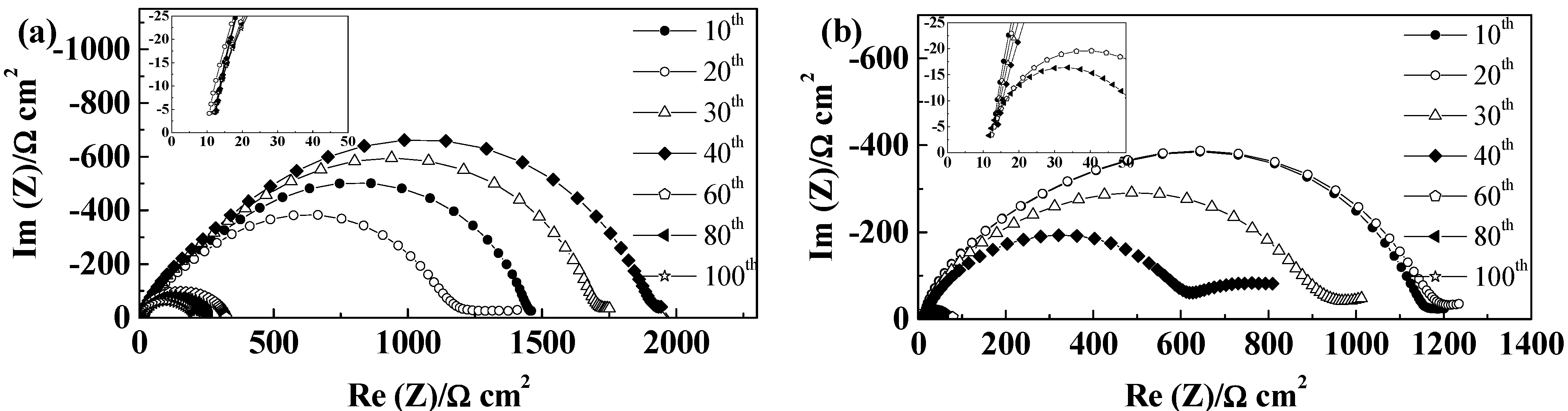
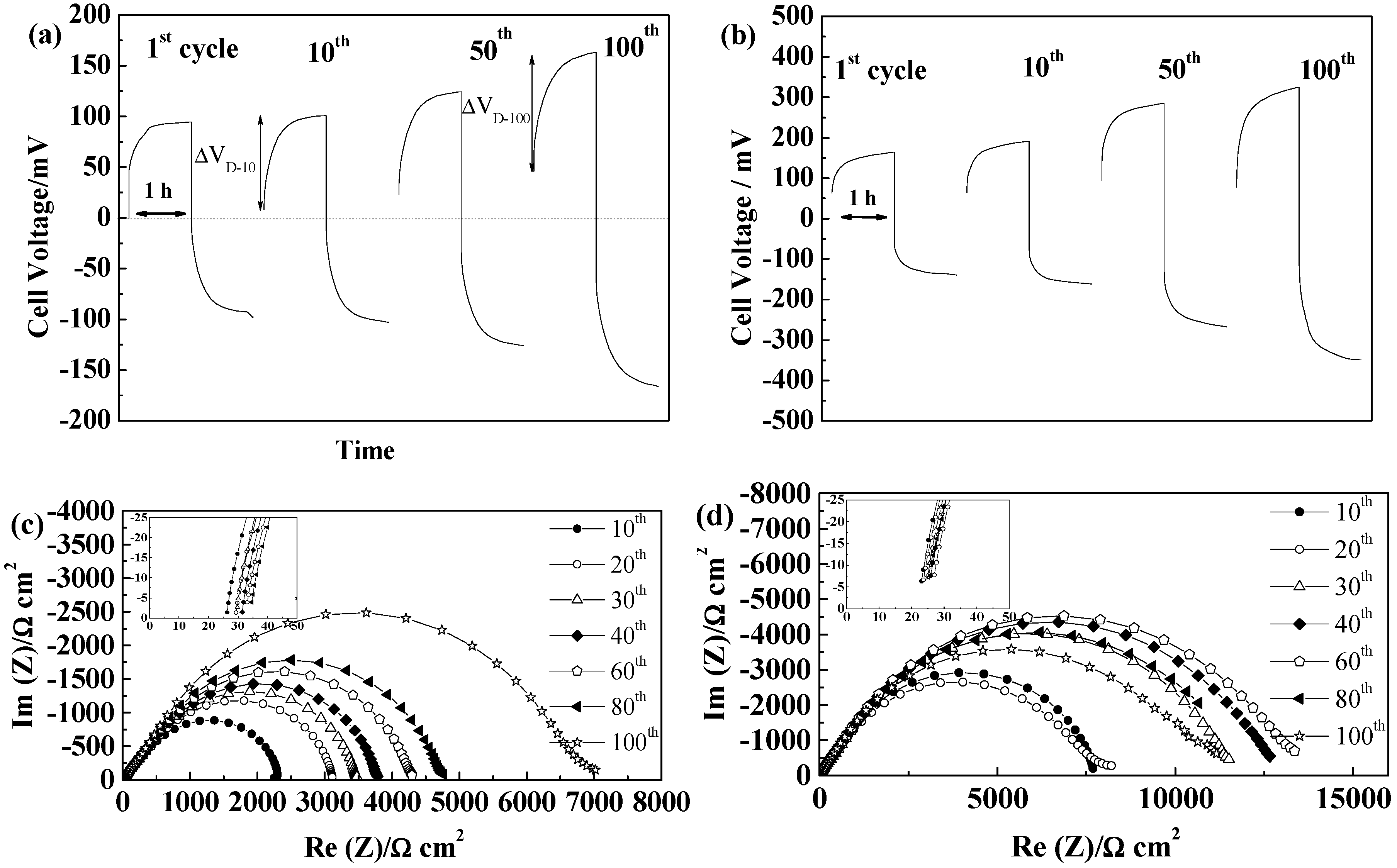
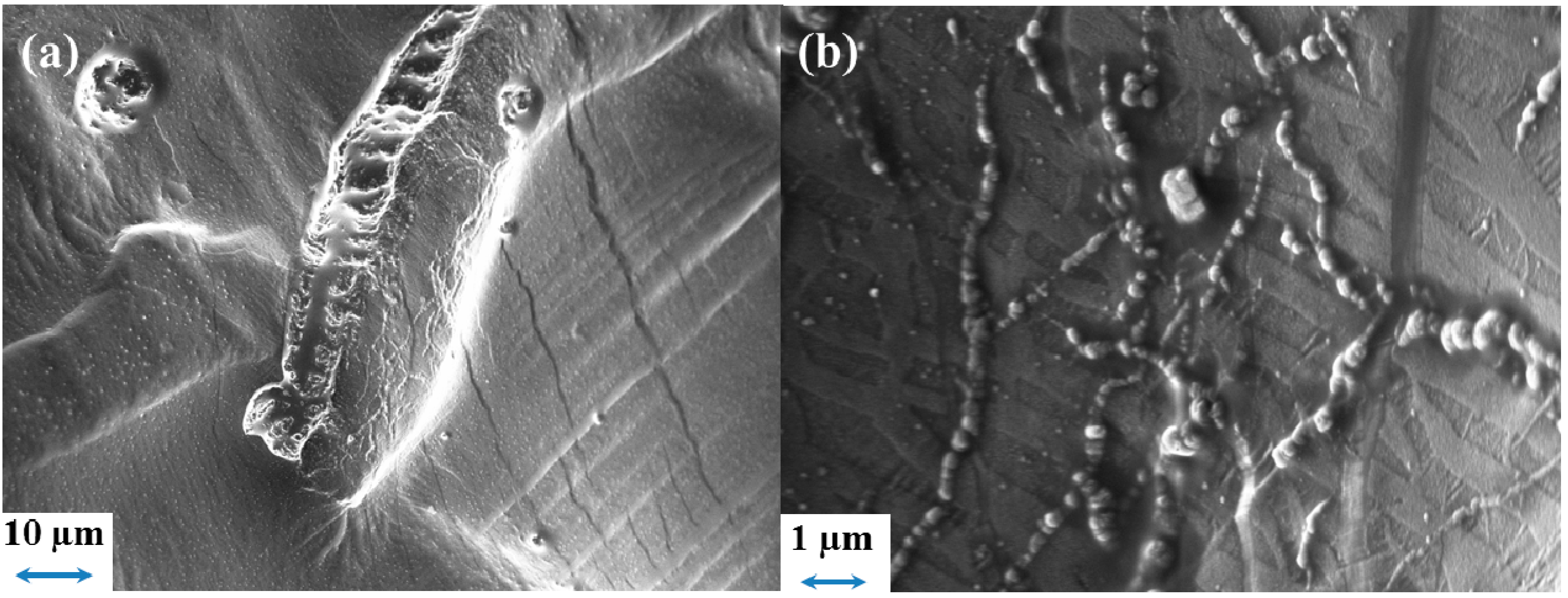
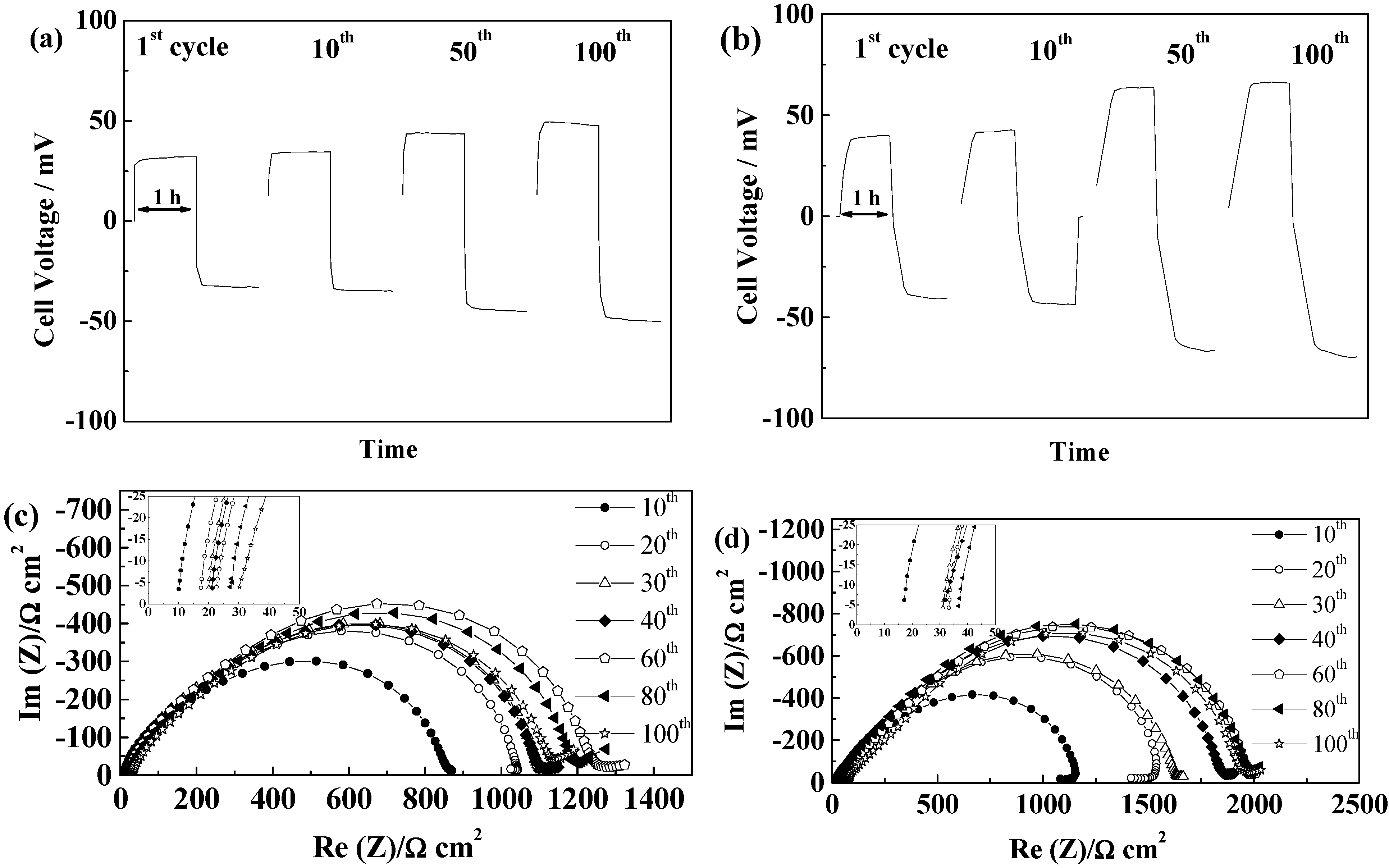
2.5.4. Separion®
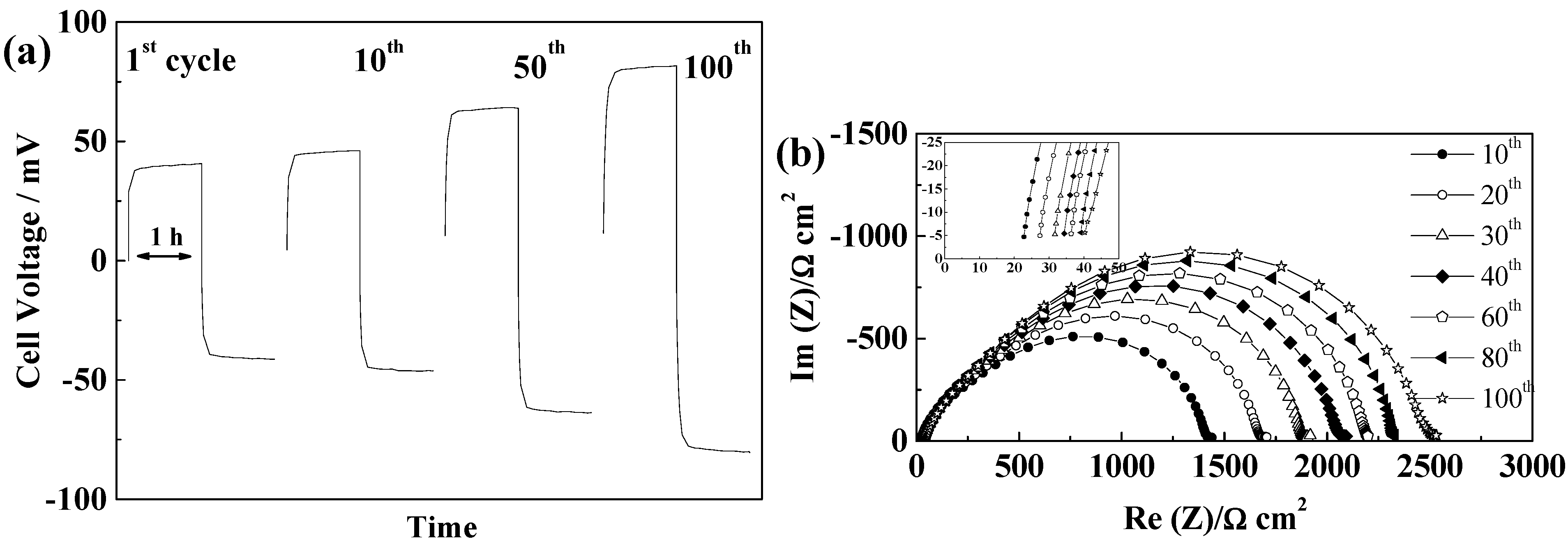
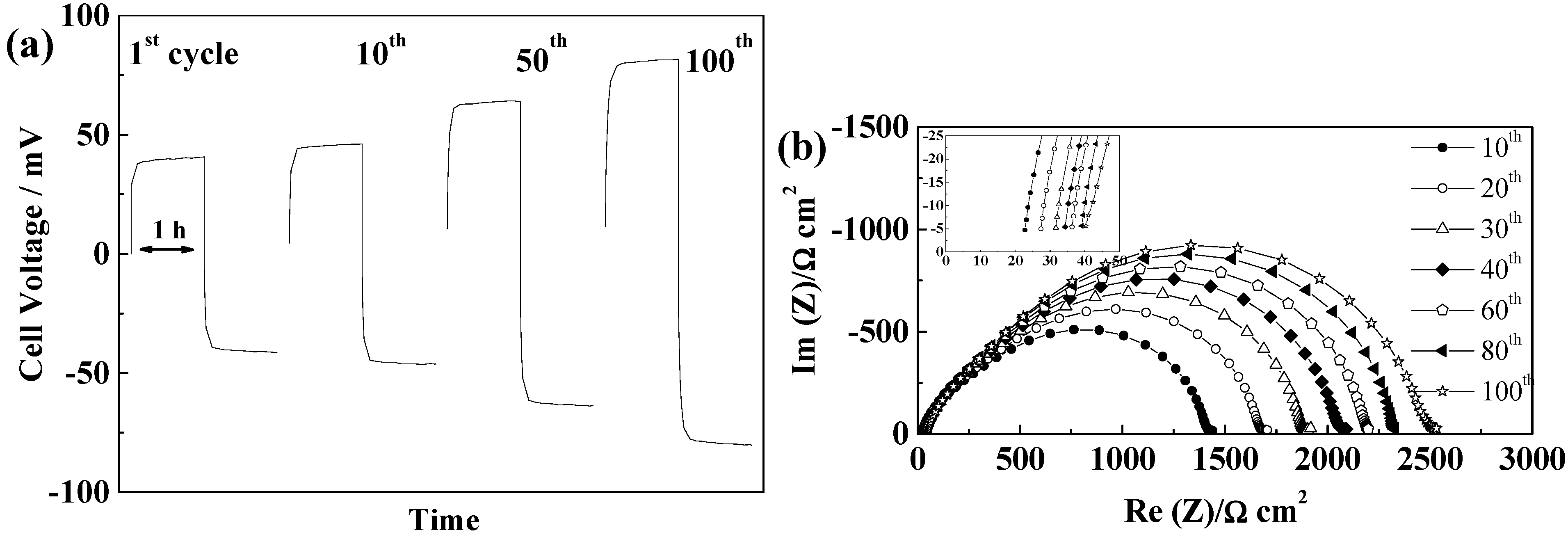
2.5.5. Cellulose “Copa Spacer”
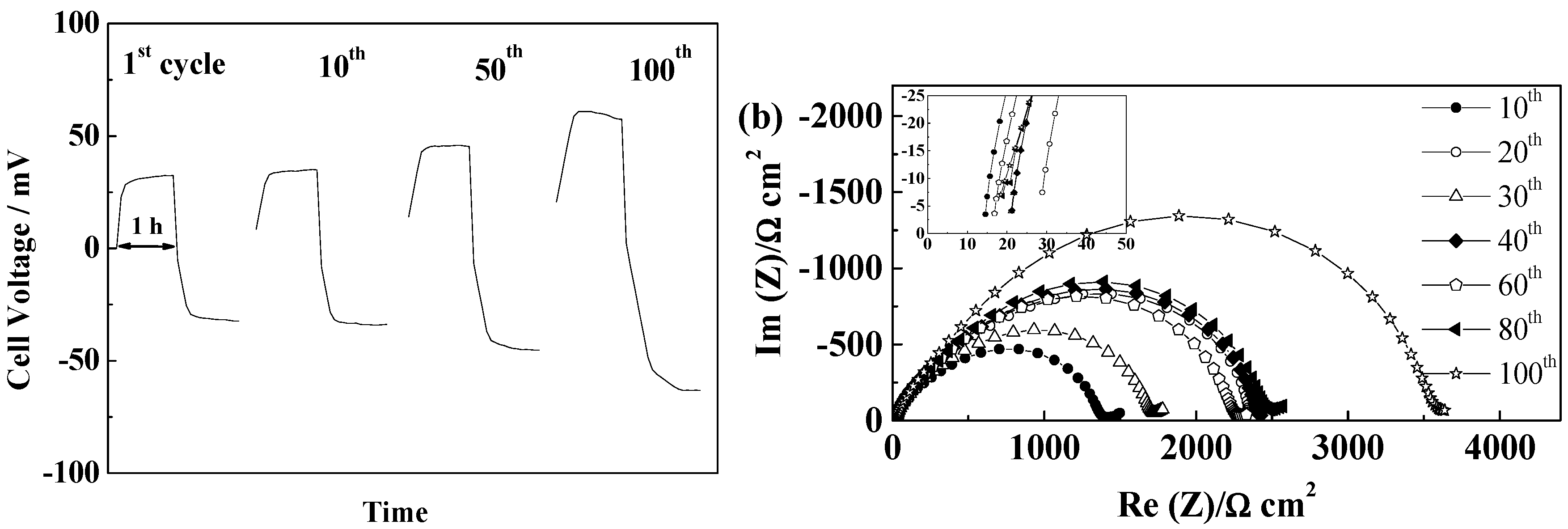
3. Experimental
3.1. Preparation of Ionic Liquid Based Electrolytes
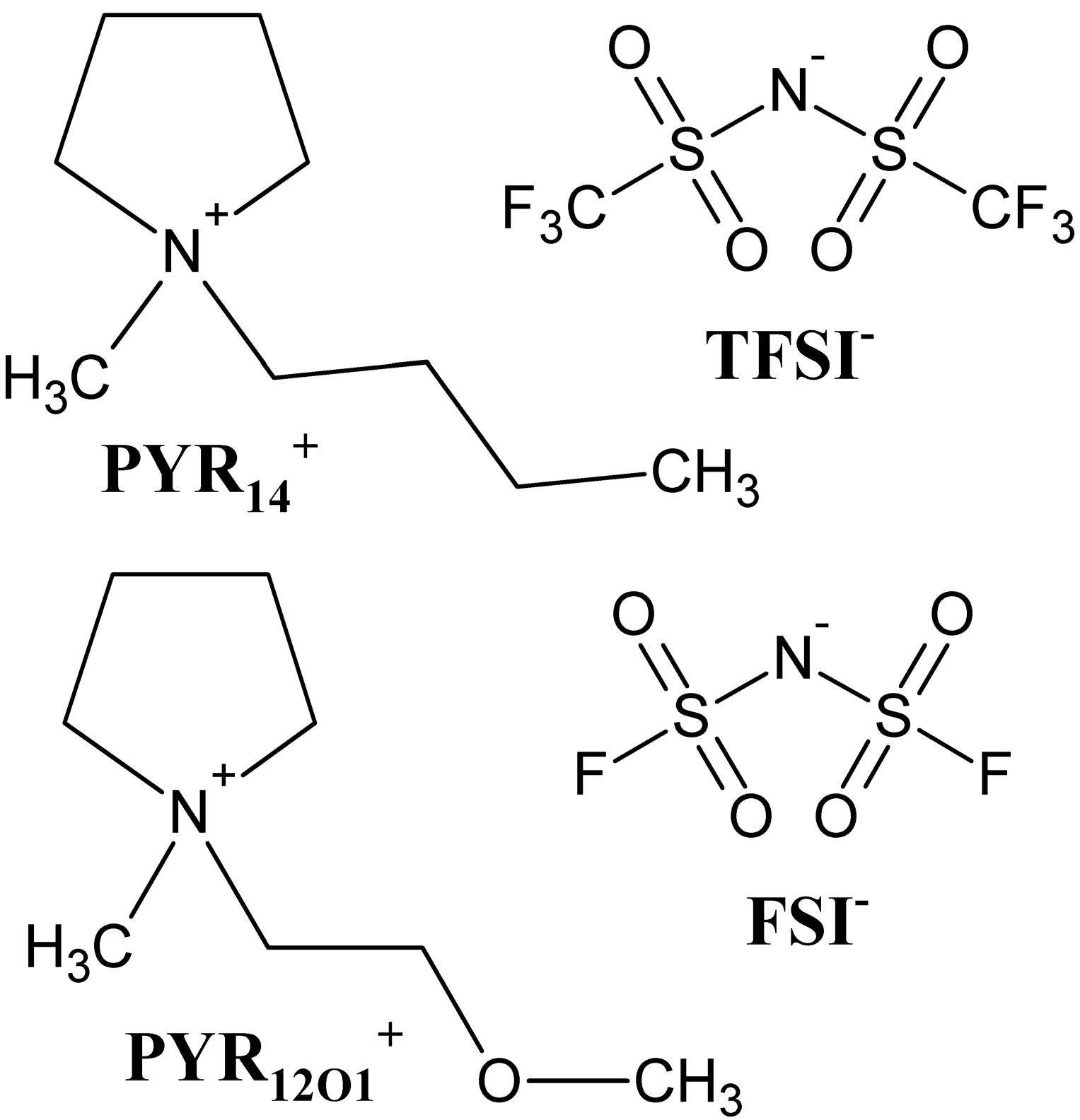
| Electrolyte | σ (20 °C)/S·cm−1 |
|---|---|
| PYR14FSI:LiTFSI (9:1) | 2.23 × 10−3 |
| PYR14TFSI:LiTFSI (9:1) | 1.07 × 10−3 |
| PYR12O1FSI:LiTFSI (9:1) | 3.94 × 10−3 |
| PYR12O1TFSI:LiTFSI (9:1) | 2.09 × 10−3 |
3.2. Separators Characterizations
3.3. Electrochemical Measurements
3.4. Scanning Electron Microscopy (SEM) Imaging
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Jacobson, M.Z.; Delucchi, M.A. A Path to sustainable energy by 2030. Sci. Am. 2009, 301, 58–65. [Google Scholar] [CrossRef]
- Jacobson, M.Z.; Delucchi, M.A. Providing all global energy with wind, water, and solar power, Part I: Technologies, energy resources, quantities and areas of infrastructure, and materials. Energy Policy 2011, 39, 1154–1169. [Google Scholar] [CrossRef]
- Delucchi, M.A.; Jacobson, M.Z. Providing all global energy with wind, water, and solar power, Part II: Reliability, system and transmission costs, and policies. Energy Policy 2011, 39, 1170–1190. [Google Scholar] [CrossRef]
- Fong, R.; von Sacken, U.; Dahn, J.R. Studies of lithium intercalation into carbons using nonaqueous electrochemical cells. J. Electrochem. Soc. 1990, 137, 2009–2013. [Google Scholar] [CrossRef]
- Peled, E. The electrochemical behavior of alkali and alkaline earth metals in nonaqueous battery systems—The solid electrolyte interphase model. J. Electrochem. Soc. 1979, 126, 2047–2051. [Google Scholar] [CrossRef]
- Doughty, D.; Roth, P. A general discussion of Li ion battery safety. Electrochem. Soc. Interface 2012, 21, 37–44. [Google Scholar]
- Arai, H.; Tsuda, M.; Saito, K.; Hayashi, M.; Sakurai, Y. Thermal reactions between delithiated lithium nickelate and electrolyte solutions. J. Electrochem. Soc. 2002, 149, 401–406. [Google Scholar] [CrossRef]
- Zinigrad, E.; Larush-Asraf, L.; Gnanaraj, J.S.; Sprecher, M.; Aurbach, D. On the thermal stability of LiPF6. Thermochim. Acta 2005, 438, 184–191. [Google Scholar] [CrossRef]
- Campion, C.L.; Li, W.; Lucht, B.L. Thermal decomposition of LiPF6-based electrolytes for lithium-ion batteries. J. Electrochem. Soc. 2005, 152, 2327–2334. [Google Scholar] [CrossRef]
- Barlowz, C.G. Reaction of water with hexafluorophosphates and with Li bis(perfluoroethylsulfonyl)imide salt. Electrochem. Solid State Lett. 1999, 2, 362–364. [Google Scholar] [CrossRef]
- Yang, H.; Zhuang, G.V.; Ross, P.N., Jr. Thermal stability of LiPF6 salt and Li-ion battery electrolytes containing LiPF6. J. Power Sources 2006, 161, 573–579. [Google Scholar] [CrossRef]
- Vetter, J.; Novak, P.; Wagner, M.R.; Veit, C.; Möller, K.-C.; Besenhard, J.O.; Winter, M.; Wohlfahrt-Mehrens, M.; Vogler, C.; Hammouche, A. Ageing mechanisms in lithium-ion batteries. J. Power Sources 2005, 147, 269–281. [Google Scholar] [CrossRef]
- Armand, M. Materials for Advanced Batteries; Plenum Press: New York, NY, USA, 1980. [Google Scholar]
- Lewandowski, A.; Swiderska-Mocek, A. Ionic liquids as electrolytes for Li-ion batteries—An overview of electrochemical studies. J. Power Sources 2009, 194, 601–609. [Google Scholar] [CrossRef]
- Armand, M.; Tarascon, J.-M. Building better batteries. Nature 2008, 451, 625–657. [Google Scholar]
- Borgel, V.; Markevich, E.; Aurbach, D.; Semrau, G.; Schmidt, M. On the thermal behavior of model Li–LixCoO2 systems containing ionic liquids in standard electrolyte solutions. J. Power Sources 2009, 189, 331–336. [Google Scholar] [CrossRef]
- Armand, M.; Endres, F.; MacFarlane, D.R.; Ohno, H.; Scrosati, B. Ionic-liquid materials for the electrochemical challenges of the future. Nat. Mater. 2009, 8, 621–629. [Google Scholar] [CrossRef]
- Holzapfel, M.; Jost, C.; Novak, P. Stable cycling of graphite in an ionic liquid based electrolyte. Chem. Commun. 2004, 18, 2098–2099. [Google Scholar] [CrossRef]
- Sato, T.; Maruo, T.; Marukane, S.; Takagi, K. Ionic liquids containing carbonate solvent as electrolytes for lithium ion cells. J. Power Sources 2004, 138, 253–261. [Google Scholar] [CrossRef]
- Ishikawa, M.; Sugimoto, T.; Kikuta, M.; Ishiko, E.; Kono, M. Pure ionic liquid electrolytes compatible with a graphitized carbon negative electrode in rechargeable lithium-ion batteries. J. Power Sources 2006, 162, 658–662. [Google Scholar] [CrossRef]
- Sugimoto, T.; Kikuta, M.; Ishiko, E.; Kono, M.; Ishikawa, M. Ionic liquid electrolytes compatible with graphitized carbon negative without additive and their effects on interfacial properties. J. Power Sources 2008, 183, 436–440. [Google Scholar] [CrossRef]
- Sugimoto, T.; Atsumi, Y.; Kikuta, M.; Ishiko, E.; Kono, M.; Ishikawa, M. Ionic liquid electrolyte systems based on bis(fluorosulfonyl)imide for lithium-ion batteries. J. Power Sources 2009, 189, 802–805. [Google Scholar] [CrossRef]
- Guerfi, A.; Duchesne, S.; Kobayashi, Y.; Vijh, A.; Zaghib, K. LiFePO4 and graphite electrodes with ionic liquids based on bis(fluorosulfonyl)imide (FSI)—For Li-ion batteries. J. Power Sources 2008, 175, 866–873. [Google Scholar] [CrossRef]
- Matsumoto, H.; Sakaebe, H.; Tatsumi, K.; Kikuta, M.; Ishiko, E.; Kono, M. Fast cycling of Li/LiCoO2 cell with low-viscosity ionic liquids based on bis(fluorosulfonyl)imide (FSI)−. J. Power Sources 2006, 160, 1308–1313. [Google Scholar] [CrossRef]
- Zhou, Q.; Henderson, W.A.; Appetecchi, G.B.; Montanino, M.; Passerini, S. Physical and electrochemical properties of N-alkyl-N-methylpyrrolidinium bis(fluorosulfonyl)imide ionic liquids: PY13FSI and PY14FSI. J. Phys. Chem. 2008, 112, 13577–13580. [Google Scholar] [CrossRef]
- Paillard, E.; Zhou, Q.; Henderson, W.A.; Appetecchi, G.B.; Montanino, M.; Passerini, S. Electrochemical and physicochemical properties of PY14FSI-based electrolytes with LiFSI. J. Electrochem. Soc. 2009, 156, A891–A895. [Google Scholar] [CrossRef]
- Howlett, P.C.; MacFarlane, D.R.; Hollenkamp, A.F. High lithium metal cycling efficiency in a room-temperature ionic liquid. Electrochem. Solid State Lett. 2004, 7, A97–A101. [Google Scholar] [CrossRef]
- Kim, G.-T.; Appetecchi, G.B.; Montanino, M.; Alessandrini, F.; Passerini, S. Long-term cyclability of lithium metal electrodes in ionic liquid-based electrolytes at room temperature. ECS Trans. 2010, 25, 127–138. [Google Scholar]
- Zhang, J.; Liu, Z.; Kong, Q.; Zhang, C.; Pang, S.; Yue, L.; Wang, X.; Yao, J.; Cui, G. Mussel inspired modification of polypropylene separators by catechol/polyamine for Li-ion batteries. ACS Appl. Mater. Interfaces 2012, 5, 128–134. [Google Scholar] [CrossRef]
- Zhang, S.S. A review on the separators of liquid electrolyte Li-ion batteries. J. Power Sources 2007, 164, 351–364. [Google Scholar] [CrossRef]
- Venugopal, G.; Moore, J.; Howard, J.; Pendalwar, S. Characterization of microporous separators for lithium-ion batteries. J. Power Sources 1999, 77, 34–41. [Google Scholar] [CrossRef]
- Arora, P.; Zhang, Z.J. Battery separators. Chem. Rev. 2004, 104, 4419–4462. [Google Scholar] [CrossRef]
- Hennige, V.; Hying, C.; Hoerpel, G.; Novak, P.; Vetter, J. Separator provided with asymmetrical pore structures for an electrochemical cell. U.S. Patent 7,709,140, 4 May 2010. [Google Scholar]
- Kuribayashi, I. Characterization of composite cellulosic separators for rechargeable lithium-ion batteries. J. Power Sources 1996, 63, 87–91. [Google Scholar] [CrossRef]
- Jabbour, L.; Destro, M.; Chaussy, D.; Gerbaldi, C.; Penazzi, N.; Bodoardo, S.; Beneventi, D. Flexible cellulose/LiFePO4 paper-cathodes: Toward eco-friendly all-paper Li-ion batteries. Cellulose 2013, 20, 571–582. [Google Scholar]
- Peled, E.; Golodnitsky, D.; Ardel, G.; Eshkenazy, V. The SEI model-application to lithium-polymer electrolyte batteries. Electrochim. Acta 1995, 40, 2197–2204. [Google Scholar] [CrossRef]
- Chazalviel, J.-N. Electrochemical aspects of the generation of ramified metallic electrodeposits. Phys. Rev. A 1990, 42, 7355–7367. [Google Scholar] [CrossRef]
- Brissot, C.; Rosso, M.; Chazalviel, J.-N.; Lascaud, S. Dendritic growth mechanisms in lithium/polymer cells. J. Power Sources 1999, 925, 81–82. [Google Scholar]
- Rosso, M.; Gobron, T.; Brissot, C.; Chazalviel, J.-N.; Lascaud, S. Onset of dendritic growth in lithium/polymer cells. J. Power Sources 2001, 804, 97–98. [Google Scholar]
- Reiter, J.; Paillard, E.; Grande, L.; Winter, M.; Passerini, S. Physicochemical properties of N-methoxyethyl-N-methylpyrrolidinum ionic liquids with perfluorinated anions. Electrochim. Acta 2013, 91, 101–107. [Google Scholar] [CrossRef]
- Maier, J. Defect chemistry and conductivity effects in heterogeneous solid electrolytes. J. Electrochem. Soc. 1987, 134, 1524–1535. [Google Scholar] [CrossRef]
- Bhattacharyya, A.J.; Maier, J. Second phase effects on the conductivity of non-aqueous salt solutions: “Soggy Sand Electrolytes”. Adv. Mater. 2004, 16, 811–814. [Google Scholar] [CrossRef]
- Capiglia, C.; Mustarelli, P.; Quartarone, E.; Tomasi, C.; Magistris, A. Effects of nanoscale SiO2 on the thermal and transport properties of solvent-free, poly(ethylene oxide) (PEO)-based polymer electrolytes. Solid State Ionics 1999, 118, 73–79. [Google Scholar] [CrossRef]
- Grande, L.; Paillard, E.; Kim, G.-T.; Monaco, S.; Passerini, S. Ionic liquid electrolytes for Li-air batteries: Lithium metal cycling. Int. J. Mol. Sci. 2014, 15, 8122–8137. [Google Scholar] [CrossRef]
- Bard, A.J.; Faulkner, L.R. Electrochemical Methods Fundamentals and Applications; Wiley and Son: New York, NY, USA, 2001. [Google Scholar]
- Appetecchi, G.B.; Scaccia, S.; Tizzani, C.; Alessandrini, F.; Passerini, S. Synthesis of hydrophobic ionic liquids for electrochemical applications. J. Electrochem. Soc. 2006, 153, A1685–A1691. [Google Scholar] [CrossRef]
- Appetecchi, G.B.; Montanino, M.; Zane, D.; Carewska, M.; Alessandrini, F.; Passerini, S. Effect of the alkyl group on the synthesis and the electrochemical properties of N-alkyl-N-methyl-pyrrolidinium bis(trifluoromethanesulfonyl)imide ionic liquids. Electrochim. Acta 2009, 54, 1325–1332. [Google Scholar] [CrossRef]
- MacMullin, R.B.; Muccini, G.A. Characteristics of porous beds and structures. AIChE J. 1956, 2, 393–403. [Google Scholar] [CrossRef]
© 2014 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Kirchhöfer, M.; Von Zamory, J.; Paillard, E.; Passerini, S. Separators for Li-Ion and Li-Metal Battery Including Ionic Liquid Based Electrolytes Based on the TFSI− and FSI− Anions. Int. J. Mol. Sci. 2014, 15, 14868-14890. https://doi.org/10.3390/ijms150814868
Kirchhöfer M, Von Zamory J, Paillard E, Passerini S. Separators for Li-Ion and Li-Metal Battery Including Ionic Liquid Based Electrolytes Based on the TFSI− and FSI− Anions. International Journal of Molecular Sciences. 2014; 15(8):14868-14890. https://doi.org/10.3390/ijms150814868
Chicago/Turabian StyleKirchhöfer, Marija, Jan Von Zamory, Elie Paillard, and Stefano Passerini. 2014. "Separators for Li-Ion and Li-Metal Battery Including Ionic Liquid Based Electrolytes Based on the TFSI− and FSI− Anions" International Journal of Molecular Sciences 15, no. 8: 14868-14890. https://doi.org/10.3390/ijms150814868
APA StyleKirchhöfer, M., Von Zamory, J., Paillard, E., & Passerini, S. (2014). Separators for Li-Ion and Li-Metal Battery Including Ionic Liquid Based Electrolytes Based on the TFSI− and FSI− Anions. International Journal of Molecular Sciences, 15(8), 14868-14890. https://doi.org/10.3390/ijms150814868





