Non-Synonymous Single Nucleotide Polymorphisms in the P2X Receptor Genes: Association with Diseases, Impact on Receptor Functions and Potential Use as Diagnosis Biomarkers
Abstract
:1. Introduction
2. Non-Synonymous Single Nucleotide Polymorphisms (NS-SNPs) in the P2RX Genes and Disease Association
2.1. NS-SNPs in the P2RX2 Gene Increase Susceptibility to Hearing Loss
2.2. NS-SNP in the P2RX4 Gene
2.2.1. 1248A>G Is Associated with High Pulse Pressure
2.2.2. 1248A>G Increases Susceptibility to Age-Related Macular Degeneration (AMD)
2.3. NS-SNPs in the P2RX7 Gene
2.3.1. 1405A>G and 1068G>A Are Implicated in Susceptibility to Affective Mood Disorders
2.3.2. 370T>C and 489C>T Increase Susceptibility to Multiple Sclerosis (MS)
2.3.3. 489C>T and 835G>A Alter Chronic Pain Sensitivity
2.3.4. 489C>T Is a Risk Factor for Childhood Febrile Seizure
2.3.5. 1513A>C Reduces Cardiovascular Risk
2.3.6. 1513A>C in Susceptibility to Tuberculosis (TB)
2.3.7. 489C>T and 1405A>G Are Potential Factors Increasing Susceptibility to Sepsis
2.3.8. 1068G>A and 1513A>C Alter Susceptibility to Toxoplasmosis
2.3.9. Multiple NS-SNPs Are Associated with Osteoporosis and Bone Fracture Risk
| rs Number | Change in Nucleotide (Amino Acid) Sequence | Implicated Conditions |
|---|---|---|
| rs17525809 | 370T>V (A76V) | Multiple sclerosis [56] |
| rs28360447 | 474G>A (G150R) | Osteoporosis [73,74] |
| rs208294 | 489C>T (H155Y) | Multiple sclerosis [56]; chronic pain [14]; severe sepsis [67]; children febrile seizures [60] |
| rs7958311 | 835G>A (R270H) | Chronic pain [14] |
| rs28360457 | 946G>A (R307Q) | Osteoporosis [71,72] |
| rs1718119 | 1068G>A (A348T) | Osteoporosis [72,73,74]; anxiety disorder [54]; toxoplasmosis [68] |
| rs2230911 | 1096C>G (T357S) | Osteoporosis [71] |
| rs2230912 | 1405A>G (Q460R) | Osteoporosis [72,73,74]; severe sepsis [67]; bipolar disorders and major depressive disorders [52,53,55,81] (but see [82,83]) † |
| rs3751143 | 1513A>C (E496A) | Osteoporosis [70,71,72,73,74]; tuberculosis [63,64,99]; cardiovascular risks [61] |
| rs1653624 | 1729T>A (I568N) | Osteoporosis [70,71,72] |
3. NS-SNP Mutational Effects on Receptor Function
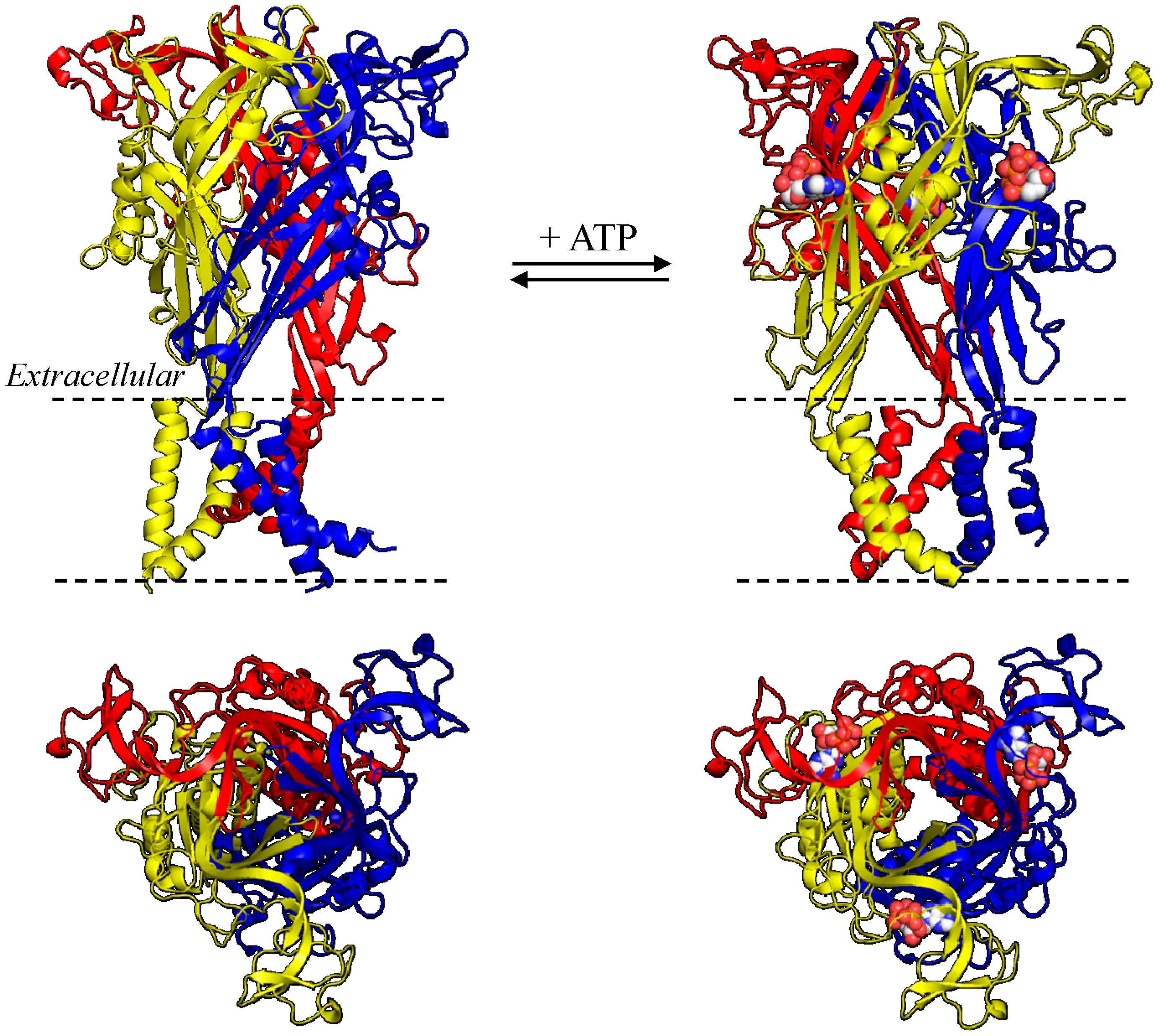
3.1. V60L and the Human P2X2 Receptor
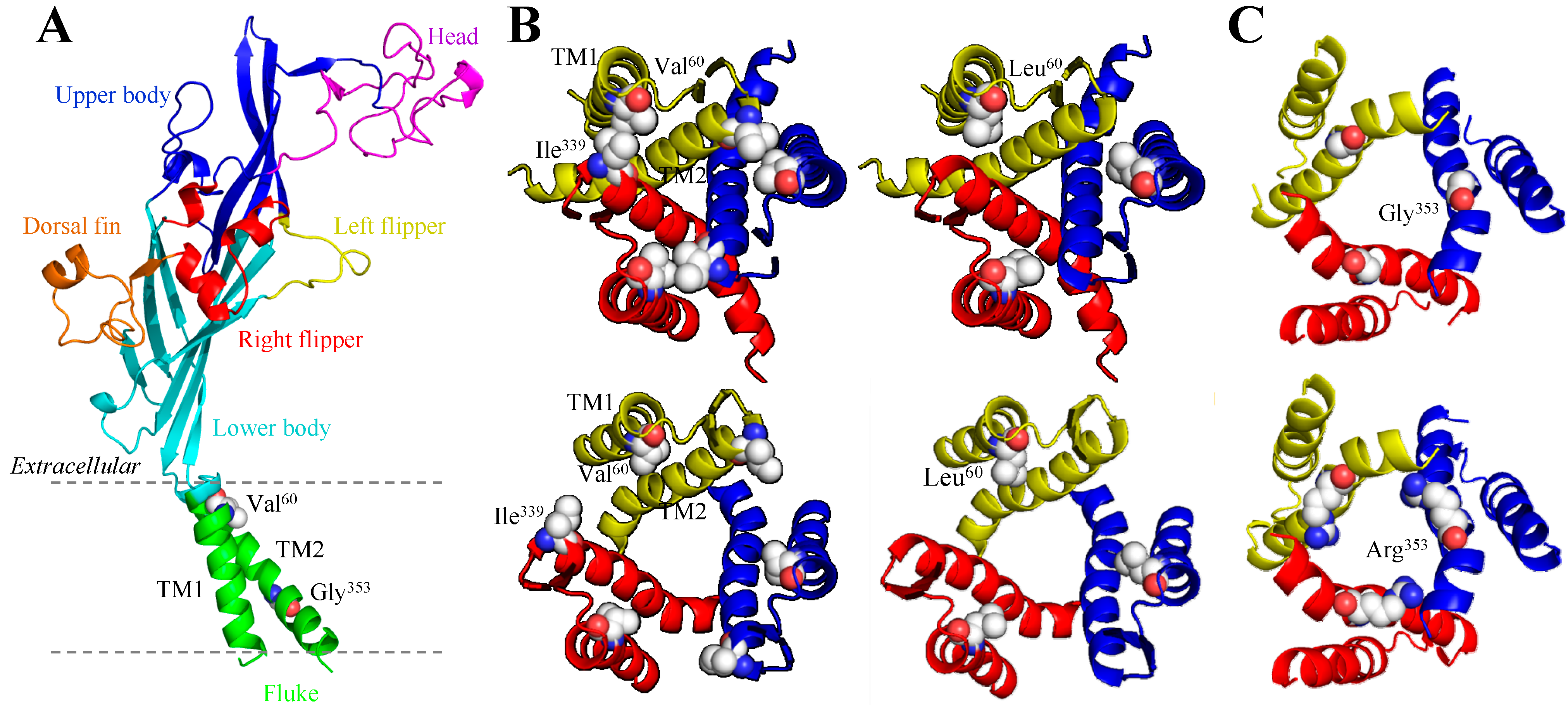
3.2. G353R and the Human P2X2 Receptor
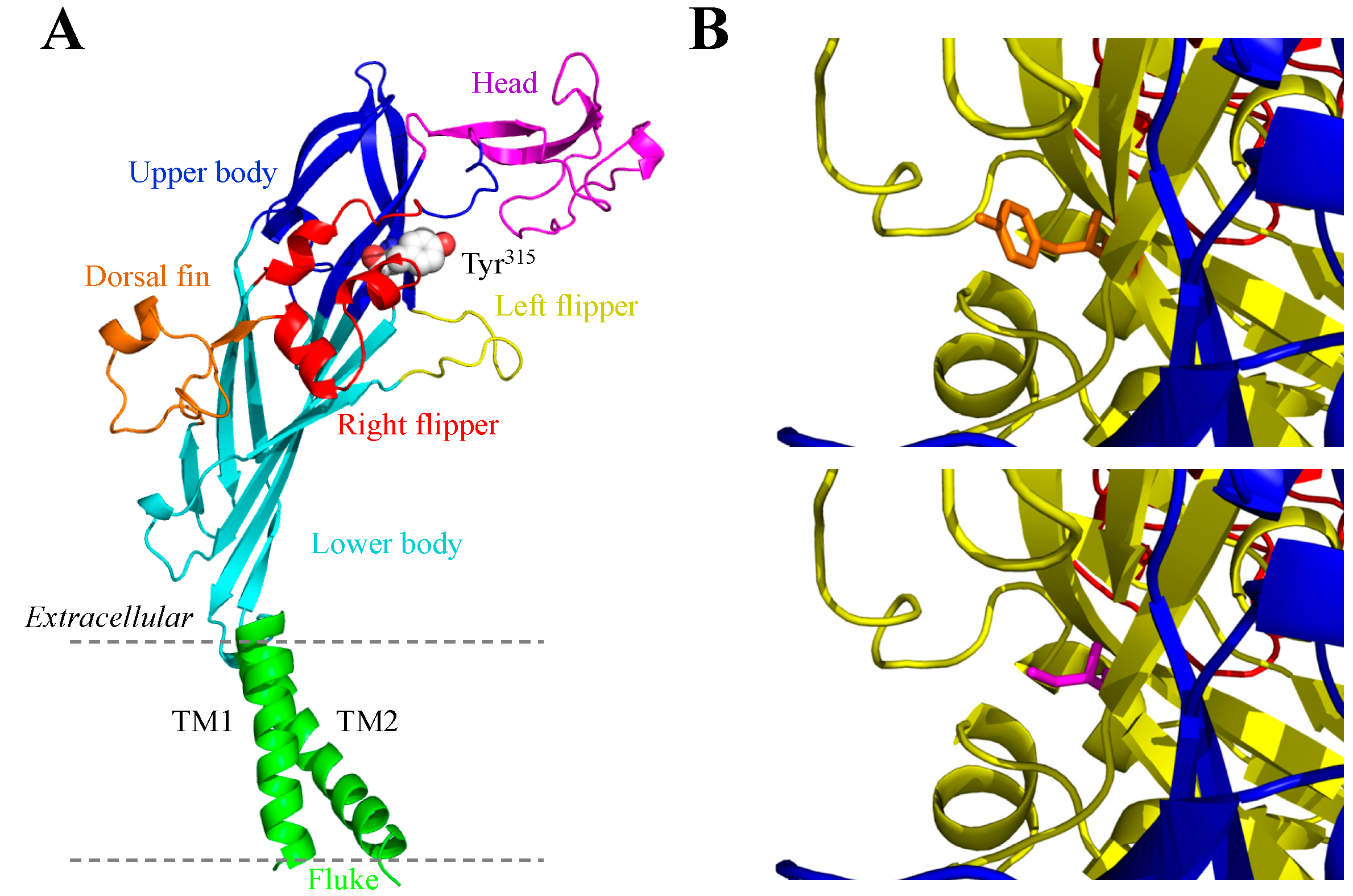
3.3. Y315C and the Human P2X4 Receptor
3.4. A76V and the Human P2X7 Receptor
3.5. G150R and the Human P2X7 Receptor
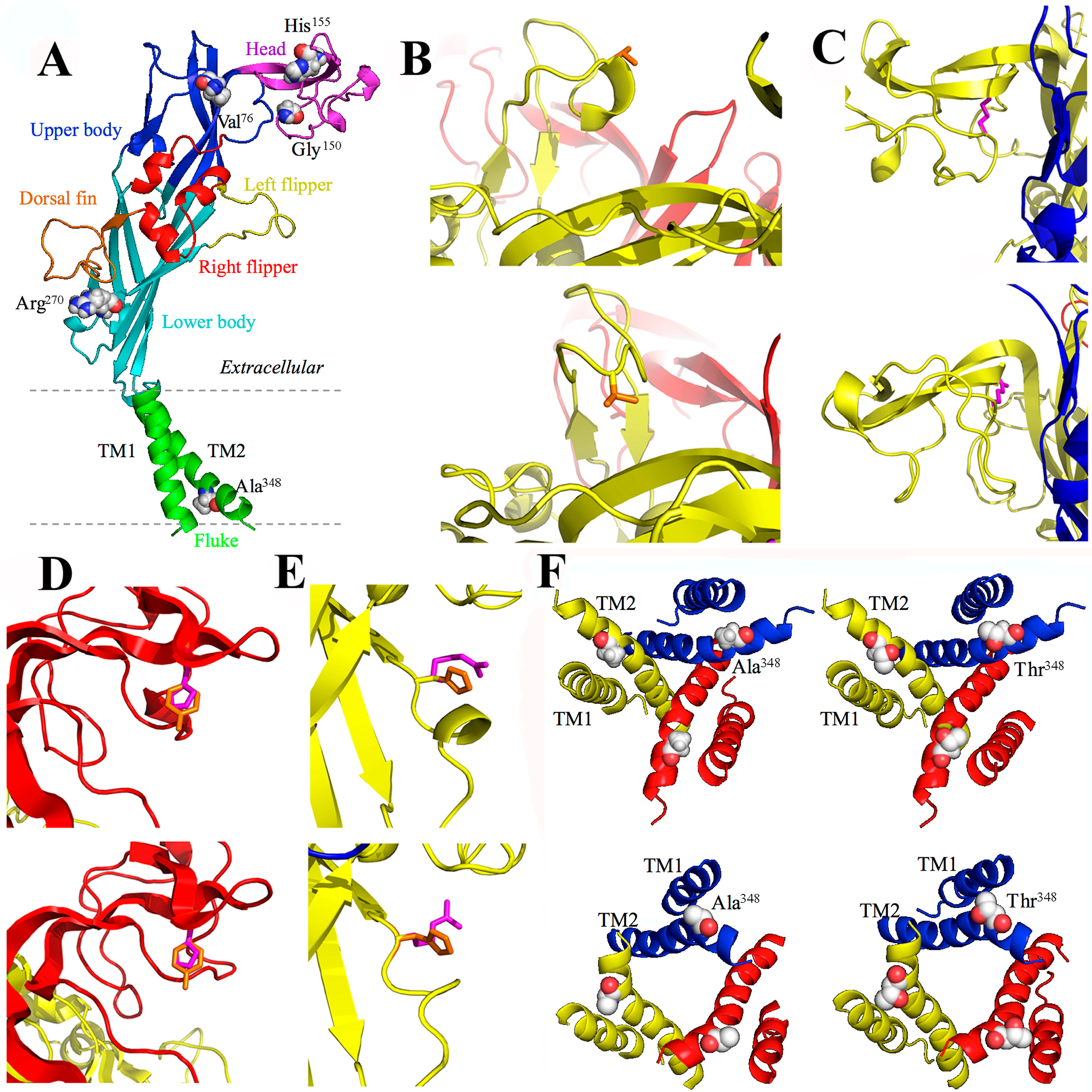
3.6. H155Y and the Human P2X7 Receptor
3.7. R270H and the Human P2X7 Receptor
3.8. A348T and the Human P2X7 Receptor
3.9. Q460R and the Human P2X7 Receptor
3.10. E496A and the Human P2X7 Receptor
3.11. I568N and the Human P2X7 Receptor
4. Concluding Remarks
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Holton, F.A.; Holton, P. The capillary dilator substances in dry powders of spinal roots—A possible role of adenosine triphosphate in chemical transmission from nerve endings. J. Physiol. 1954, 126, 124–140. [Google Scholar]
- Burnstock, G. Purinergic nerves. Pharmacol. Rev. 1972, 24, 509–581. [Google Scholar]
- Webb, T.E.; Simon, J.; Krishek, B.J.; Bateson, A.N.; Smart, T.G.; King, B.F.; Burnstock, G.; Barnard, E.A. Cloning and functional expression of a brain G-protein-coupled ATP receptor. FEBS Lett. 1993, 324, 219–225. [Google Scholar] [CrossRef]
- Lustig, K.D.; Shiau, A.K.; Brake, A.J.; Julius, D. Expression cloning of an ATP receptor from mouse neuroblastoma cells. Proc. Natl. Acad. Sci. USA 1993, 90, 5113–5117. [Google Scholar]
- Valera, S.; Hussy, N.; Evans, R.J.; Adami, N.; North, R.A.; Surprenant, A.; Buell, G. A new class of ligand-gated ion channel defined by P2X receptor for extracellular ATP. Nature 1994, 371, 516–519. [Google Scholar] [CrossRef]
- Brake, A.J.; Wagenbach, M.J.; Julius, D. New structural motif for ligand-gated ion channels defined by an ionotropic ATP receptor. Nature 1994, 371, 519–523. [Google Scholar]
- Ralevic, V.; Burnstock, G. Receptors for purines and pyrimidines. Pharmacol. Rev. 1998, 50, 413–492. [Google Scholar]
- North, R.A. Molecular physiology of P2X receptors. Physiol. Rev. 2002, 82, 1013–1067. [Google Scholar]
- Jiang, L.H.; Baldwin, J.M.; Roger, S.; Baldwin, S.A. Insights into the molecular mechanisms underlying mammalian P2X7 receptor functions and contributions in diseases, revealed by structural modeling and single nucleotide polymorphisms. Front. Pharmacol. 2013, 4, 55. [Google Scholar]
- Surprenant, A.; North, R.A. Signaling at purinergic P2X receptors. Annu. Rev. Physiol. 2009, 71, 333–359. [Google Scholar]
- Khakh, B.S.; North, R.A. Neuromodulation by extracellular ATP and P2X receptors in the CNS. Neuron 2012, 76, 51–69. [Google Scholar]
- North, R.A.; Jarvis, M.F. P2X receptors as drug targets. Mol. Pharmacol. 2013, 83, 759–769. [Google Scholar]
- Jiang, L.-H. P2X receptormediated ATP purinergic signalling in health and disease. Cell Health Cytoskelet. 2012, 4, 83–101. [Google Scholar]
- Sorge, R.E.; Trang, T.; Dorfman, R.; Smith, S.B.; Beggs, S.; Ritchie, J.; Austin, J.-S.; Zaykin, D.V.; vander Meulen, H.; Costigan, M. Genetically determined P2X7 receptor pore formation regulates variability in chronic pain sensitivity. Nat. Med. 2012, 18, 595–599. [Google Scholar] [CrossRef]
- Lynch, K.J.; Touma, E.; Niforatos, W.; Kage, K.L.; Burgard, E.C.; van Biesen, T.; Kowaluk, E.A.; Jarvis, M.F. Molecular and functional characterization of human P2X2 receptors. Mol. Pharmacol. 1999, 56, 1171–1181. [Google Scholar]
- Salih, S.G.; Housley, G.D.; Burton, L.D.; Greenwood, D. P2X2 receptor subunit expression in a subpopulation of cochlear type I spiral ganglion neurones. Neuroreport 1998, 9, 279–282. [Google Scholar]
- Housley, G.D.; Luo, L.; Ryan, A.F. Localization of mRNA encoding the P2X2 receptor subunit of the adenosine 5'-triphosphate-gated ion channel in the adult and developing rat inner ear by in situ hybridization. J. Comp. Neurol. 1998, 393, 403–414. [Google Scholar] [CrossRef]
- Housley, G.D.; Kanjhan, R.; Raybould, N.P.; Greenwood, D.; Salih, S.G.; Jarlebark, L.; Burton, L.D.; Setz, V.C.M.; Cannell, M.B.; Soeller, C.; et al. Expression of the P2X2 receptor subunit of the ATP-gated ion channel in the cochlea: Implications for sound transduction and auditory neurotransmission. J. Neurosci. 1999, 19, 8377–8388. [Google Scholar]
- Wang, J.C.C.; Raybould, N.P.; Luo, L.; Ryan, A.F.; Cannell, M.B.; Thorne, P.R.; Housley, G.D. Noise induces up-regulation of P2X2 receptor subunit of ATP-gated ion channels in the rat cochlea. Neuroreport 2003, 14, 817–823. [Google Scholar]
- Salih, S.G.; Housley, G.D.; Raybould, N.P.; Thorne, P.R. ATP-gated ion channel expression in primary auditory neurones. Neuroreport 1999, 10, 2579–2586. [Google Scholar]
- Yu, N.; Zhao, H.-B. ATP activates P2X receptors and requires extracellular Ca2++ participation to modify outer hair cell nonlinear capacitance. Pflüg. Arch.-Eur. J. Physiol. 2008, 457, 453–461. [Google Scholar] [CrossRef]
- Zhu, Y.; Zhao, H.-B. ATP activates P2X receptors to mediate gap junctional coupling in the cochlea. Biochem. Biophys. Res. Commun. 2012, 426, 528–532. [Google Scholar] [CrossRef]
- Zhu, Y.; Zhao, H.-B. ATP-mediated potassium recycling in the cochlear supporting cells. Purinergic Signal. 2010, 6, 221–229. [Google Scholar] [CrossRef]
- Housley, G.D.; Morton-Jones, R.; Vlajkovic, S.M.; Telang, R.S.; Paramananthasivam, V.; Tadros, S.F.; Wong, A.C.Y.; Froud, K.E.; Cederholm, J.M.E.; Sivakumaran, Y.; et al. ATP-gated ion channels mediate adaptation to elevated sound levels. Proc. Natl. Acad. Sci. USA 2013, 110, 7494–7499. [Google Scholar] [CrossRef]
- Yan, D.; Zhu, Y.; Walsh, T.; Xie, D.; Yuan, H.; Sirmaci, A.; Fujikawa, T.; Wong, A.C.Y.; Loh, T.L.; Du, L.; et al. Mutation of the ATP-gated P2X2 receptor leads to progressive hearing loss and increased susceptibility to noise. Proc. Natl. Acad. Sci. USA 2013, 110, 2228–2233. [Google Scholar] [CrossRef]
- Blanton, S.H.; Liang, C.Y.; Cai, M.W.; Pandya, A.; Du, L.L.; Landa, B.; Mummalanni, S.; Li, K.S.; Chen, Z.Y.; Qin, X.N.; et al. A novel locus for autosomal dominant non-syndromic deafness (DFNA41) maps to chromosome 12q24-qter. J. Med. Genet. 2002, 39, 567–570. [Google Scholar]
- Faletra, F.; Girotto, G.; D’Adamo, A.P.; Vozzi, D.; Morgan, A.; Gasparini, P. A novel P2RX2 mutation in an Italian family affected by autosomal dominant nonsyndromic hearing loss. Gene 2014, 534, 236–239. [Google Scholar]
- Buell, G.N.; Talabot, F.; Gos, A.; Lorenz, J.; Lai, E.; Morris, M.A.; Antonarakis, S.E. Gene structure and chromosomal localization of the human P2X7 receptor. Recept. Channels 1998, 5, 347–354. [Google Scholar]
- Garcia-Guzman, M.; Soto, F.; Gomez-Hernandez, J.M.; Lund, P.-E.; Stühmer, W. Characterization of recombinant human P2X4 receptor reveals pharmacological differences to the rat homologue. Mol. Pharmacol. 1997, 51, 109–118. [Google Scholar]
- Yamamoto, K.; Korenaga, R.; Kamiya, A.; Qi, Z.; Sokabe, M.; Ando, J. P2X4 receptors mediate ATP-induced calcium influx in human vascular endothelial cells. Am. J. Physiol.-Heart Circ. Physiol. 2000, 279, H285–H292. [Google Scholar]
- Yamamoto, K.; Korenaga, R.; Kamiya, A.; Ando, J. Fluid shear stress activates Ca2++ influx into human endothelial cells via P2X4 purinoceptors. Circ. Res. 2000, 87, 385–391. [Google Scholar]
- Yamamoto, K.; Furuya, K.; Nakamura, M.; Kobatake, E.; Sokabe, M.; Ando, J. Visualization of flow-induced ATP release and triggering of Ca2++ waves at caveolae in vascular endothelial cells. J. Cell Sci. 2011, 124, 3477–3483. [Google Scholar]
- Yamamoto, K.; Sokabe, T.; Matsumoto, T.; Yoshimura, K.; Shibata, M.; Ohura, N.; Fukuda, T.; Sato, T.; Sekine, K.; Kato, S. Impaired flow-dependent control of vascular tone and remodeling in P2X4-deficient mice. Nat. Med. 2005, 12, 133–137. [Google Scholar]
- Stokes, L.; Scurrah, K.; Ellis, J.A.; Cromer, B.A.; Skarratt, K.K.; Gu, B.J.; Harrap, S.B.; Wiley, J.S. A loss-of-function polymorphism in the human P2X4 receptor is associated with increased pulse pressure. Hypertension 2011, 58, 1086–1092. [Google Scholar]
- Ting, A.Y.; Lee, T.K.; MacDonald, I.M. Genetics of age-related macular degeneration. Curr. Opin. Ophthalmol. 2009, 20, 369–376. [Google Scholar]
- Gu, B.J.; Baird, P.N.; Vessey, K.A.; Skarratt, K.K.; Fletcher, E.L.; Fuller, S.J.; Richardson, A.J.; Guymer, R.H.; Wiley, J.S. A rare functional haplotype of the P2RX4 and P2RX7 genes leads to loss of innate phagocytosis and confers increased risk of age-related macular degeneration. FASEB J. 2013, 27, 1479–1487. [Google Scholar]
- Monif, M.; Reid, C.A.; Powell, K.L.; Smart, M.L.; Williams, D.A. The P2X7 receptor drives microglial activation and proliferation: A trophic role for P2X7R pore. J. Neurosci. 2009, 29, 3781–3791. [Google Scholar]
- Gu, B.J.; Saunders, B.M.; Petrou, S.; Wiley, J.S. P2X7 is a scavenger receptor for apoptotic cells in the absence of its ligand, extracellular ATP. J. Immunol. 2011, 187, 2365–2375. [Google Scholar]
- Jiang, L.-H. Inhibition of P2X7 receptors by divalent cations: Old action and new insight. Eur. Biophys. J. 2009, 38, 339–346. [Google Scholar]
- Panupinthu, N.; Zhao, L.; Possmayer, F.; Ke, H.Z.; Sims, S.M.; Dixon, S.J. P2X7 nucleotide receptors mediate blebbing in osteoblasts through a pathway involving lysophosphatidic acid. J. Biol. Chem. 2007, 282, 3403–3412. [Google Scholar]
- Panupinthu, N.; Rogers, J.T.; Zhao, L.; Solano-Flores, L.P.; Possmayer, F.; Sims, S.M.; Dixon, S.J. P2X7 receptors on osteoblasts couple to production of lysophosphatidic acid: A signaling axis promoting osteogenesis. J. Cell Biol. 2008, 181, 859–871. [Google Scholar]
- Li, J.; Liu, D.; Ke, H.Z.; Duncan, R.L.; Turner, C.H. The P2X7 nucleotide receptor mediates skeletal mechanotransduction. J. Biol. Chem. 2005, 280, 42952–42959. [Google Scholar]
- Okumura, H.; Shiba, D.; Kubo, T.; Yokoyama, T. P2X7 receptor as sensitive flow sensor for ERK activation in osteoblasts. Biochem. Biophys. Res. Commun. 2008, 372, 486–490. [Google Scholar]
- Jørgensen, N.R.; Henriksen, Z.; Sørensen, O.H.; Eriksen, E.F.; Civitelli, R.; Steinberg, T.H. Intercellular calcium signaling occurs between human osteoblasts and osteoclasts and requires activation of osteoclast P2X7 receptors. J. Biol. Chem. 2002, 277, 7574–7580. [Google Scholar]
- Ke, H.Z.; Qi, H.; Weidema, A.F.; Zhang, Q.; Panupinthu, N.; Crawford, D.T.; Grasser, W.A.; Paralkar, V.M.; Li, M.; Audoly, L.P. Deletion of the P2X7 nucleotide receptor reveals its regulatory roles in bone formation and resorption. Mol. Endocrinol. 2003, 17, 1356–1367. [Google Scholar]
- Thunberg, U.; Tobin, G.; Johnson, A.; Söderberg, O.; Padyukov, L.; Hultdin, M.; Klareskog, L.; Enblad, G.; Sundström, C.; Roos, G. Polymorphism in the P2X7 receptor gene and survival in chronic lymphocytic leukaemia. Lancet 2002, 360, 1935–1939. [Google Scholar] [CrossRef]
- Wiley, J.S.; Dao-Ung, L.P.; Gu, B.J.; Sluyter, R.; Shemon, A.N.; Li, C.; Taper, J.; Gallo, J.; Manoharan, A. A loss-of-function polymorphic mutation in the cytolytic P2X7 receptor gene and chronic lymphocytic leukaemia: A molecular study. Lancet 2002, 359, 1114–1119. [Google Scholar]
- Starczynski, J.; Pepper, C.; Pratt, G.; Hooper, L.; Thomas, A.; Hoy, T.; Milligan, D.; Bentley, P.; Fegan, C. The P2X7 receptor gene polymorphism 1513 A→C has no effect on clinical prognostic markers, in vitro sensitivity to fludarabine, Bcl-2 family protein expression or survival in B-cell chronic lymphocytic leukaemia. Br. J. Haematol. 2003, 123, 66–71. [Google Scholar]
- Dao-Ung, L.P.; Fuller, S.J.; Sluyter, R.; SkarRatt, K.K.; Thunberg, U.; Tobin, G.; Byth, K.; Ban, M.; Rosenquist, R.; Stewart, G.J. Association of the 1513C polymorphism in the P2X7 gene with familial forms of chronic lymphocytic leukaemia. Br. J. Haematol. 2004, 125, 815–817. [Google Scholar]
- Nückel, H.; Frey, U.; Dürig, J.; Dührsen, U.; Siffert, W. Methylenetetrahydrofolate reductase (MTHFR) gene 677C>T and 1298A>C polymorphisms are associated with differential apoptosis of leukemic B cells in vitro and disease progression in chronic lymphocytic leukemia. Leukemia 2004, 18, 1816–1823. [Google Scholar]
- Zhang, L.; Ibbotson, R.; Orchard, J.; Gardiner, A.; Seear, R.; Chase, A.; Oscier, D.; Cross, N. P2X7 polymorphism and chronic lymphocytic leukaemia: Lack of correlation with incidence, survival and abnormalities of chromosome 12. Leukemia 2003, 17, 2097–2100. [Google Scholar]
- Barden, N.; Harvey, M.; Gagné, B.; Shink, E.; Tremblay, M.; Raymond, C.; Labbé, M.; Villeneuve, A.; Rochette, D.; Bordeleau, L.; et al. Analysis of single nucleotide polymorphisms in genes in the chromosome 12Q24.3. 31 region points to P2RX7 as a susceptibility gene to bipolar affective disorder. Am. J. Med. Genet. Part B 2006, 141B, 374–382. [Google Scholar]
- Lucae, S.; Salyakina, D.; Barden, N.; Harvey, M.; Gagné, B.; Labbé, M.; Binder, E.B.; Uhr, M.; Paez-Pereda, M.; Sillaber, I. P2RX7, a gene coding for a purinergic ligand-gated ion channel, is associated with major depressive disorder. Hum. Mol. Genet. 2006, 15, 2438–2445. [Google Scholar]
- Erhardt, A.; Lucae, S.; Unschuld, P.G.; Ising, M.; Kern, N.; Salyakina, D.; Lieb, R.; Uhr, M.; Binder, E.B.; Keck, M.E. Association of polymorphisms in P2RX7 and CaMKKb with anxiety disorders. J. Affect. Disord. 2007, 101, 159–168. [Google Scholar]
- McQuillin, A.; Bass, N.; Choudhury, K.; Puri, V.; Kosmin, M.; Lawrence, J.; Curtis, D.; Gurling, H. Case-control studies show that a non-conservative amino-acid change from a glutamine to arginine in the P2RX7 purinergic receptor protein is associated with both bipolar-and unipolar-affective disorders. Mol. Psychiatry 2008, 14, 614–620. [Google Scholar]
- Oyanguren-Desez, O.; Rodriguez-Antigueedad, A.; Villoslada, P.; Domercq, M.; Alberdi, E.; Matute, C. Gain-of-function of P2X7 receptor gene variants in multiple sclerosis. Cell Calcium 2011, 50, 468–472. [Google Scholar]
- Al-Shukaili, A.; Al-Kaabi, J.; Hassan, B.; Al-Araimi, T.; Al-Tobi, M.; Al-Kindi, M.; Al-Maniri, A.; Al-Gheilani, A.; Al-Ansari, A. P2X7 receptor gene polymorphism analysis in rheumatoid arthritis. Int. J. Immunogenet. 2011, 38, 389–396. [Google Scholar]
- Portales-Cervantes, L.; Nino-Moreno, P.; Salgado-Bustamante, M.; Garcia-Hernandez, M.H.; Baranda-Candido, L.; Reynaga-Hernandez, E.; Barajas-Lopez, C.; Gonzalez-Amaro, R.; Portales-Perez, D.P. The His155Tyr (489C>T) single nucleotide polymorphism of P2RX7 gene confers an enhanced function of P2X7 receptor in immune cells from patients with rheumatoid arthritis. Cell. Immunol. 2012, 276, 168–175. [Google Scholar]
- Forchap, S.L.; Anandacoomarasamy, A.; Wicks, J.; di Virgilio, F.; Baricordi, O.R.; Rubbini, M.; Trotta, F.; Wiley, J.; Manolios, N. P2X7 gene polymorphisms do not appear to be a susceptibility gene locus in sporadic cases of systemic lupus erythematosus. Tissue Antigens 2008, 72, 487–490. [Google Scholar]
- Emsley, H.C.; Appleton, R.E.; Whitmore, C.L.; Jury, F.; Lamb, J.A.; Martin, J.E.; Ollier, W.E.; de la Morandière, K.P.; Southern, K.W.; Allan, S.M. Variations in inflammation-related genes may be associated with childhood febrile seizure susceptibility. Seizure 2014, 23, 457–461. [Google Scholar]
- Gidlof, O.; Smith, J.G.; Melander, O.; Lovkvist, H.; Hedblad, B.; Engstrom, G.; Nilsson, P.; Carlson, J.; Berglund, G.; Olsson, S.; et al. A common missense variant in the ATP receptor P2X7 is associated with reduced risk of cardiovascular events. PLoS One 2012, 7, e37491. [Google Scholar]
- Li, C.M.; Campbell, S.J.; Kumararatne, D.S.; Bellamy, R.; Ruwende, C.; McAdam, K.P.; Hill, A.V.; Lammas, D.A. Association of a polymorphism in the P2X7 gene with tuberculosis in a Gambian population. J. Infect. Dis. 2002, 186, 1458–1462. [Google Scholar] [CrossRef]
- Fernando, S.L.; Saunders, B.M.; Sluyter, R.; Skarratt, K.K.; Goldberg, H.; Marks, G.B.; Wiley, J.S.; Britton, W.J. A polymorphism in the P2X7 gene increases susceptibility to extrapulmonary tuberculosis. Am. J. Respir. Crit. Care Med. 2007, 175, 360–366. [Google Scholar] [CrossRef]
- Nino-Moreno, P.; Portales-Perez, D.; Hernandez-Castro, B.; Portales-Cervantes, L.; Flores-Meraz, V.; Baranda, L.; Gomez-Gomez, A.; Acuna-Alonzo, V.; Granados, J.; Gonzalez-Amaro, R. P2X7 and NRAMP1/SLC11 A1 gene polymorphisms in Mexican mestizo patients with pulmonary tuberculosis. Clin. Exp. Immunol. 2007, 148, 469–477. [Google Scholar] [CrossRef]
- Xiao, J.; Sun, L.; Jiao, W.W.; Li, Z.N.; Zhao, S.Y.; Li, H.M.; Jin, J.; Jiao, A.X.; Guo, Y.J.; Jiang, Z.F.; et al. Lack of association between polymorphisms in the P2X7 gene and tuberculosis in a Chinese Han population. FEMS Immunol. Med. Microbiol. 2009, 55, 107–111. [Google Scholar] [CrossRef]
- Sambasivan, V.; Murthy, K.J.R.; Reddy, R.; Vijayalakshimi, V.; Hasan, Q. P2X7 gene polymorphisms and risk assessment for pulmonary tuberculosis in Asian Indians. Dis. Mark. 2010, 28, 43–48. [Google Scholar] [CrossRef]
- Geistlinger, J.; Du, W.; Groll, J.; Liu, F.; Hoegel, J.; Foehr, K.J.; Pasquarelli, A.; Schneider, E.M. P2RX7 genotype association in severe sepsis identified by a novel Multi-Individual Array for rapid screening and replication of risk SNPs. Clin. Chim. Acta 2012, 413, 39–47. [Google Scholar] [CrossRef]
- Jamieson, S.E.; Peixoto-Rangel, A.L.; Hargrave, A.C.; de Roubaix, L.A.; Mui, E.J.; Boulter, N.R.; Miller, E.N.; Fuller, S.J.; Wiley, J.S.; Castellucci, L.; et al. Evidence for associations between the purinergic receptor P2X7 (P2RX7) and toxoplasmosis. Genes Immun. 2010, 11, 374–383. [Google Scholar] [CrossRef]
- Lees, M.P.; Fuller, S.J.; McLeod, R.; Boulter, N.R.; Miller, C.M.; Zakrzewski, A.M.; Mui, E.J.; Witola, W.H.; Coyne, J.J.; Hargrave, A.C.; et al. P2X7 receptor-mediated killing of an intracellular parasite, Toxoplasma gondii, by human and murine macrophages. J. Immunol. 2010, 184, 7040–7046. [Google Scholar] [CrossRef]
- Ohlendorff, S.D.; Tofteng, C.L.; Jensen, J.-E.B.; Petersen, S.; Civitelli, R.; Fenger, M.; Abrahamsen, B.; Hermann, A.P.; Eiken, P.; Jorgensen, N.R. Single nucleotide polymorphisms in the P2X7 gene are associated to fracture risk and to effect of estrogen treatment. Pharmacogenet. Genomics 2007, 17, 555–567. [Google Scholar]
- Gartland, A.; Skarratt, K.K.; Hocking, L.J.; Parsons, C.; Stokes, L.; Jørgensen, N.R.; Fraser, W.D.; Reid, D.M.; Gallagher, J.A.; Wiley, J.S. Polymorphisms in the P2X7 receptor gene are associated with low lumbar spine bone mineral density and accelerated bone loss in post-menopausal women. Eur. J. Hum. Genet. 2012, 20, 559–564. [Google Scholar] [CrossRef]
- Jørgensen, N.R.; Husted, L.B.; Skarratt, K.K.; Stokes, L.; Tofteng, C.L.; Kvist, T.; Jensen, J.-E.B.; Eiken, P.; Brixen, K.; Fuller, S. Single-nucleotide polymorphisms in the P2X7 receptor gene are associated with post-menopausal bone loss and vertebral fractures. Eur. J. Hum. Genet. 2012, 20, 675–681. [Google Scholar] [CrossRef]
- Husted, L.; Harsløf, T.; Stenkjær, L.; Carstens, M.; Jørgensen, N.; Langdahl, B.L. Functional polymorphisms in the P2X7 receptor gene are associated with osteoporosis. Osteoporos. Int. 2013, 24, 949–959. [Google Scholar] [CrossRef]
- Wesselius, A.; Bours, M.J.L.; Henriksen, Z.; Syberg, S.; Petersen, S.; Schwarz, P.; Jorgensen, N.R.; van Helden, S.; Dagnelie, P.C. Association of P2X7 receptor polymorphisms with bone mineral density and osteoporosis risk in a cohort of Dutch fracture patients. Osteoporos. Int. 2013, 24, 1235–1246. [Google Scholar] [CrossRef]
- Kessler, R.C.; Berglund, P.; Demler, O.; Jin, R.; Koretz, D.; Merikangas, K.R.; Rush, A.J.; Walters, E.E.; Wang, P.S. The epidemiology of major depressive disorder: Results from the National Comorbidity Survey Replication (NCS-R). JAMA 2003, 289, 3095–3105. [Google Scholar] [CrossRef]
- Jacobi, F.; Wittchen, H.-U.; Holting, C.; Hofler, M.; Pfister, H.; Muller, N.; Lieb, R. Prevalence, co-morbidity and correlates of mental disorders in the general population: Results from the German Health Interview and Examination Survey (GHS). Psychol. Med. 2004, 34, 597–612. [Google Scholar]
- Grant, B.F.; Hasin, D.S.; Stinson, F.S.; Dawson, D.A.; Ruan, W.J.; Goldstein, R.B.; Smith, S.M.; Saha, T.D.; Huang, B. Prevalence, correlates, co-morbidity, and comparative disability of DSM-IV generalized anxiety disorder in the USA: Results from the national epidemiologic survey on alcohol and related conditions. Psychol. Med. 2005, 35, 1747. [Google Scholar] [CrossRef]
- McDowell, I.; Lindsay, J.; Sykes, E.; Verreault, R.; Laurin, D.; Hendrie, H.C.; Hall, K.S.; Ogunniyi, A.; Gao, S.; Frassati, D. Prevalence and incidence studies of mood disorders: A systematic review of the literature. Can. J. Psychiatry 2004, 49, 124–138. [Google Scholar]
- Somers, J.M.; Goldner, E.M.; Waraich, P.; Hsu, L. Prevalence and incidence studies of anxiety disorders: A systematic review of the literature. Can. J. Psychiatry 2006, 51, 100. [Google Scholar]
- Shink, E.; Harvey, M.; Tremblay, M.; Gagné, B.; Belleau, P.; Raymond, C.; Labbé, M.; Dubé, M.P.; Lafrenière, R.G.; Barden, N. Analysis of microsatellite markers and single nucleotide polymorphisms in candidate genes for susceptibility to bipolar affective disorder in the chromosome 12Q24. 31 region. Am. J. Med. Genet. Part B 2005, 135, 50–58. [Google Scholar]
- Hejjas, K.; Szekely, A.; Domotor, E.; Halmai, Z.; Balogh, G.; Schilling, B.; Sarosi, A.; Faludi, G.; Sasvari-Szekely, M.; Nemoda, Z. Association between depression and the Gln460Arg polymorphism of P2RX7 gene: A dimensional approach. Am. J. Med. Genet. Part B 2009, 150, 295–299. [Google Scholar]
- Green, E.K.; Grozeva, D.; Raybould, R.; Elvidge, G.; Macgregor, S.; Craig, I.; Farmer, A.; McGuffin, P.; Forty, L.; Jones, L. P2RX7: A bipolar and unipolar disorder candidate susceptibility gene? Am. J. Med. Genet. Part B 2009, 150, 1063–1069. [Google Scholar]
- Grigoroiu-Serbanescu, M.; Herms, S.; Mühleisen, T.W.; Georgi, A.; Diaconu, C.C.; Strohmaier, J.; Czerski, P.; Hauser, J.; Leszczynska-Rodziewicz, A.; Jamra, R.A. Variation in P2RX7 candidate gene (rs2230912) is not associated with bipolar I disorder and unipolar major depression in four European samples. Am. J. Med. Genet. Part B 2009, 150, 1017–1021. [Google Scholar]
- Feng, W.-P.; Zhang, B.; Li, W.; Liu, J. Lack of association of P2RX7 gene rs2230912 polymorphism with mood disorders: A meta-analysis. PLoS One 2014, 9, e88575. [Google Scholar] [CrossRef]
- Pamela, S.; Stephan, R.; Laura, J.S.; Ole, A.A.; Sven, C.; Nick, C.; Howard, J.E.; John, I.N.; Marcella, R.; Douglas, B.; et al. Large-scale genome-wide association analysis of bipolar disorder identifies a new susceptibility locus near ODZ4. Nat. Genet. 2011, 43, 977–983. [Google Scholar] [CrossRef]
- Sklar, P.; Smoller, J.W.; Fan, J.; Ferreira, M.A.; Perlis, R.H.; Chambert, K.; Nimgaonkar, V.L.; McQueen, M.B.; Faraone, S.V.; Kirby, A.; et al. Whole-genome association study of bipolar disorder. Mol. Psychiatry 2008, 13, 558–569. [Google Scholar] [CrossRef]
- Ferreira, M.A.; O’Donovan, M.C.; Meng, Y.A.; Jones, I.R.; Ruderfer, D.M.; Jones, L.; Fan, J.; Kirov, G.; Perlis, R.H.; Green, E.K.; et al. Collaborative genome-wide association analysis supports a role for ANK3 and CACNA1C in bipolar disorder. Nat. Genet. 2008, 40, 1056–1058. [Google Scholar] [CrossRef]
- Love, S.; Louis, D.; Ellison, D.W. Greenfield’s Neuropathology (2-Volume Set); CRC Press: Boca Raton, FL, USA, 2008. [Google Scholar]
- Narcisse, L.; Scemes, E.; Zhao, Y.; Lee, S.C.; Brosnan, C.F. The cytokine IL-1β transiently enhances P2X7 receptor expression and function in human astrocytes. Glia 2005, 49, 245–258. [Google Scholar] [CrossRef]
- Yiangou, Y.; Facer, P.; Durrenberger, P.; Chessell, I.P.; Naylor, A.; Bountra, C.; Banati, R.R.; Anand, P. COX-2, CB2 and P2X7-immunoreactivities are increased in activated microglial cells/macrophages of multiple sclerosis and amyotrophic lateral sclerosis spinal cord. BMC Neurol. 2006, 6, 12. [Google Scholar] [CrossRef]
- Matute, C.; Torre, I.; Pérez-Cerdá, F.; Pérez-Samartín, A.; Alberdi, E.; Etxebarria, E.; Arranz, A.M.; Ravid, R.; Rodríguez-Antigüedad, A.; Sánchez-Gómez, M. P2X7 receptor blockade prevents ATP excitotoxicity in oligodendrocytes and ameliorates experimental autoimmune encephalomyelitis. J. Neurosci. 2007, 27, 9525–9533. [Google Scholar] [CrossRef]
- Chessell, I.P.; Hatcher, J.P.; Bountra, C.; Michel, A.D.; Hughes, J.P.; Green, P.; Egerton, J.; Murfin, M.; Richardson, J.; Peck, W.L. Disruption of the P2X7 purinoceptor gene abolishes chronic inflammatory and neuropathic pain. Pain 2005, 114, 386–396. [Google Scholar] [CrossRef]
- Honore, P.; Donnelly-Roberts, D.; Namovic, M.T.; Hsieh, G.; Zhu, C.Z.; Mikusa, J.P.; Hernandez, G.; Zhong, C.; Gauvin, D.M.; Chandran, P. A-740003 [N-(1-{[(cyanoimino)(5-quinolinylamino) methyl]amino}-2,2-dimethylpropyl)-2-(3,4-dimethoxyphenyl)acetamide], a novel and selective P2X7 receptor antagonist, dose-dependently reduces neuropathic pain in the rat. J. Pharmacol. Exp. Ther. 2006, 319, 1376–1385. [Google Scholar] [CrossRef]
- Chen, Y.; Zhang, X.; Wang, C.; Li, G.; Gu, Y.; Huang, L.Y. Activation of P2X7 receptors in glial satellite cells reduces pain through downregulation of P2X3 receptors in nociceptive neurons. Proc. Natl. Acad. Sci. USA 2008, 105, 16773–16778. [Google Scholar] [CrossRef]
- Chan, J.; Xing, Y.; Magliozzo, R.S.; Bloom, B.R. Killing Of virulent Mycobacterium-tuberculosis by reactive nitrogen intermediates produced by activated murine macrophages. J. Exp. Med. 1992, 175, 1111–1122. [Google Scholar] [CrossRef]
- Lammas, D.A.; Stober, C.; Harvey, C.J.; Kendrick, N.; Panchalingam, S.; Kumararatne, D.S. ATP-induced killing of mycobacteria by human macrophages is mediated by purinergic P2Z(P2X7) receptors. Immunity 1997, 7, 433–444. [Google Scholar] [CrossRef]
- Kusner, D.J.; Barton, J.A. ATP stimulates human macrophages to kill intracellular virulent Mycobacterium tuberculosis via calcium-dependent phagosome-lysosome fusion. J. Immunol. 2001, 167, 3308–3315. [Google Scholar] [CrossRef]
- Kusner, D.J.; Adams, J. ATP-induced killing of virulent Mycobacterium tuberculosis within human macrophages requires phospholipase D. J. Immunol. 2000, 164, 379–388. [Google Scholar] [CrossRef]
- Wu, G.; Zhao, M.; Gu, X.; Yao, Y.; Liu, H.; Song, Y. The effect of P2X7 receptor 1513 polymorphism on susceptibility to tuberculosis: A meta-analysis. Infect. Genet. Evol. 2014, 23, 84–91. [Google Scholar]
- McAuley, J. Early and longitudinal evaluations of treated infants and children and untreated historical patients with congenital toxoplasmosis—The chicago collaborative treatment trial. Clin. Infect. Dis. 1994, 18, 38–72. [Google Scholar] [CrossRef]
- McLeod, R.; Kieffer, F.; Sautter, M.; Hosten, T.; Pelloux, H. Why prevent, diagnose and treat congenital toxoplasmosis? Memorias Do Instituto Oswaldo Cruz 2009, 104, 320–344. [Google Scholar] [CrossRef]
- Peck, W.; Burckhardt, P.; Christiansen, C.; Fleisch, H.; Genant, H.; Gennari, C.; Martin, T.; Martini, L.; Morita, R.; Ogata, E. Consensus Development Conference—Diagnosis, prophylaxis, and treatment of osteoporosis. Am. J. Med. 1993, 94, 646–650. [Google Scholar] [CrossRef]
- Kanis, J.A.; Melton, L.J.; Christiansen, C.; Johnston, C.C.; Khaltaev, N. The diagnosis of osteoporosis. J. Bone Miner. Res. 1994, 9, 1137–1141. [Google Scholar]
- Syberg, S.; Schwarz, P.; Petersen, S.; Steinberg, T.H.; Jensen, J.-E.B.; Teilmann, J.; Jørgensen, N.R. Association between P2X7 receptor polymorphisms and bone status in mice. J. Osteoporos. 2012, 2012, 637986. [Google Scholar]
- Kawate, T.; Michel, J.C.; Birdsong, W.T.; Gouaux, E. Crystal structure of the ATP-gated P2X4 ion channel in the closed state. Nature 2009, 460, 592–598. [Google Scholar] [CrossRef]
- Hattori, M.; Gouaux, E. Molecular mechanism of ATP binding and ion channel activation in P2X receptors. Nature 2012, 485, 207–212. [Google Scholar] [CrossRef]
- Jiang, R.; Taly, A.; Grutter, T. Moving through the gate in ATP-activated P2X receptors. Trends Biochem. Sci. 2013, 38, 20–29. [Google Scholar] [CrossRef]
- Browne, L.E.; Jiang, L.-H.; North, R.A. New structure enlivens interest in P2X receptors. Trends Pharmacol. Sci. 2010, 31, 229–237. [Google Scholar] [CrossRef]
- Evans, R.J. Structural interpretation of P2X receptor mutagenesis studies on drug action. Br. J. Pharmacol. 2010, 161, 961–971. [Google Scholar] [CrossRef]
- Jiang, L.-H.; Rassendren, F.; Spelta, V.; Surprenant, A.; North, R.A. Amino acid residues involved in gating identified in the first membrane-spanning domain of the rat P2X2 receptor. J. Biol. Chem. 2001, 276, 14902–14908. [Google Scholar]
- Cao, L.; Broomhead, H.E.; Young, M.T.; North, R.A. Polar residues in the second transmembrane domain of the rat P2X2 receptor that affect spontaneous gating, unitary conductance, and rectification. J. Neurosci. 2009, 29, 14257–14264. [Google Scholar] [CrossRef]
- Rassendren, F.; Buell, G.; Newbolt, A.; North, R.A.; Surprenant, A. Identification of amino acid residues contributing to the pore of a P2X receptor. EMBO J. 1997, 16, 3446–3454. [Google Scholar] [CrossRef]
- Roger, S.; Mei, Z.Z.; Baldwin, J.M.; Dong, L.; Bradley, H.; Baldwin, S.A.; Surprenant, A.; Jiang, L.H. Single nucleotide polymorphisms that were identified in affective mood disorders affect ATP-activated P2X7 receptor functions. J. Psychiatr. Res. 2010, 44, 347–355. [Google Scholar] [CrossRef]
- Stokes, L.; Fuller, S.J.; Sluyter, R.; Skarratt, K.K.; Gu, B.J.; Wiley, J.S. Two haplotypes of the P2X7 receptor containing the Ala-348 to Thr polymorphism exhibit a gain-of-function effect and enhanced interleukin-1β secretion. FASEB J. 2010, 24, 2916–2927. [Google Scholar] [CrossRef]
- Denlinger, L.C.; Coursin, D.B.; Schell, K.; Angelini, G.; Green, D.N.; Guadarrama, A.G.; Halsey, J.; Prabhu, U.; Hogan, K.J.; Bertics, P.J. Human P2X7 pore function predicts allele linkage disequilibrium. Clin. Chem. 2006, 52, 995–1004. [Google Scholar]
- Huang, L.-D.; Fan, Y.-Z.; Tian, Y.; Yang, Y.; Liu, Y.; Wang, J.; Zhao, W.-S.; Zhou, W.-C.; Cheng, X.-Y.; Cao, P. Inherent dynamics of head domain correlates with atp-recognition of P2X4 receptors: Insights gained from molecular simulations. PLoS One 2014, 9, e97528. [Google Scholar]
- Cabrini, G.; Falzoni, S.; Forchap, S.L.; Pellegatti, P.; Balboni, A.; Agostini, P.; Cuneo, A.; Castoldi, G.; Baricordi, O.R.; di Virgilio, F. A His-155 to Tyr polymorphism confers gain-of-function to the human P2X7 receptor of human leukemic lymphocytes. J. Immunol. 2005, 175, 82–89. [Google Scholar] [CrossRef]
- Worthington, R.; Smart, M.; Gu, B.; Williams, D.; Petrou, S.; Wiley, J.; Barden, J. Point mutations confer loss of ATP-induced human P2X7 receptor function. FEBS Lett. 2002, 512, 43–46. [Google Scholar] [CrossRef]
- Bradley, H.J.; Baldwin, J.M.; Goli, G.R.; Johnson, B.; Zou, J.; Sivaprasadarao, A.; Baldwin, S.A.; Jiang, L.-H. Residues 155 and 348 contribute to the determination of P2X7 receptor function via distinct mechanisms revealed by single-nucleotide polymorphisms. J. Biol. Chem. 2011, 286, 8176–8187. [Google Scholar] [CrossRef]
- Ursu, D.; Ebert, P.; Langron, E.; Ruble, C.; Munsie, L.; Zou, W.; Fijal, B.; Qian, Y.W.; McNearney, T.A.; Mogg, A.; et al. Gain and loss of function of P2X7 receptors: Mechanisms, pharmacology and relevance to diabetic neuropathic pain. Mol. Pain 2014, 10, 37. [Google Scholar] [CrossRef]
- Spildrejorde, M.; Bartlett, R.; Stokes, L.; Jalilian, I.; Peranec, M.; Sluyter, V.; Curtis, B.L.; Skarratt, K.K.; Skora, A.; Bakhsh, T.; et al. A R270C polymorphism leads to loss of function of the canine P2X7 receptor. Physiol. Genomics. Available online: http://www.ncbi.nlm.nih.gov/pubmed/24824213 (accessed on 13 May 2014). [CrossRef]
- Li, M.; Kawate, T.; Silberberg, S.D.; Swartz, K.J. Pore-opening mechanism in trimeric P2X receptor channels. Nat. Commun. 2012, 1, 44. [Google Scholar]
- Gu, B.J.; Zhang, W.Y.; Worthington, R.A.; Sluyter, R.; Dao-Ung, P.; Petrou, S.; Barden, J.A.; Wiley, J.S. A Glu-496 to Ala polymorphism leads to loss of function of the human P2X7 receptor. J. Biol. Chem. 2001, 276, 11135–11142. [Google Scholar] [CrossRef]
- Boldt, W.; Klapperstuck, M.; Buttner, C.; Sadtler, S.; Schmalzing, N.; Markwardt, F. Glu(496) Ala polymorphism of human P2X7 receptor does not affect its electrophysiological phenotype. Am. J. Physiol.-Cell. Physiol. 2003, 284, C749–C756. [Google Scholar] [CrossRef]
- Sluyter, R.; Dalitz, J.; Wiley, J. P2X7 receptor polymorphism impairs extracellular adenosine 5'-triphosphate-induced interleukin-18 release from human monocytes. Genes Immun. 2004, 5, 588–591. [Google Scholar] [CrossRef]
- Wesselius, A.; Bours, M.J.L.; Arts, I.C.W.; Theunisz, E.H.E.; Geusens, P.; Dagnelie, P.C. The P2X7 loss-of-function Glu496Ala polymorphism affects ex vivo cytokine release and protects against the cytotoxic effects of high ATP-levels. BMC Immunol. 2012, 13, 64. [Google Scholar]
- Wiley, J.S.; Dao-Ung, L.P.; Li, C.P.; Shemon, A.N.; Gu, B.J.; Smart, M.L.; Fuller, S.J.; Barden, J.A.; Petrou, S.; Sluyter, R. An Ile-568 to Asn polymorphism prevents normal trafficking and function of the human P2X7 receptor. J. Biol. Chem. 2003, 278, 17108–17113. [Google Scholar] [CrossRef]
- Smart, M.L.; Gu, B.; Panchal, R.G.; Wiley, J.; Cromer, B.; Williams, D.A.; Petrou, S. P2X7 receptor cell surface expression and cytolytic pore formation are regulated by a distal C-terminal region. J. Biol. Chem. 2003, 278, 8853–8860. [Google Scholar]
- Tittle, R.K.; Hume, R.I. Opposite effects of zinc on human and rat P2X2 receptors. J. Neurosci. 2008, 28, 11131–11140. [Google Scholar] [CrossRef]
- Rassendren, F.; Buell, G.; Virginio, C.; North, R.A.; Surprenant, A. The permeabilizing ATP receptor, P2X7 cloning and expression of a human cDNA. J. Biol. Chem. 1997, 272, 5482–5486. [Google Scholar]
- Bo, X.; Jiang, L.-H.; Wilson, H.L.; Kim, M.; Burnstock, G.; Surprenant, A.; North, R.A. Pharmacological and biophysical properties of the human P2X5 receptor. Mol. Pharmacol. 2003, 63, 1407–1416. [Google Scholar] [CrossRef]
- Bradley, H.J.; Browne, L.E.; Yang, W.; Jiang, L.H. Pharmacological properties of the rhesus macaque monkey P2X7 receptor. Br. J. Pharmacol. 2011, 164, 743–754. [Google Scholar] [CrossRef]
- Jiang, L.-H.; Mackenzie, A.B.; North, R.A.; Surprenant, A. Brilliant blue G selectively blocks ATP-gated rat P2X7 receptors. Mol. Pharmacol. 2000, 58, 82–88. [Google Scholar]
- Stokes, L.; Jiang, L.H.; Alcaraz, L.; Bent, J.; Bowers, K.; Fagura, M.; Furber, M.; Mortimore, M.; Lawson, M.; Theaker, J. Characterization of a selective and potent antagonist of human P2X7 receptors, AZ11645373. Br. J. Pharmacol. 2006, 149, 880–887. [Google Scholar] [CrossRef]
- Humphreys, B.D.; Virginio, C.; Surprenant, A.; Rice, J.; Dubyak, G.R. Isoquinolines as antagonists of the P2X7 nucleotide receptor: High selectivity for the human versus rat receptor homologues. Mol. Pharmacol. 1998, 54, 22–32. [Google Scholar]
© 2014 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Caseley, E.A.; Muench, S.P.; Roger, S.; Mao, H.-J.; Baldwin, S.A.; Jiang, L.-H. Non-Synonymous Single Nucleotide Polymorphisms in the P2X Receptor Genes: Association with Diseases, Impact on Receptor Functions and Potential Use as Diagnosis Biomarkers. Int. J. Mol. Sci. 2014, 15, 13344-13371. https://doi.org/10.3390/ijms150813344
Caseley EA, Muench SP, Roger S, Mao H-J, Baldwin SA, Jiang L-H. Non-Synonymous Single Nucleotide Polymorphisms in the P2X Receptor Genes: Association with Diseases, Impact on Receptor Functions and Potential Use as Diagnosis Biomarkers. International Journal of Molecular Sciences. 2014; 15(8):13344-13371. https://doi.org/10.3390/ijms150813344
Chicago/Turabian StyleCaseley, Emily A., Stephen P. Muench, Sebastien Roger, Hong-Ju Mao, Stephen A. Baldwin, and Lin-Hua Jiang. 2014. "Non-Synonymous Single Nucleotide Polymorphisms in the P2X Receptor Genes: Association with Diseases, Impact on Receptor Functions and Potential Use as Diagnosis Biomarkers" International Journal of Molecular Sciences 15, no. 8: 13344-13371. https://doi.org/10.3390/ijms150813344
APA StyleCaseley, E. A., Muench, S. P., Roger, S., Mao, H.-J., Baldwin, S. A., & Jiang, L.-H. (2014). Non-Synonymous Single Nucleotide Polymorphisms in the P2X Receptor Genes: Association with Diseases, Impact on Receptor Functions and Potential Use as Diagnosis Biomarkers. International Journal of Molecular Sciences, 15(8), 13344-13371. https://doi.org/10.3390/ijms150813344









