Clinical Significance of POU5F1P1 rs10505477 Polymorphism in Chinese Gastric Cancer Patients Receving Cisplatin-Based Chemotherapy after Surgical Resection
Abstract
:1. Introduction
2. Results
2.1. Patients’ Characteristics
2.2. Associations of POU5F1P1 rs10505477 with Prognosis of Gastric Cancer (GC) Patients
| Variables | Patients, n = 909 | Deaths, n = 418 | MST (Months) | log-Rank p | HR (95% CI) | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Age (years) | |||||||||||
| ≤60 | 429 | 195 | 97 | 0.354 | 1.000 | ||||||
| >60 | 480 | 223 | 62 | 1.095 (0.903–1.327) | |||||||
| Sex | |||||||||||
| Male | 700 | 320 | 74 | 0.57 | 1.000 | ||||||
| Female | 209 | 98 | 67 | 1.067 (0.851–1.338) | |||||||
| Tumor size | |||||||||||
| ≤5 cm | 564 | 236 | 74 | <0.001 | 1.000 | ||||||
| >5 cm | 345 | 182 | 51 | 1.409 (1.616–1.710) | |||||||
| Location | |||||||||||
| Non-Cardia | 601 | 280 | 70 | 0.371 | 1.000 | ||||||
| Cardia | 308 | 138 | 77 | 0.912 (0.744–1.118) | |||||||
| Histological types | |||||||||||
| Intestinal | 387 | 149 | 77 | <0.001 | 1.000 | ||||||
| Diffuse | 518 | 266 | 52 | 1.453 (1.189–1.776) | |||||||
| Others | 4 | 3 | 11 | 2.732 (0.871–8.571) | |||||||
| Differentiation a | |||||||||||
| Well-to-moderate | 297 | 125 | 80 | 0.49 | 1.000 | ||||||
| Poorly | 472 | 228 | 62 | 1.158 (0.931–1.441) | |||||||
| Mucinous/signet-ring cell | 65 | 32 | 62 | 1.202 (0.815–1.772) | |||||||
| Others | 75 | 33 | 67 | 0.986 (0.671–1.448) | |||||||
| Depth of invasion b | |||||||||||
| T1 | 177 | 57 | N/A1 | <0.001 | 1.000 | ||||||
| T2 | 130 | 56 | 78 | 1.452(1.004–2.101) | |||||||
| T3 | 6 | 3 | 70 | 1.427(0.447–4.559) | |||||||
| T4 | 578 | 291 | 52 | 1.839(1.383–2.446) | |||||||
| Lymph node metastasis c | |||||||||||
| N0 | 359 | 128 | N/A1 | <0.001 | 1.000 | ||||||
| N1/N2/N3 | 529 | 277 | 48 | 1.731 (1.403–2.136) | |||||||
| Distant metastasis | |||||||||||
| M0 | 891 | 407 | 74 | 0.296 | 1.000 | ||||||
| M1 | 16 | 9 | 40 | 1.417 (0.732–2.743) | |||||||
| TNM stage | |||||||||||
| I | 239 | 80 | N/A1 | <0.001 | 1.000 | ||||||
| II | 195 | 77 | N/A1 | 1.241 (0.907–1.698) | |||||||
| III | 447 | 244 | 41 | 1.993 (1.547–2.568) | |||||||
| IV | 22 | 11 | 47 | 1.823 (0.970–3.424) | |||||||
| Chemotherapy | |||||||||||
| No | 618 | 293 | 62 | 0.344 | 1.000 | ||||||
| Yes | 291 | 125 | 98 | 0.904 (0.734–1.115) | |||||||
| Chemotherapy regimes | |||||||||||
| l-OHP | 109 | 38 | 60 | 0.082 | 1.000 | ||||||
| DDP | 179 | 89 | 51 | 1.398 (0.954–2.048) | |||||||
| Smoking | |||||||||||
| Non-smoker | 833 | 386 | 67 | 0.432 | 1.000 | ||||||
| Smoker | 76 | 32 | 97 | 0.866 (0.604–1.243) | |||||||
| Drinking | |||||||||||
| Non-drinker | 850 | 389 | 70 | 0.691 | 1.000 | ||||||
| Drinker | 59 | 29 | 63 | 1.079 (0.740–1.574) |
| Genetic Model | Genotypes | Patients | Deaths | MST (Months) | log-Rank p | HR (95% CI) * |
|---|---|---|---|---|---|---|
| Codominant model | GG | 315 | 136 | 77 | 0.185 | 1.000 |
| GA | 434 | 215 | 60 | 1.200 (0.968–1.488) | ||
| AA | 160 | 67 | 69 | 1.014 (0.757–1.359) | ||
| Dominant model | GG | 315 | 136 | 77 | 0.177 | 1.000 |
| GA/AA | 594 | 282 | 63 | 1.150 (0.937–1.411) | ||
| Recessive model | GG/GA | 749 | 351 | 67 | 0.478 | 1.000 |
| AA | 160 | 67 | N/A1 | 0.910 (0.701–1.182) |
| Variables | Genotypes (Dominant Model) | HR (95% CI) a | p Heterogeneity | |
|---|---|---|---|---|
| GG | GA/AA | |||
| Total (n = 909) | 315 | 594 | 1.150 (0.937–1.411) | 0.177 |
| Tumor size | ||||
| ≤5 cm | 202 | 362 | 1.248 (0.949–1.640) | 0.112 |
| >5 cm | 113 | 232 | 0.989 (0.726–1.348) | 0.945 |
| Tumor site | ||||
| Non-Cardia | 207 | 394 | 1.226 (0.951–1.581) | 0.116 |
| Cardia | 108 | 200 | 1.038 (0.733–1.469) | 0.835 |
| Lauren classification | ||||
| Intestinal type | 145 | 242 | 0.996 (0.715–1.387) | 0.98 |
| Diffuse type | 170 | 352 | 1.224 (0.941–1.593) | 0.132 |
| Differentiation | ||||
| Well to moderate | 114 | 183 | 0.988 (0.689–1.418) | 0.949 |
| Poorly | 160 | 312 | 1.102 (0.835–1.454) | 0.494 |
| Mucinous/signet-ring cell | 19 | 46 | 2.075 (0.850–5.065) | 0.109 |
| Others | 22 | 53 | 1.674 (0.754–3.718) | 0.206 |
| Depth of invasion | ||||
| T1 | 66 | 111 | 1.072 (0.634–1.813) | 0.796 |
| T2 | 47 | 83 | 1.502 (0.842–2.680) | 0.168 |
| T3 | 2 | 4 | 0.627 (0.138–2.837) | 0.544 |
| T4 | 194 | 384 | 1.115 (0.872–1.425) | 0.385 |
| Lymph node metastasis | ||||
| N0 | 137 | 222 | 1.181 (0.842–1.655) | 0.335 |
| N1/N2/N3 | 170 | 369 | 1.135 (0.877–1.469) | 0.336 |
| Distant metastasis | ||||
| M0 | 309 | 582 | 1.130 (0.914–1.398) | 0.259 |
| M1 | 6 | 10 | 1.544 (0.721–3.305) | 0.264 |
| TNM stage | ||||
| I | 93 | 149 | 1.238 (0.789–1.942) | 0.352 |
| II | 64 | 195 | 1.032 (0.640–1.664) | 0.896 |
| III | 150 | 299 | 1.080 (0.826–1.412) | 0.574 |
| IV | 8 | 14 | 2.301 (0.600–8.818) | 0.224 |
| Chemotherapy | ||||
| No | 208 | 410 | 1.144 (0.893–1.466) | 0.286 |
| Yes | 107 | 184 | 1.414 (0.792–1.645) | 0.478 |
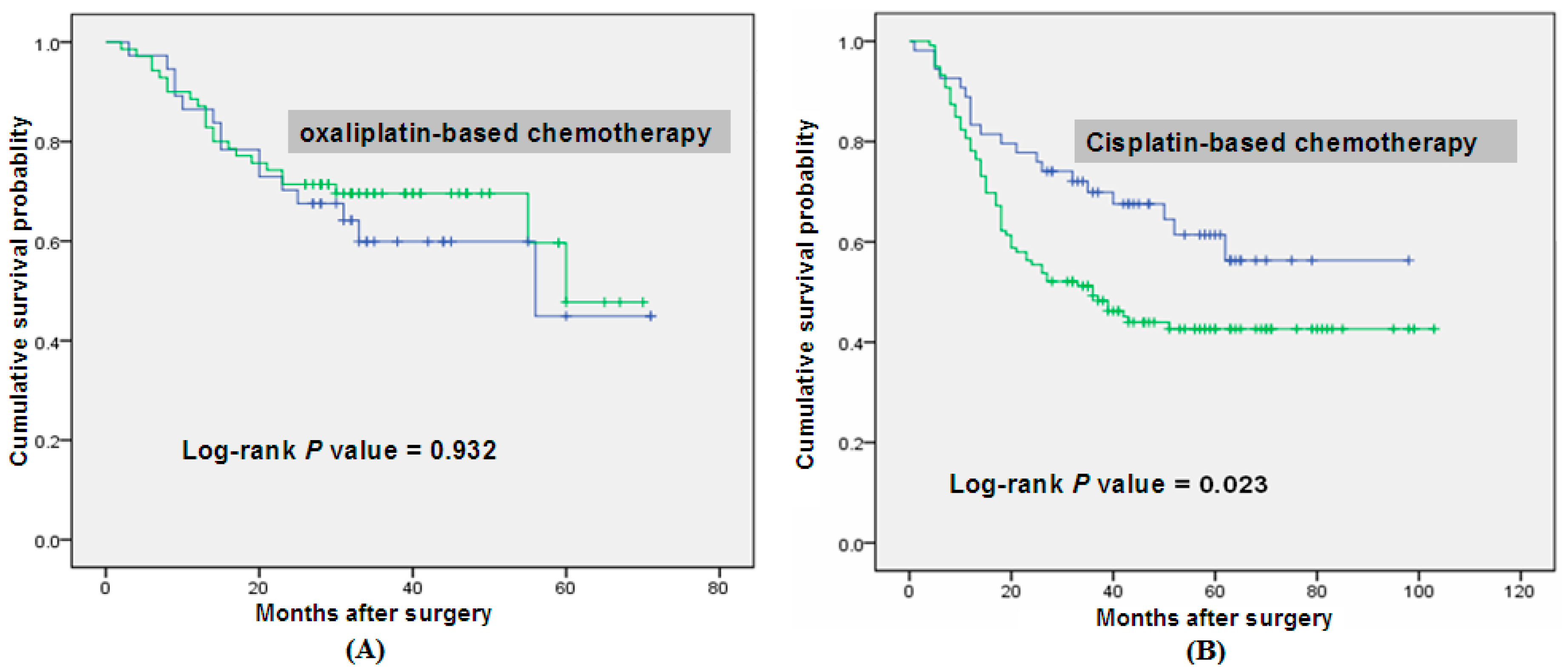
| Chemotherapy Based on l-OHP | |||||
|---|---|---|---|---|---|
| Genotype | Patients, n =108 | Deaths, n = 38 | MST (Months) | log-Rank p | HR (95% CI) a |
| GG | 39 | 14 | 55 | 0.932 | 1.000 |
| GA/AA | 69 | 24 | 60 | 2.038 (0.954–3.041) | |
| Chemotherapy Based on CDDP | |||||
| Genotype | Patients, n =173 | Deaths, n = 86 | MST (Months) | log-Rank p | HR (95% CI) a |
| GG | 54 | 20 | N/A1 | 0.023 | 1.000 |
| GA/AA | 119 | 66 | 36 | 1.764 (1.069–2.911) | |
| Variables | B | SE | HR | 95% CI | p Value |
|---|---|---|---|---|---|
| Age | 0.316 | 0.189 | 1.371 | 0.947–1.985 | 0.094 |
| Sex | 0.267 | 0.228 | 1.306 | 0.835–2.042 | 0.242 |
| Histological types | −0.145 | 0.193 | 0.865 | 0.592–1.264 | 0.454 |
| Regimes (l-OHP vs. DDP) | 0.391 | 0.198 | 1.479 | 1.003–2.180 | 0.048 |
| Dominant model (GG vs. GA/AA) | 0.17 | 0.121 | 1.393 | 0.929–2.089 | 0.109 |
3. Discussion
4. Material and Methods
4.1. Ethics Statement
4.2. Study Subjects
4.3. Genotyping
4.4. Statistical Method
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Siegel, R.; Ma, J.; Zou, Z.; Jemal, A. Cancer statistics. CA Cancer J. Clin. 2014, 64, 9–29. [Google Scholar] [CrossRef]
- Ferlay, J.; Shin, H.R.; Bray, F.; Forman, D.; Mathers, C.; Parkin, D.M. Estimates of worldwide burden of cancer in 2008: GLOBOCAN 2008. Int. J. Cancer 2010, 127, 2893–2917. [Google Scholar] [CrossRef]
- Santoro, R.; Carboni, F.; Lepiane, P.; Ettorre, G.M.; Santoro, E. Clinicopathological features and prognosis of gastric cancer in young European adults. Br. J. Surg. 2007, 94, 737–742. [Google Scholar]
- Takaishi, S.; Okumura, T.; Wang, T.C. Gastric cancer stem cells. J. Clin. Oncol. 2008, 26, 2876–2882. [Google Scholar] [CrossRef]
- Takaishi, S.; Okumura, T.; Tu, S.; Wang, S.S.; Shibata, W.; Vigneshwaran, R.; Gordon, S.A.; Shimada, Y.; Wang, T.C. Identification of gastric cancer stem cells using the cell surface marker CD44. Stem Cells 2009, 27, 1006–1020. [Google Scholar]
- Han, M.E.; Jeon, T.Y.; Hwang, S.H.; Lee, Y.S.; Kim, H.J.; Shim, H.E.; Yoon, S.; Baek, S.Y.; Kin, B.S.; Kang, C.D.; et al. Cancer spheres from gastric cancer patients provide an ideal model system for cancer stem cell research. Cell. Mol. Life Sci. 2011, 68, 3589–3605. [Google Scholar] [CrossRef]
- Tatematsu, M.; Tsukamoto, T.; Inada, K. Stem cells and gastric cancer: Role of gastric and intestinal mixed intestinal metaplasia. Cancer Sci. 2003, 94, 135–141. [Google Scholar] [CrossRef]
- Zhang, C.; Li, C.; He, F.; Cai, Y.; Yang, H. Identification of CD44+CD24+ gastric cancer stem cells. J. Cancer Res. Clin. Oncol. 2011, 137, 1679–1686. [Google Scholar] [CrossRef]
- Alison, M.R.; Poulsom, R.; Forbes, S.; Wright, N.A. An introduction to stem cells. J. Pathol. 2002, 197, 419–423. [Google Scholar] [CrossRef]
- Pan, G.J.; Chang, Z.Y.; Scholer, H.R.; Pei, D. Stem cell pluripotency and transcription factor OCT4. Cell Res. 2002, 12, 321–329. [Google Scholar] [CrossRef]
- Boyer, L.A.; Lee, T.I.; Cole, M.F.; Johnstone, S.E.; Levine, S.S.; Boyer, LAZucker, J.P.; Guenther, M.G.; Kumar, R.M.; Murray, H.L.; et al. Core transcriptional regulatory circuitry in human embryonic stem cells. Cell 2005, 122, 947–956. [Google Scholar] [CrossRef]
- Cheng, C.J.; Wu, Y.C.; Shu, J.A.; Ling, T.Y.; Kuo, H.C.; Wu, J.Y.; Chang, E.E.; Chang, S.C.; Huang, Y.H. Aberrant expression and distribution of the OCT-4 transcription factor in seminomas. J. Biomed. Sci. 2007, 14, 797–807. [Google Scholar] [CrossRef]
- Matsuoka, J.; Yashiro, M.; Sakurai, K.; Kubo, N.; Tanaka, H.; Muguruma, K.; Sawada, T.; Ohira, M.; Hirakawa, K. Role of the stemness factors Sox2, OCT3/4 and nanog in gastric carcinoma. J. Surg. Res. 2012, 174, 130–135. [Google Scholar] [CrossRef]
- Suo, G.; Han, J.; Wang, X.; Zhang, J.; Zhao, Y.; Zhao, Y.; Dai, J. OCT4 pseudogenes are transcribed in cancers. Biochem. Biophys. Res. Commun. 2005, 337, 1047–1051. [Google Scholar] [CrossRef]
- Panagopoulos, I.; Moller, E.; Collin, A.; Mertens, F. The POU5F1P1 pseudogene encodes a putative protein similar to POU5F1 isoform 1. Oncol. Rep. 2008, 20, 1029–1033. [Google Scholar]
- Pal, P.; Xi, H.; Guha, S.; Sun, G.; Helfand, B.T.; Meeks, J.J.; Suarez, B.K.; Catalona, W.J.; Deka, R. Common variants in 8q24 are associated with risk for prostate cancer and tumor aggressiveness in men of European ancestry. Prostate 2009, 69, 1548–1556. [Google Scholar] [CrossRef]
- Wei, W.; Jiang, M.; Luo, L.; Li, Z.; Wang, P.; Dong, W.Q. Colorectal cancer susceptibility variants alter risk of breast cancer in a Chinese Han population. Genet. Mol. Res. 2013, 12, 6268–6274. [Google Scholar]
- Gruber, S.B.; Moreno, V.; Rozek, L.S.; Rennerts, H.S.; Lejbkowicz, F.; Bonner, J.D.; Greenson, J.K.; Giordano, T.J.; Fearson, E.R.; Rennert, G. Genetic variation in 8q24 associated with risk of colorectal cancer. Cancer Biol. Ther. 2007, 6, 1143–1147. [Google Scholar]
- Poynter, J.N.; Figueiredo, J.C.; Conti, D.V.; Kennedy, K.; Gallinger, S.; Siegmund, K.D.; Casey, G.; Thibodeau, S.N.; Jenkins, M.A.; Hopper, J.L.; et al. Variants on 9p24 and 8q24 are associated with risk of colorectal cancer: Results from the colon cancer family registry. Cancer Res. 2007, 67, 11128–11132. [Google Scholar]
- Schafmayer, C.; Buch, S.; Volzke, H.; von Schonfels, W.; Egberts, J.H.; Schniewind, B.; Brosch, M.; Ruether, A.; Franke, A.; Mathiak, M.; et al. Investigation of the colorectal cancer susceptibility region on chromosome 8q24.21 in a large German case-control sample. Int. J. Cancer 2009, 124, 75–80. [Google Scholar]
- Dai, J.; Gu, J.; Huang, M.; Eng, C.; Kopetz, E.S.; Ellis, L.M.; Hawk, E.; Wu, X. GWAS-identified colorectal cancer susceptibility loci associated with clinical outcomes. Carcinogenesis 2012, 33, 1327–1331. [Google Scholar] [CrossRef]
- Hutter, C.M.; Slattery, M.L.; Duggan, D.J.; Muehling, J.; Curtin, K.; Beresford, S.A.; Rajkovic, A.; Sarto, G.E.; Marshall, J.R.; et al. Characterization of the association between 8q24 and colon cancer: Gene-environment exploration and meta-analysis. BMC Cancer 2010, 10, 670. [Google Scholar] [CrossRef]
- White, K.L.; Sellers, T.A.; Fridley, B.L.; Vierkant, R.A.; Phelan, C.M.; Tsai, Y.Y.; Kalli, K.R.; Berchuck, A.; Iversen, E.S.; Hartmann, L.C.; et al. Variation at 8q24 and 9p24 and risk of epithelial ovarian cancer. Twin. Res. Hum. Genet. 2010, 13, 43–56. [Google Scholar] [CrossRef]
- Lochhead, P.; Ng, M.T.H.; Hold, G.L.; Rabkin, C.S.; Vaughan, T.L.; Gammon, M.D.; Risch, H.A.; Lissowska, J.; Mukhopadhya, I.; Chow, W.H.; et al. Possible association between a genetic polymorphism at 8q24 and risk of upper gastrointestinal cancer. Eur. J. Cancer. Prev. 2011, 20, 54–57. [Google Scholar] [CrossRef]
- Zhang, Y.; Wang, M.; Gu, D.; Wu, D.; Zhang, X.; Gong, W.; Tan, Y.; Zhou, J.; Wu, X.; Tang, C; et al. Association of XRCC1 gene polymorphisms with the survival and clinicopathological characteristics of gastric cancer. DNA Cell Biol. 2013, 32, 111–118. [Google Scholar] [CrossRef]
- Zhang, X.; Zhu, H.; Wu, X.; Wang, M.; Gu, D.; Gu, D.; Gong, W.; Xu, Z.; Tan, Y.; Gong, Y.; et al. A genetic polymorphism in TOX3 is associated with survival of gastric cancer in a Chinese population. PLoS One 2013, 8, e72186. [Google Scholar]
- Zhang, Y.; Zhu, H.; Zhang, X.; Gu, D.; Zhou, X.; Wang, M.; Cao, C.; Zhang, X.; Wu, X.; Gong, W.; et al. linical significance of MYT1L gene polymorphisms in Chinese patients with gastric cancer. PLoS One 2013, 8, e71979. [Google Scholar]
- Spradling, A.; Drummond-Barbosa, D.; Kai, T. Stem cells find their niche. Nature 2001, 414, 98–104. [Google Scholar] [CrossRef]
- Ezeh, U.I.; Turek, P.J.; Reijo, R.A.; Clark, A.T. Human embryonic stem cell genes OCT4, NANOG, STELLAR, and GDF3 are expressed in both seminoma and breast carcinoma. Cancer 2005, 104, 2255–2265. [Google Scholar] [CrossRef]
- Wen, K.; Fu, Z.; Wu, X.; Feng, J.; Chen, W.; Qian, J. OCT-4 is required for an antiapoptotic behavior of chemoresistant colorectal cancer cells enriched for cancer stem cells: Effects associated with STAT3/Survivin. Cancer Lett. 2013, 333, 56–65. [Google Scholar] [CrossRef]
- Davidson, K.C.; Adams, A.M.; Goodson, J.M.; McDonald, C.E.; Potter, J.C.; Berndt, J.D.; Biechele, T.L.; Taylor, R.J.; Moon, R.T. Wnt/β-catenin signaling promotes differentiation not self-renewal of human embryonic stem cells and is repressed by OCT4. Proc. Natl. Acad. Sci. USA 2012, 109, 4485–4490. [Google Scholar] [CrossRef]
- Wang, X.Q.; Ongkeko, W.M.; Chen, L.; Yang, Z.F.; Lu, P.; Chen, K.K.; Lopez, J.P.; Poon, R.T.; Fan, S.T. Octamer 4 (Oct4) mediates chemotherapeutic drug resistance in liver cancer cells through a potential Oct4–AKT–ATP-binding cassette G2 pathway. Hepatology 2010, 52, 528–539. [Google Scholar] [CrossRef]
- Kastler, S.; Honold, L.; Luedeke, M.; Kuefer, R.; Moller, P.; Hoegel, J.; Vogel, W.; Maier, C.; Assum, G. POU5F1P1, a putative cancer susceptibility gene, is overexpressed in prostatic carcinoma. Prostate 2010, 7, 666–674. [Google Scholar]
- Cunningham, D.; Rao, S.; Starling, N.; Iveson, T.; Nicolson, M.; Coxon, F.; Middleton, G.; Daniel, F.; Oates, J.; Norman, A.R. Randomised multicentre phase III study comparingcapecitabine with fluorouracil and oxaliplatin with cisplatin in patients with advanced oesophagogastric (OG) cancer: The REAL 2 trial. J. Clin. Oncol. 2006, 24, 18S. [Google Scholar] [CrossRef]
- Kim, Y.S.; Sym, S.J.; Park, S.H.; Park, I.; Hong, J.; Ahn, H.K.; Park, J.; Cho, E.K.; Lee, W.K.; Chung, M.; et al. A randomized phase II study of weekly docetaxel/cisplatin vs. weekly docetaxel/oxaliplatin as first-line therapy for patients with advanced gastric cancer. Cancer Chemother. Pharmacol. 2014, 73, 163–169. [Google Scholar] [CrossRef]
- Al-Batran, S.E.; Hartmann, J.T.; Probst, S.; Schmalenberg, H.; Hollerbach, S.; Hofheinz, R.; Rethwisch, V.; Seipelt, G.; Homann, N.; Wilhelm, G.; et al. Phase III trial in metastatic gastroesophageal adenocarcinoma with fluorouracil, leucovorin plus either oxaliplatin or cisplatin: A study of the arbeitsgemeinschaft internistische onkologie. J. Clin. Oncol. 2008, 26, 1435–1442. [Google Scholar] [CrossRef]
- Gottesman, M.M.; Fojo, T.; Bates, S.E. Multidrug resistance in cancer: Role of ATP-dependent transporters. Nat. Rev. Cancer 2002, 2, 48–58. [Google Scholar] [CrossRef]
- Stewart, D.J. Mechanisms of resistance to cisplatin and carboplatin. Crit. Rev. Oncol. Hematol. 2007, 63, 12–31. [Google Scholar] [CrossRef]
- Chu, W.; Pak, B.J.; Bani, M.R.; Kapoor, M.; Lu, S.J.; Tamir, A.; Kerbel, R.S.; Ben-David, Y. Tyrosinase-related protein 2 as a mediator of melanoma specific resistance to cis-diamminedichloroplatinum(II): Therapeutic implications. Oncogene 2000, 19, 395–402. [Google Scholar] [CrossRef]
- Mizutani, Y.; Bonavida, B. Overcoming cis-diamminedichloroplatinum(II) resistance of human ovarian tumor cells by combination treatment with cis-diamminedichloroplatinum(II) and tumor necrosis factor-α. Cancer 1993, 72, 809–818. [Google Scholar] [CrossRef]
- Liu, L.Z.; Zhou, X.D.; Qian, G.; Shi, X.; Fang, J.; Jiang, B.H. AKT1 amplification regulates cisplatin resistance in human lung cancer cells through the mammalian target of rapamycin/p70S6K1 pathway. Cancer Res. 2007, 67, 6325–6332. [Google Scholar]
- Meijer, C.; Mulder, N.H.; Timmer-Bosscha, H.; Sluiter, W.J.; Meersma, G.J.; de Vries, E.G. Relationship of cellular glutathione to the cytotoxicity and resistance of seven platinum compounds. Cancer Res. 1992, 52, 6885–6889. [Google Scholar]
- Zhang, L.L.; Zhang, J.; Shen, L.; Xu, X.M.; Yu, H.G. Overexpression of AKT decreases the chemosensitivity of gastric cancer cells to cisplatin in vitro and in vivo. Mol. Med. Rep. 2013, 7, 1387–1390. [Google Scholar]
- Huang, S.; Chen, M.; Shen, Y.; Shen, W.; Guo, H.; Gao, Q.; Zuo, X. Inhibition of activated Stat3 reverses drug resistance to chemotherapeutic agents in gastric cancer cells. Cancer Lett. 2012, 315, 198–205. [Google Scholar] [CrossRef]
- Xu, N.; Shen, C.; Luo, Y.; Xia, L.; Xue, F.; Xia, Q.; Zhang, J. Upregulated miR-130a increases drug resistance by regulating RUNX3 and Wnt signaling in cisplatin-treated HCC cell. Biochem. Biophys. Res. Commun. 2012, 425, 468–472. [Google Scholar] [CrossRef]
- Gosepath, E.M.; Eckstein, N.; Hamacher, A.; Servan, K.; von Jonquieres, G.; Lage, H.; Györffy, B.; Royer, H.D.; Kassack, M.U. Acquired cisplatin resistance in the head-neck cancer cell line Cal27 is associated with decreased DKK1 expression and can partially be reversed by overexpression of DKK1. Int. J. Cancer 2008, 123, 2013–2019. [Google Scholar] [CrossRef]
- Biroccio, A.; Benassi, B.; Fiorentino, F.; Zupi, G. Glutathione depletion induced by c-Myc downregulation triggers apoptosis on treatment with alkylating agents. Neoplasia 2004, 6, 195–206. [Google Scholar] [CrossRef]
© 2014 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Shen, L.; Du, M.; Wang, C.; Gu, D.; Wang, M.; Zhang, Q.; Zhao, T.; Zhang, X.; Tan, Y.; Huo, X.; et al. Clinical Significance of POU5F1P1 rs10505477 Polymorphism in Chinese Gastric Cancer Patients Receving Cisplatin-Based Chemotherapy after Surgical Resection. Int. J. Mol. Sci. 2014, 15, 12764-12777. https://doi.org/10.3390/ijms150712764
Shen L, Du M, Wang C, Gu D, Wang M, Zhang Q, Zhao T, Zhang X, Tan Y, Huo X, et al. Clinical Significance of POU5F1P1 rs10505477 Polymorphism in Chinese Gastric Cancer Patients Receving Cisplatin-Based Chemotherapy after Surgical Resection. International Journal of Molecular Sciences. 2014; 15(7):12764-12777. https://doi.org/10.3390/ijms150712764
Chicago/Turabian StyleShen, Lili, Mulong Du, Chun Wang, Dongying Gu, Meilin Wang, Qi Zhang, Tingting Zhao, Xunlei Zhang, Yongfei Tan, Xinying Huo, and et al. 2014. "Clinical Significance of POU5F1P1 rs10505477 Polymorphism in Chinese Gastric Cancer Patients Receving Cisplatin-Based Chemotherapy after Surgical Resection" International Journal of Molecular Sciences 15, no. 7: 12764-12777. https://doi.org/10.3390/ijms150712764
APA StyleShen, L., Du, M., Wang, C., Gu, D., Wang, M., Zhang, Q., Zhao, T., Zhang, X., Tan, Y., Huo, X., Gong, W., Xu, Z., Chen, J., & Zhang, Z. (2014). Clinical Significance of POU5F1P1 rs10505477 Polymorphism in Chinese Gastric Cancer Patients Receving Cisplatin-Based Chemotherapy after Surgical Resection. International Journal of Molecular Sciences, 15(7), 12764-12777. https://doi.org/10.3390/ijms150712764




