Continuous Flow Atmospheric Pressure Laser Desorption/Ionization Using a 6–7-µm-Band Mid-Infrared Tunable Laser for Biomolecular Mass Spectrometry
Abstract
:1. Introduction
2. Results and Discussion
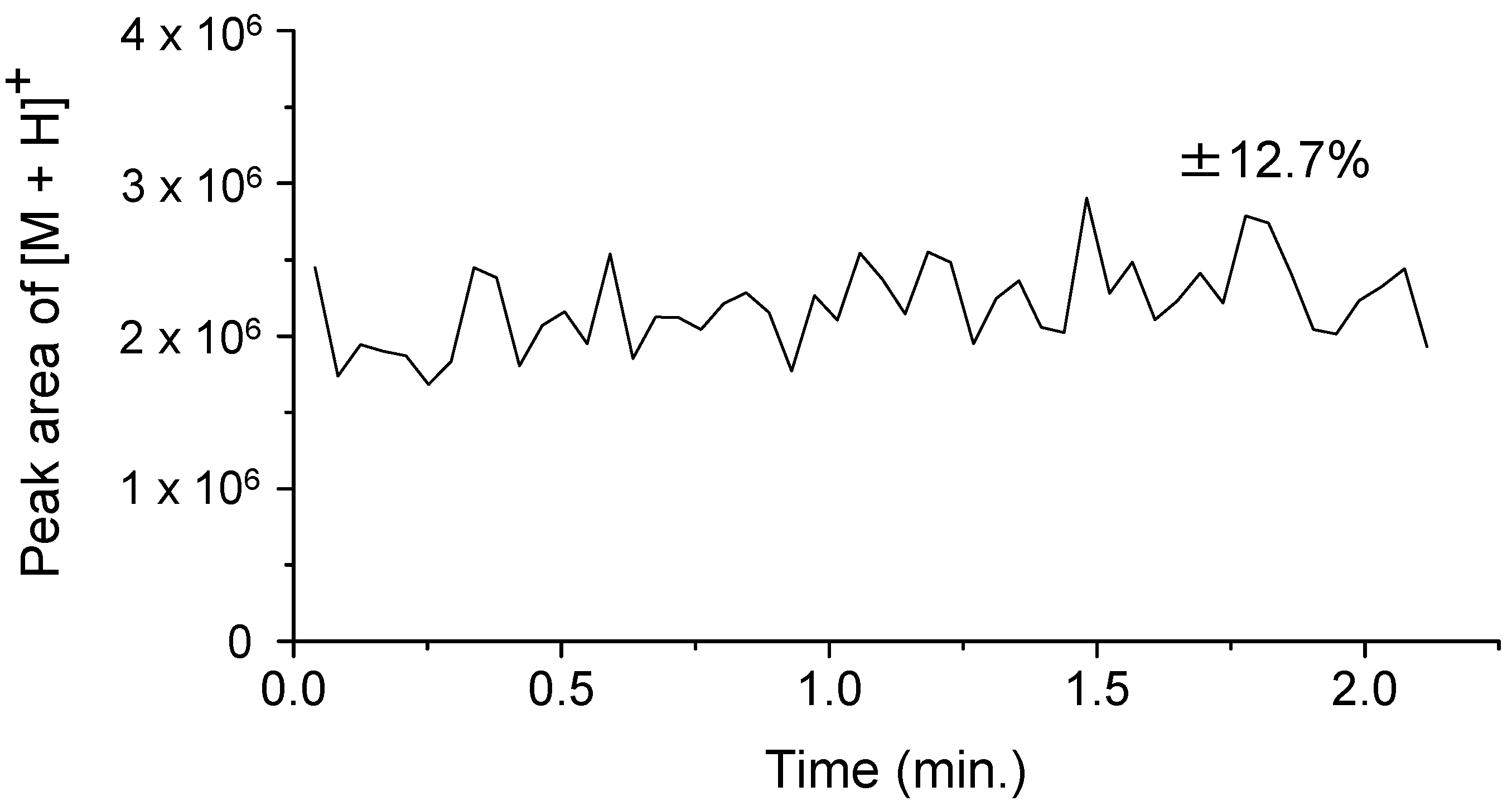
2.1. Temporal Stability of Continuous Flow (CF) Ionization of Peptides
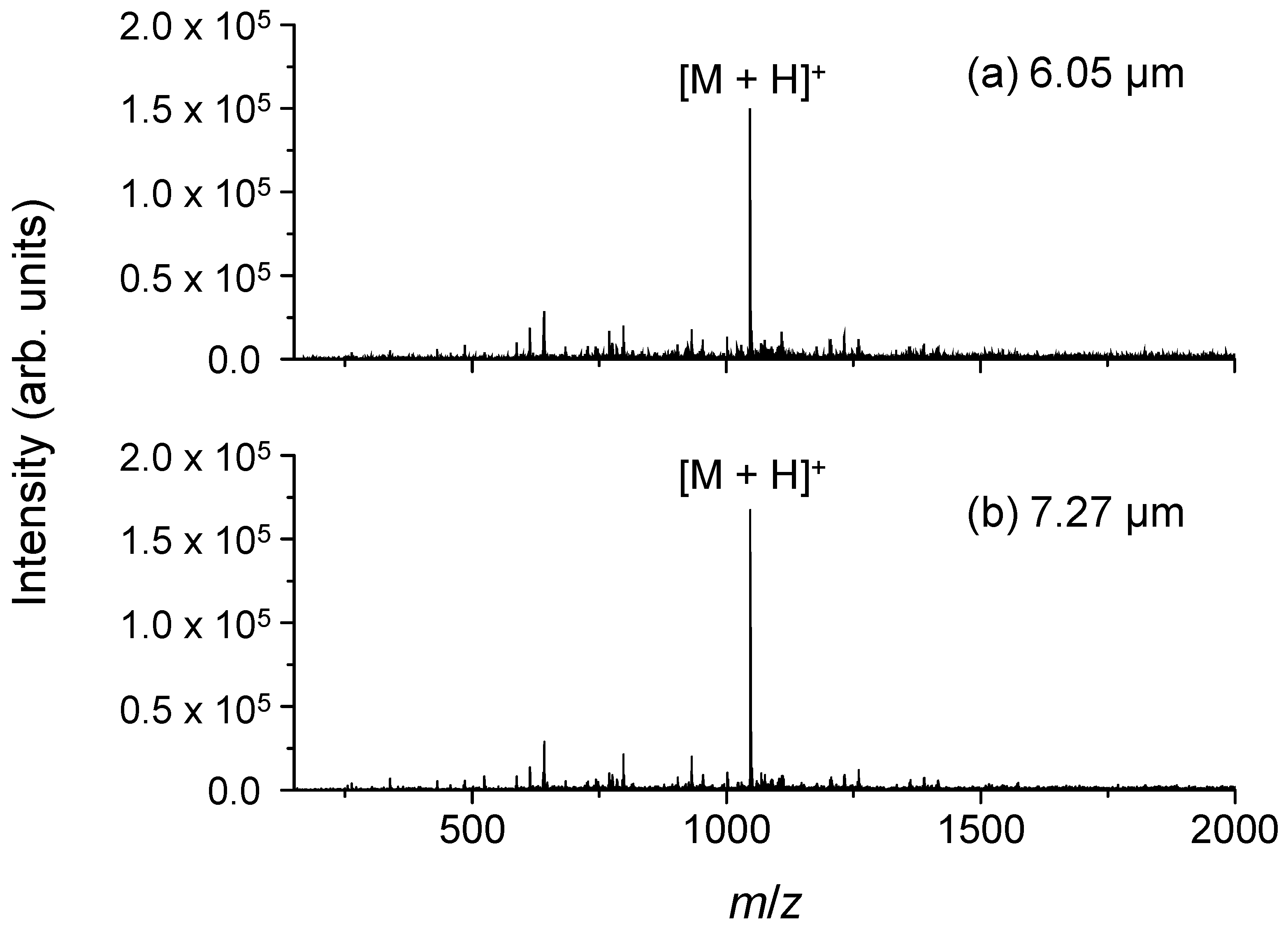
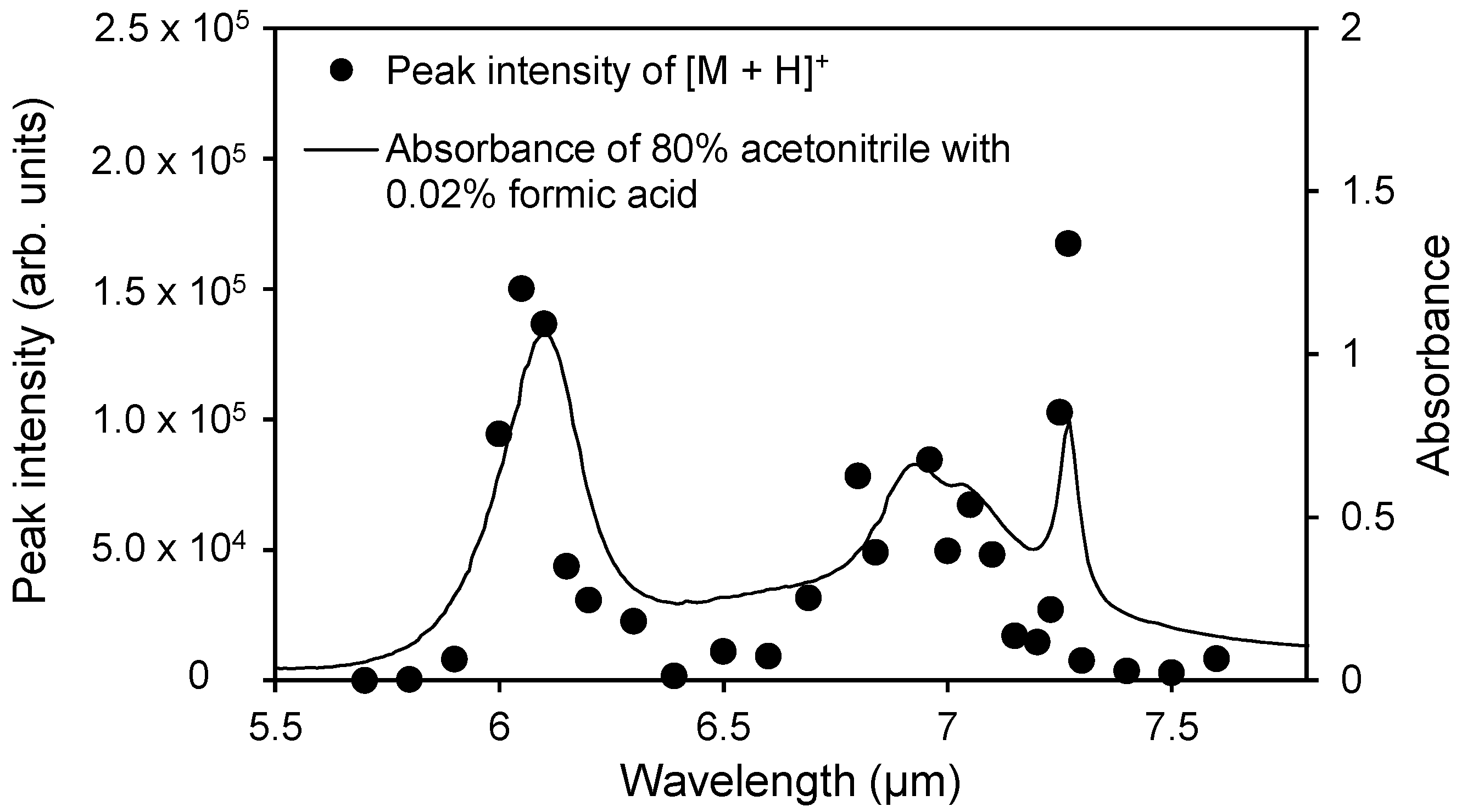
2.2. Wavelength Dependence of the Ion Signal Intensity

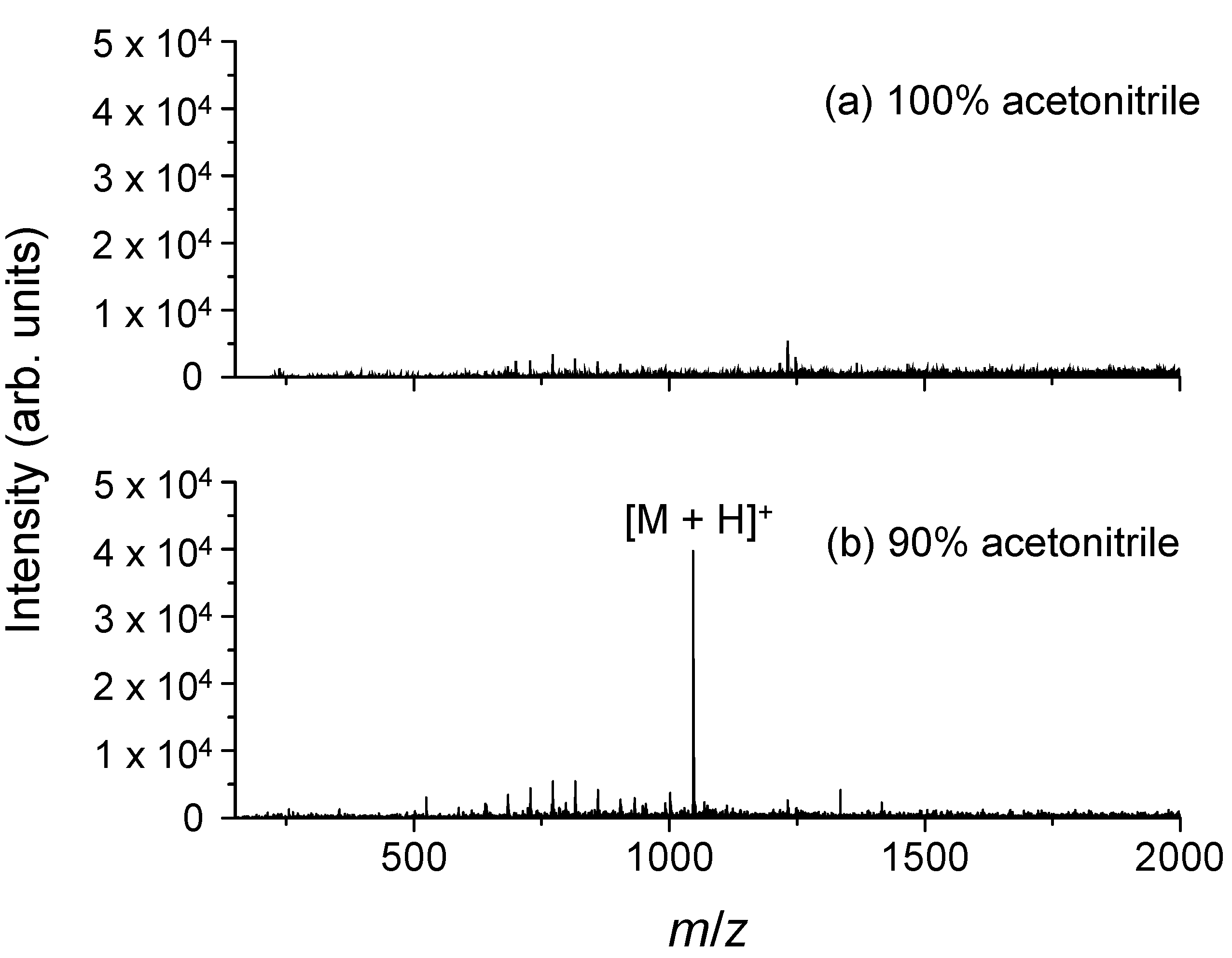
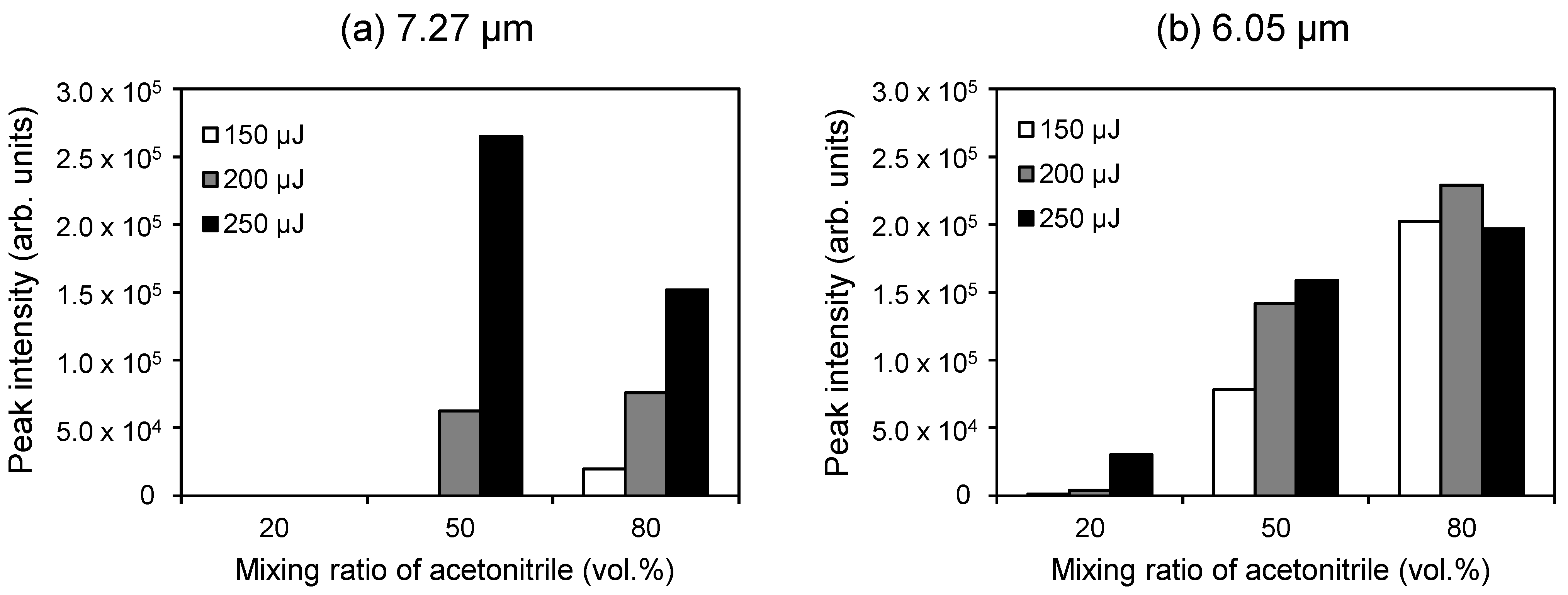
2.3. Dependence of the Ionization Efficiency on the Mixing Ratio of Water and Organic Solvent
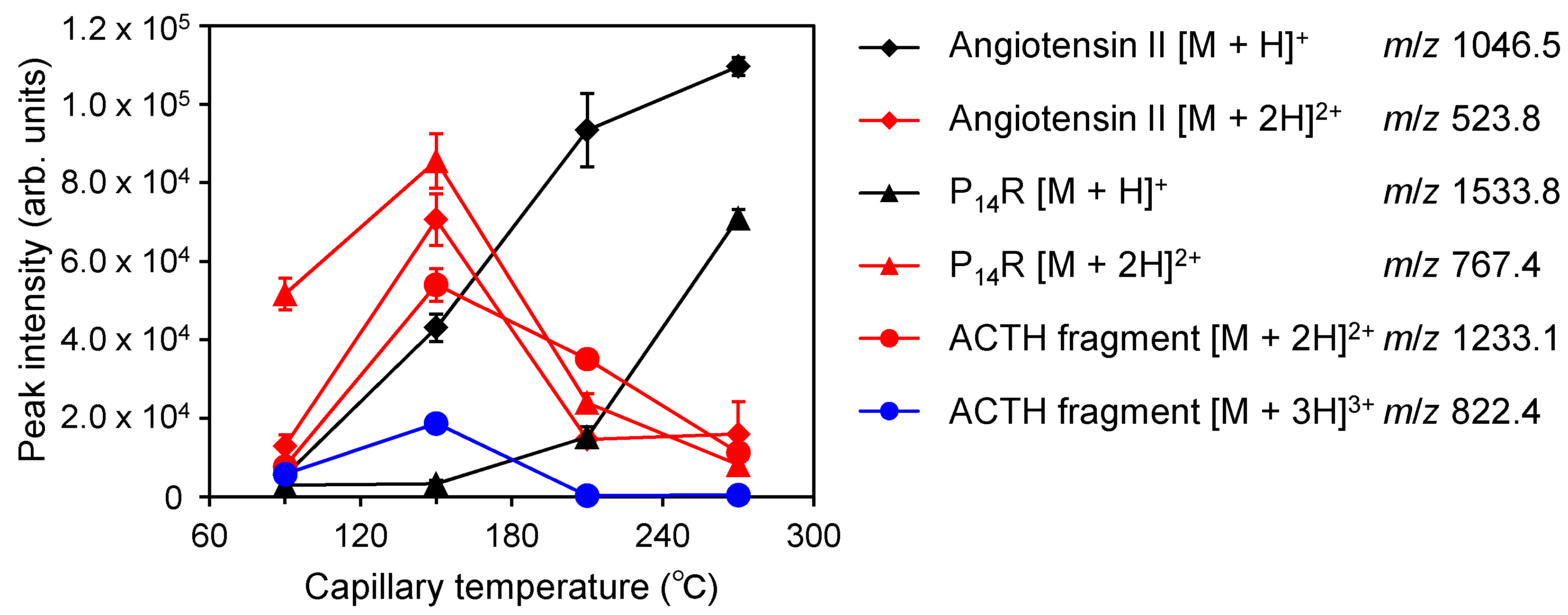
2.4. Relationship between the Generation of Multiply Charged Ions and the Desolvation Temperature
3. Experimental Section
3.1. Mid-Infrared Tunable Laser Using Difference-Frequency Generation (DFG)
3.2. Ion Trap Mass Spectrometer with an Atmospheric Pressure Laser Ion Source
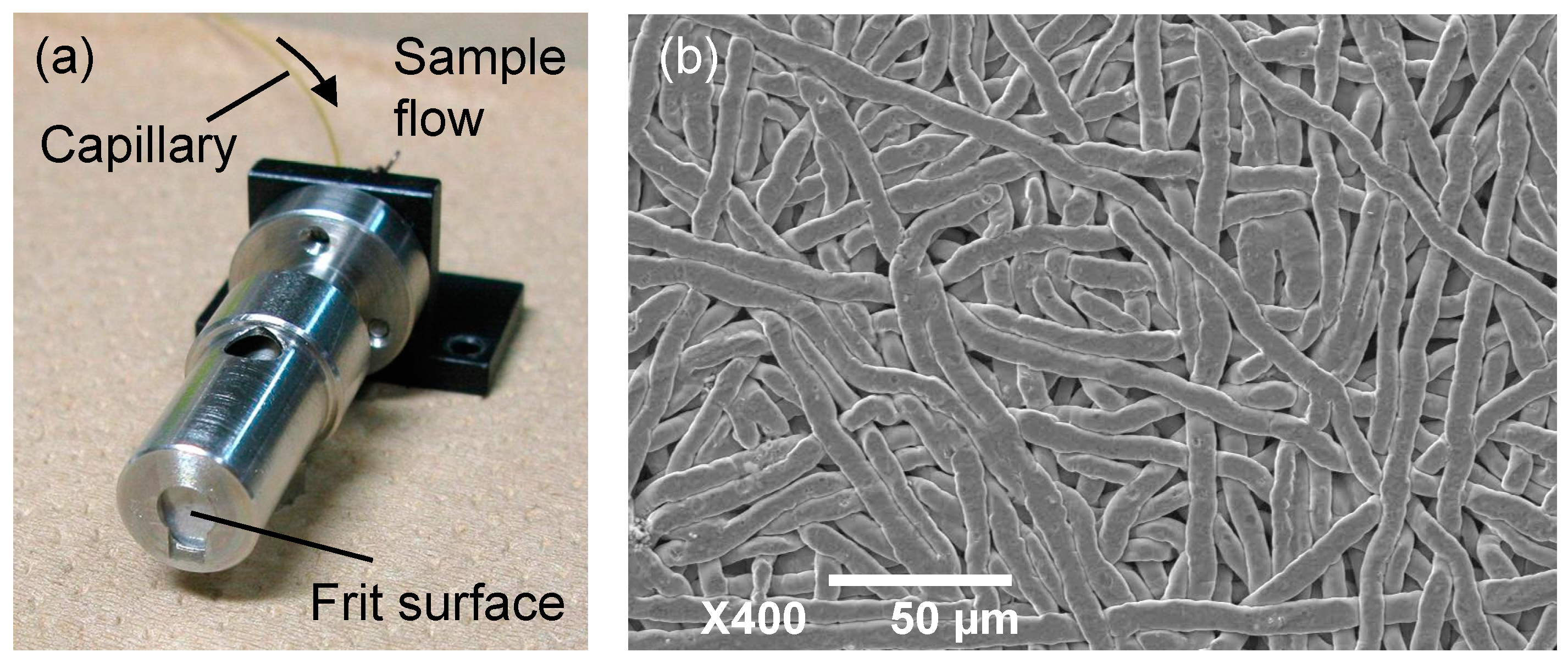
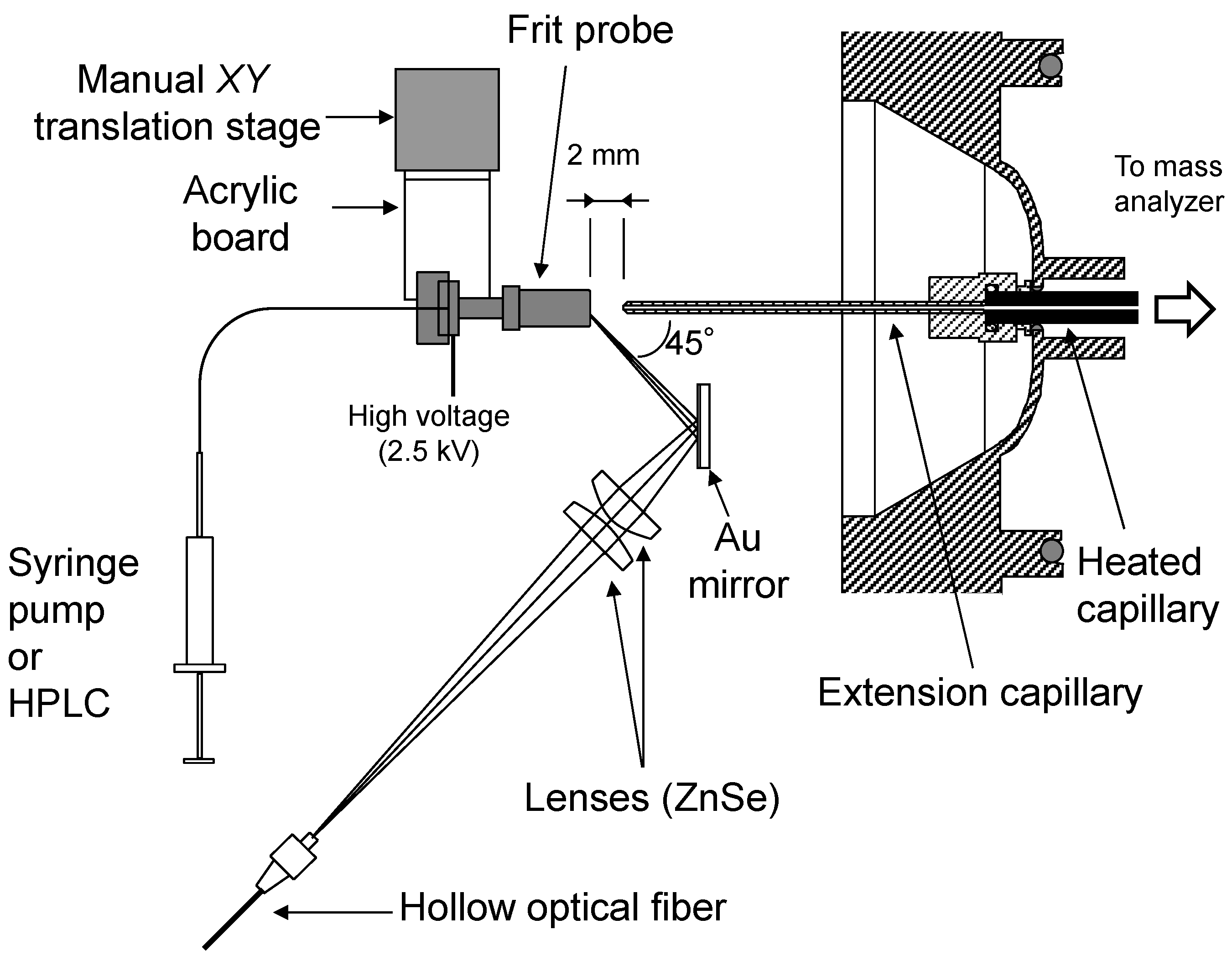
3.3. Materials and Methods
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Fenn, J.B.; Mann, M.; Meng, C.K.; Wong, S.F.; Whitehouse, C.M. Electrospray ionization for mass spectrometry of large biomolecules. Science 1989, 246, 64–71. [Google Scholar]
- Fenn, J.B.; Mann, M.; Meng, C.K.; Wong, S.F. Electrospray ionization–principles and practice. Mass Spectrom. Rev. 1990, 9, 37–70. [Google Scholar] [CrossRef]
- Karas, M.K.; Bachmann, D.; Bahr, U.; Hillenkamp, F. Matrix-assisted ultraviolet laser desorption of non-volatile compounds. Int. J. Mass Spectrom. Ion Process. 1987, 78, 53–68. [Google Scholar] [CrossRef]
- Karas, M.; Hillenkamp, F. Laser desorption ionization of proteins with molecular masses exceeding 10,000 daltons. Anal. Chem. 1988, 60, 2299–2301. [Google Scholar] [CrossRef]
- Huang, M.Z.; Hsu, H.J.; Lee, J.Y.; Jeng, J.J.; Shiea, J. Direct protein detection from biological media through electrospray-assisted laser desorption ionization/mass spectrometry. J. Proteome Res. 2006, 5, 1107–1116. [Google Scholar]
- Sampson, J.S.; Hawkridge, A.M.; Muddiman, D.C. Generation and detection of multiply-charged peptides and proteins by matrix-assisted laser desorption electrospray ionization (MALDESI) fourier transform ion cyclotron resonance mass spectrometry. J. Am. Soc. Mass Spectrom. 2006, 17, 1712–1716. [Google Scholar] [CrossRef]
- Nemes, P.; Vertes, A. Laser ablation electrospray ionization for atmospheric pressure, in vivo, and imaging mass spectrometry. Anal. Chem. 2007, 79, 8098–8106. [Google Scholar] [CrossRef]
- Takats, Z.; Wiseman, J.M.; Gologan, B.; Cooks, R.G. Mass spectrometry sampling under ambient conditions with desorption electrospray ionization. Science 2004, 306, 471–473. [Google Scholar] [CrossRef]
- Kertesz, V.; Ford, M.J.; Berkel, G.V. Automation of a surface sampling probe/electrospray mass spectrometry system. Anal. Chem. 2005, 77, 7183–7189. [Google Scholar] [CrossRef]
- Hiraoka, K.; Nishidate, K.; Mori, K.; Asakawa, D.; Suzuki, S. Development of probe electrospray using a solid needle. Rapid Commun. Mass Spectrom. 2007, 21, 3139–3144. [Google Scholar] [CrossRef]
- Mandal, M.K.; Chen, L.C.; Hashimoto, Y.; Yu, Z.; Hiraoka, K. Detection of biomolecules from solutions with high concentration of salts using probe electrospray and nano-electrospray ionization mass spectrometry. Anal. Methods 2010, 2, 1905–1912. [Google Scholar] [CrossRef]
- Overberg, A.; Karas, M.; Bahr, U.; Kaufmann, R.; Hillenkamp, F. Matrixassisted infrared-laser (2.94 µm) desorption/ionization mass spectrometry of large biomolecules. Rapid Commun. Mass Spectrom. 1990, 4, 293–296. [Google Scholar] [CrossRef]
- Overberg, A.; Karas, M.; Hillenkamp, F. Matrix-assisted laser desorption of large biomolecules with a TEA-CO2-laser. Rapid Commun. Mass Spectrom. 1991, 5, 128–131. [Google Scholar] [CrossRef]
- Sheffer, J.D.; Murray, K.K. Infrared matrix-assisted laser desorption/ionization using OH, NH and CH vibrational absorption. Rapid Commun. Mass Spectrom. 1998, 12, 1685–1690. [Google Scholar] [CrossRef]
- Laiko, V.V.; Baldwin, M.A.; Burlingame, A.L. Atmospheric pressure matrix-assisted laser desorption/ionization mass spectrometry. Anal. Chem. 2000, 72, 652–657. [Google Scholar] [CrossRef]
- Laiko, V.V.; Moyer, S.C.; Cotter, R.J. Atmospheric pressure MALDI/ion trap mass spectrometry. Anal. Chem. 2000, 72, 5239–5243. [Google Scholar] [CrossRef]
- Moyer, S.C.; Marzilli, L.A.; Woods, A.S.; Laiko, V.V.; Doroshenko, V.M.; Cotter, R.J. Atmospheric pressure matrix-assisted laser desorption/ionization (AP MALDI) on a quadrupole ion trap mass spectrometer. Int. J. Mass Spectrom. 2003, 226, 133–150. [Google Scholar] [CrossRef]
- Daniel, J.M.; Ehala, S.; Friess, S.D.; Zenobi, R. On-line atmospheric pressure matrix-assisted laser desorption/ionization mass spectrometry. Analyst 2004, 129, 574–578. [Google Scholar] [CrossRef]
- Daniel, J.M.; Laiko, V.V.; Droshenko, V.M.; Zenobi, R. Interfacing liquid chromatography with atmospheric pressure MALDI-MS. Anal. Bioanal. Chem. 2005, 383, 895–902. [Google Scholar] [CrossRef]
- Laiko, V.V.; Taranenko, N.I.; Berkout, V.D.; Yakshin, M.A.; Prasad, C.R.; Lee, H.S.; Doroshenko, V.M. Desorption/ionization of biomolecules from aqueous solution at atmospheric pressure using an infrared laser at 3 µm. J. Am. Soc. Mass Spectrom. 2002, 13, 354–361. [Google Scholar] [CrossRef]
- Rapp, E.; Charvat, A.; Beinsen, A.; Reichl, U.; Morgenstern, A.S.; Urlaub, H.; Abel, B. Atmospheric pressure free liquid infrared MALDI mass spectrometry: Toward a combined ESI/MALDI-liquid chromatography interface. Anal. Chem. 2009, 81, 443–452. [Google Scholar]
- Tajiri, M.; Takeuchi, T.; Wada, Y. Distinct features of matrix-assisted 6 µm infrared laser desorption/ionization mass spectrometry in biomolecular analysis. Anal. Chem. 2009, 81, 6750–6755. [Google Scholar] [CrossRef]
- Tajiri, M.; Wada, Y. Infrared matrix-assisted laser desorption/ionization mass spectrometry for quantification of glycosaminoglycans and gangliosides. Int. J. Mass Spectrom. 2011, 305, 164–169. [Google Scholar] [CrossRef]
- Naito, Y.; Yoshihashi-Suzuki, S.; Ishii, K.; Kanai, T.; Awazu, K. Matrix-assisted laser desorption/ionization of protein samples containing a denaturant at high concentration using a mid-infrared free-electron laser (MIR-FEL). Int. J. Mass Spectrom. 2005, 241, 49–56. [Google Scholar]
- Yoshihashi-Suzuki, S.; Sato, I.; Awazu, K. Wavelength dependence of matrix-assisted laser desorption and ionization using a tunable mid-infrared laser. Int. J. Mass Spectrom. 2008, 270, 134–138. [Google Scholar] [CrossRef]
- Hazama, H.; Furukawa, S.; Awazu, K. Effect of solvent on ionization efficiency in matrix-assisted laser desorption/ionization mass spectrometry of peptides. Chem. Phys. 2013, 419, 196–199. [Google Scholar] [CrossRef]
- Konig, S.; Kollas, O.; Dreisewerd, K. Generation of highly charged peptide and protein ions by atmospheric pressure matrix-assisted infrared laser desorption/ionization ion trap mass Spectrometry. Anal. Chem. 2007, 79, 5484–5488. [Google Scholar]
- Cramer, R.; Pirkl, A.; Hillenkamp, F.; Dreisewerd, K. Liquid AP-UV-MALDI enables stable ion yields of multiply charged peptide and protein ions for sensitive analysis by mass spectrometry. Angew. Chem. Int. Ed. 2013, 52, 2364–2367. [Google Scholar] [CrossRef]
- Winger, B.E.; Light-Wahl, K.J.; Loo, R.R.O.; Udseth, H.R.; Smith, R.D. Observation and implications of high mass-to-charge ratio ions from electrospray ionization mass spectrometry. J. Am. Soc. Mass Spectrom. 1993, 4, 536–545. [Google Scholar] [CrossRef]
- Fenn, J.B. Ion formation from charged droplets: Roles of geometry, energy, and time. J. Am. Soc. Mass Spectrom. 1993, 4, 524–535. [Google Scholar] [CrossRef]
- Hazama, H.; Takatani, Y.; Awazu, K. Integrated ultraviolet and tunable mid-infrared laser source for analyses of proteins. Proc. SPIE 2007, 6455, 645507. [Google Scholar] [CrossRef]
- Caprioli, R.M.; Fan, T.; Fan, T. Continuous-flow sample probe for fast atom bombardment mass spectrometry. Anal. Chem. 1986, 58, 2949–2954. [Google Scholar] [CrossRef]
- Hau, J.; Schrader, W.; Linscheid, M. Continuous-flow fast atom bombardment mass spectrometry: A concept to improve the sensitivity. Org. Mass Spectrom. 1993, 28, 216–222. [Google Scholar] [CrossRef]
- Ito, Y.; Takeuchi, T.; Ishii, D.; Goto, M. Direct coupling of micro high-performance liquid chromatography with FAB-MS. J. Chromatogr. 1985, 346, 161–166. [Google Scholar] [CrossRef]
- Takeuchi, T.; Watanabe, S.; Kondo, N.; Ishii, D.; Goto, M. Improvement of the interface for coupling of FAB-MS and micro high-performance liquid chromatography. J. Chromatogr. 1988, 435, 482–488. [Google Scholar] [CrossRef]
- Siethoff, C.; Nigge, W.; Linscheid, M.W. The determination of ifosfamide in human blood serum using LC/MS. Fresenius J. Anal. Chem. 1995, 352, 801–805. [Google Scholar] [CrossRef]
- Lawson, S.J.; Murray, K.K. Continuous flow infrared matrix-assisted laser desorption/ionization with a solvent matrix. Rapid Commun. Mass Spectrom. 2000, 14, 129–134. [Google Scholar] [CrossRef]
- Laiko, V.V.; Taranenko, N.I.; Droshenko, V.M. On the mechanism of ion formation from the aqueous solutions irradiated with 3 µm IR laser pulses under atmospheric pressure. J. Mass Spectrom. 2006, 41, 1315–1321. [Google Scholar] [CrossRef]
- Droshenko, V.M.; Laiko, V.V.; Taranenko, N.I.; Berkout, V.D.; Lee, H.S. Recent developments in atmospheric pressure MALDI mass spectrometry. Int. J. Mass Spectrom. 2002, 221, 39–58. [Google Scholar] [CrossRef]
© 2014 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Hiraguchi, R.; Hazama, H.; Senoo, K.; Yahata, Y.; Masuda, K.; Awazu, K. Continuous Flow Atmospheric Pressure Laser Desorption/Ionization Using a 6–7-µm-Band Mid-Infrared Tunable Laser for Biomolecular Mass Spectrometry. Int. J. Mol. Sci. 2014, 15, 10821-10834. https://doi.org/10.3390/ijms150610821
Hiraguchi R, Hazama H, Senoo K, Yahata Y, Masuda K, Awazu K. Continuous Flow Atmospheric Pressure Laser Desorption/Ionization Using a 6–7-µm-Band Mid-Infrared Tunable Laser for Biomolecular Mass Spectrometry. International Journal of Molecular Sciences. 2014; 15(6):10821-10834. https://doi.org/10.3390/ijms150610821
Chicago/Turabian StyleHiraguchi, Ryuji, Hisanao Hazama, Kenichirou Senoo, Yukinori Yahata, Katsuyoshi Masuda, and Kunio Awazu. 2014. "Continuous Flow Atmospheric Pressure Laser Desorption/Ionization Using a 6–7-µm-Band Mid-Infrared Tunable Laser for Biomolecular Mass Spectrometry" International Journal of Molecular Sciences 15, no. 6: 10821-10834. https://doi.org/10.3390/ijms150610821
APA StyleHiraguchi, R., Hazama, H., Senoo, K., Yahata, Y., Masuda, K., & Awazu, K. (2014). Continuous Flow Atmospheric Pressure Laser Desorption/Ionization Using a 6–7-µm-Band Mid-Infrared Tunable Laser for Biomolecular Mass Spectrometry. International Journal of Molecular Sciences, 15(6), 10821-10834. https://doi.org/10.3390/ijms150610821



