The Effect of Size on Ag Nanosphere Toxicity in Macrophage Cell Models and Lung Epithelial Cell Lines Is Dependent on Particle Dissolution
Abstract
:1. Introduction
2. Results and Discussion
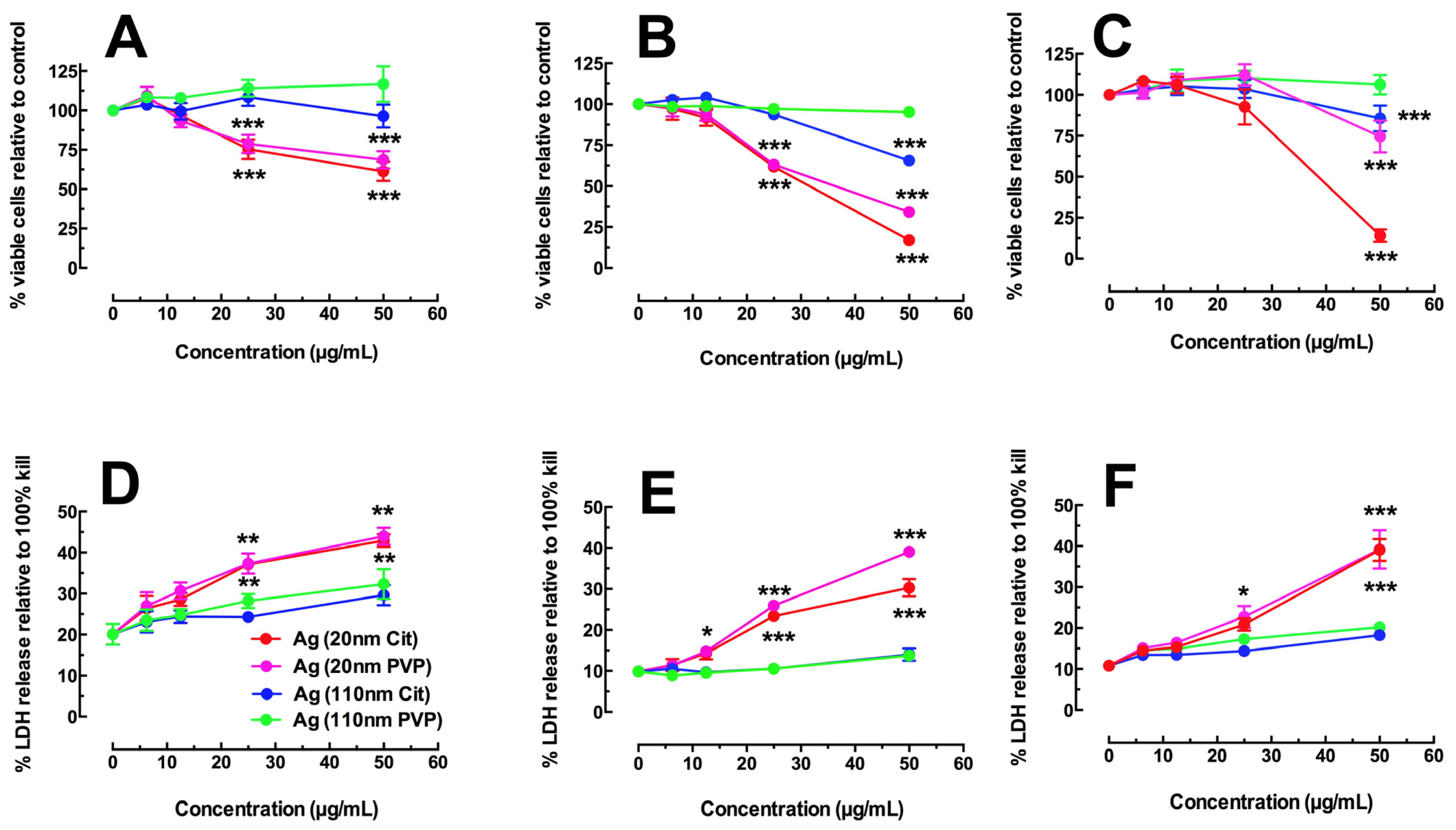
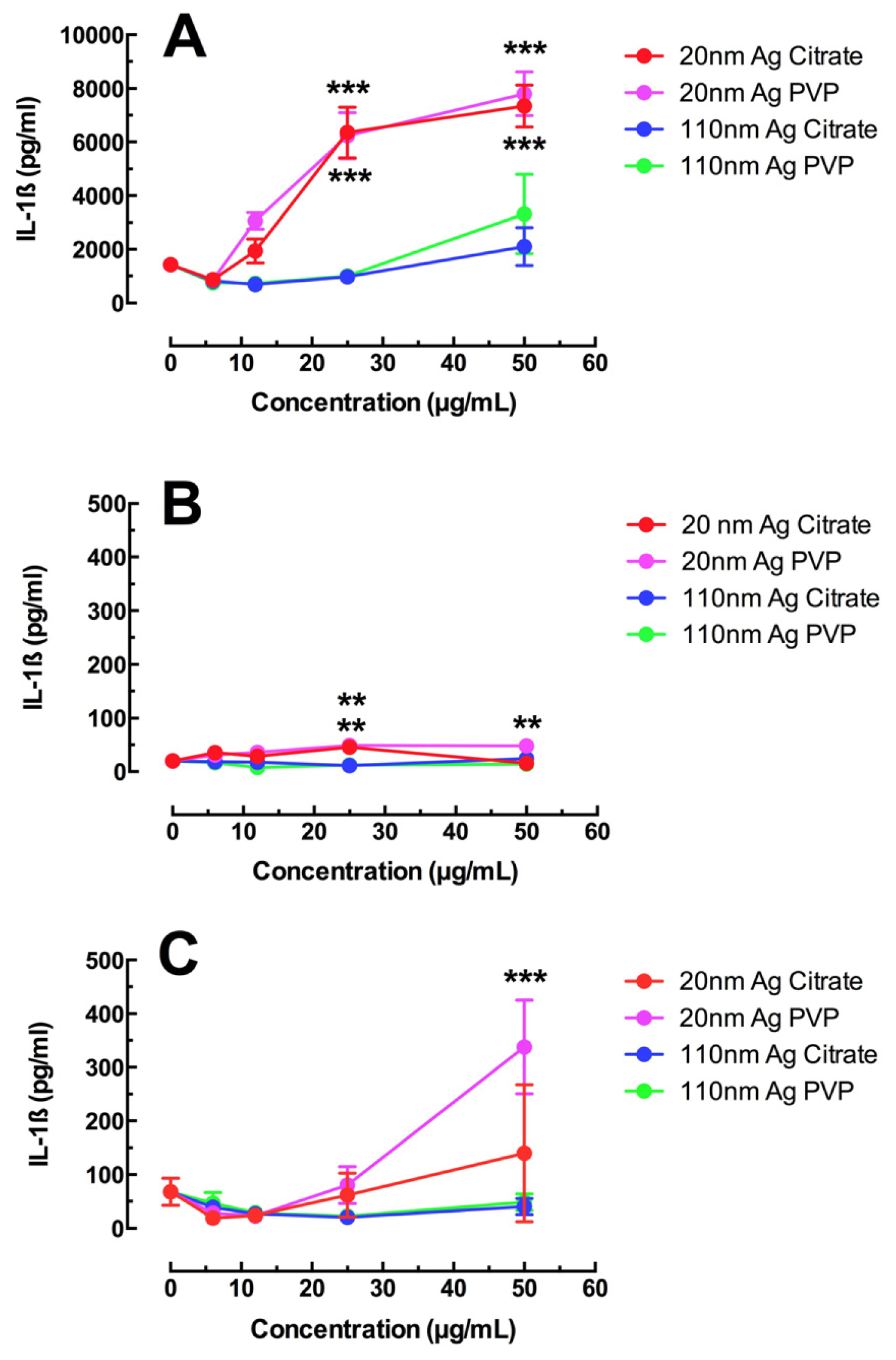
2.1. Toxicity and NLRP3 Inflammasome Activation in Macrophages Exposed to Ag Nanospheres
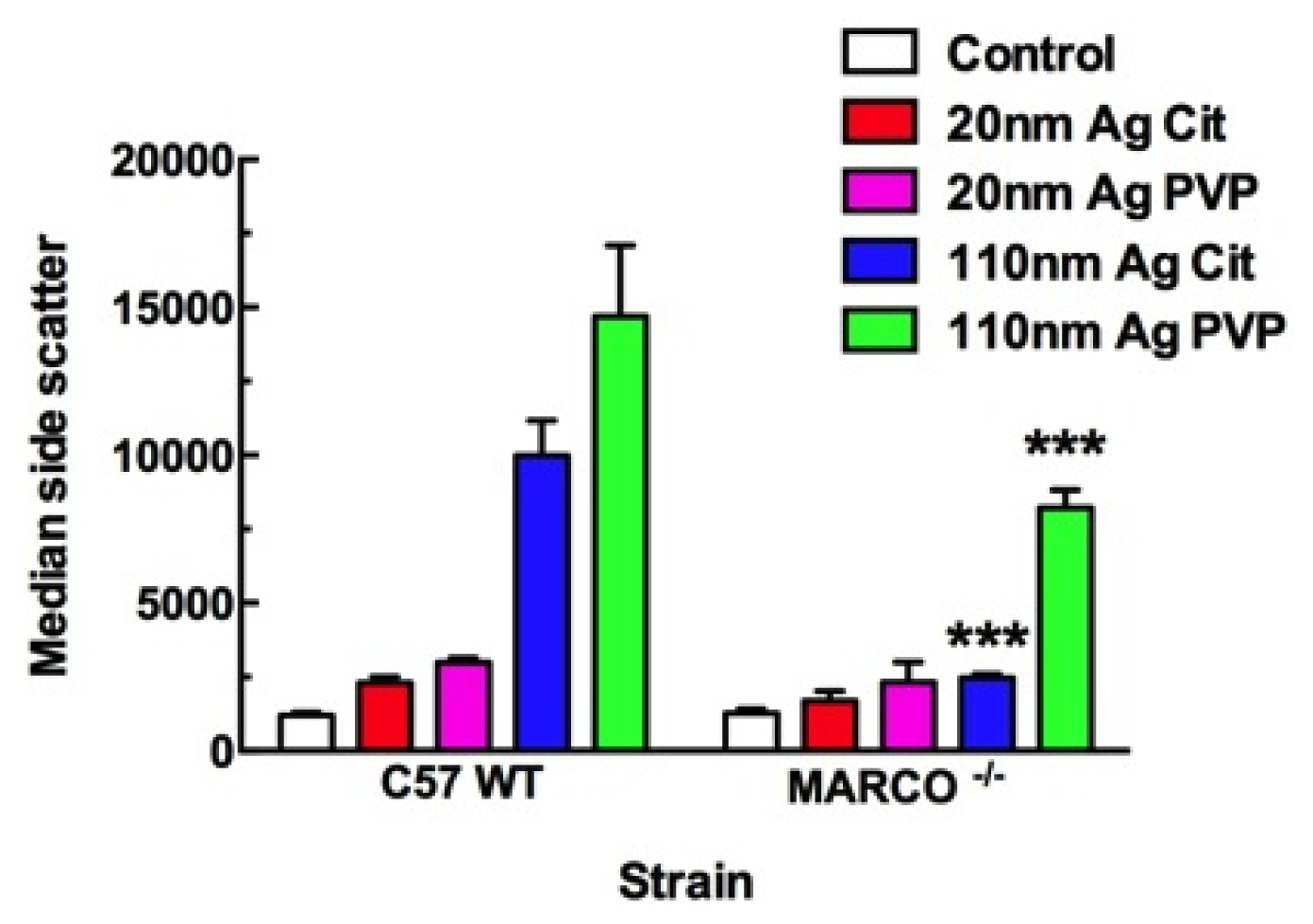
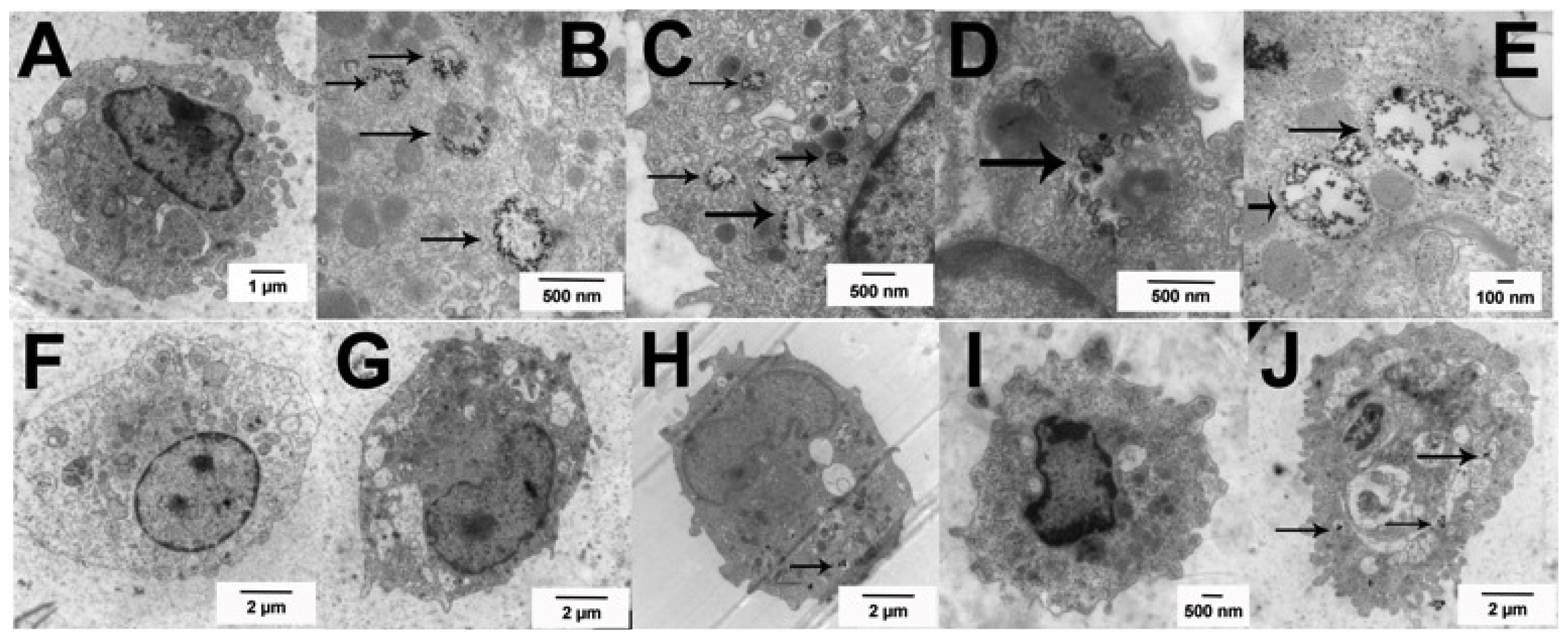
2.2. Uptake of Ag Nanospheres by Murine Macrophages
2.3. Toxicity of Ag Nanospheres in Murine Lung Epithelial Cell Lines
2.4. Uptake of Ag Nanospheres in Murine Lung Epithelial Cell Lines
3. Experimental Section
3.1. Silver Nanoparticle Characterization
3.2. Cell Isolation and Culture
3.3. Silver Nanoparticle Exposure
3.4. Cytotoxicity
3.5. IL-1β Release
3.6. Dynamic Light Scattering (DLS) to Assess Ag Aggregates in Media
3.7. Silver Nanoparticle Uptake
3.8. Statistical Analyses
4. Conclusions
Acknowledgments
Conflicts of Interest
- Author ContributionsR.F.H. conducted all of the macrophage model experiments and wrote the manuscript with A.H. S.B. conducted all of the epithelial cell line experiments. A.H. conceived the experimental designs.
References
- Wang, X.; Ji, Z.; Chang, C.H.; Zhang, H.; Wang, M.; Liao, Y.P.; Lin, S.; Meng, H.; Li, R.; Sun, B.; et al. Use of coated silver nanoparticles to understand the relationship of particle dissolution and bioavailability to cell and lung toxicological potential. Small 2014, 10, 385–398. [Google Scholar]
- Yang, E.J.; Kim, S.; Kim, J.S.; Choi, I.H. Inflammasome formation and IL-1beta release by human blood monocytes in response to silver nanoparticles. Biomaterials 2012, 33, 6858–6867. [Google Scholar]
- Carlson, C.; Hussain, S.M.; Schrand, A.M.; Braydich-Stolle, L.K.; Hess, K.L.; Jones, R.L.; Schlager, J.J. Unique cellular interaction of silver nanoparticles: Size-dependent generation of reactive oxygen species. J. Phys. Chem. B 2008, 112, 13608–13619. [Google Scholar]
- Park, M.S.; Park, J.; Jeon, S.K.; Yoon, T.H. The effects of sedimentation and dissolution on the cytotoxicity of Ag nanoparticles. J. Nanosci. Nanotechnol 2013, 13, 7264–7270. [Google Scholar]
- Schluesener, J.K.; Schluesener, H.J. Nanosilver: Application and novel aspects of toxicology. Arch. Toxicol 2013, 87, 569–576. [Google Scholar]
- Behra, R.; Sigg, L.; Clift, M.J.; Herzog, F.; Minghetti, M.; Johnston, B.; Petri-Fink, A.; Rothen-Rutishauser, B. Bioavailability of silver nanoparticles and ions: From a chemical and biochemical perspective. J. R. Soc. Interface 10, 0396.
- Liu, J.; Wang, Z.; Liu, F.D.; Kane, A.B.; Hurt, R.H. Chemical transformations of nanosilver in biological environments. ACS Nano 2012, 6, 9887–9899. [Google Scholar]
- Bertinato, J.; Cheung, L.; Hoque, R.; Plouffe, L.J. Ctr1 transports silver into mammalian cells. J. Trace Elem. Med. Biol 2010, 24, 178–184. [Google Scholar]
- Farkas, J.; Christian, P.; Gallego-Urrea, J.A.; Roos, N.; Hassellov, M.; Tollefsen, K.E.; Thomas, K.V. Uptake and effects of manufactured silver nanoparticles in rainbow trout (Oncorhynchus mykiss) gill cells. Aquat. Toxicol 2011, 101, 117–125. [Google Scholar]
- Trefry, J.C.; Wooley, D.P. Silver Nanoparticles Inhibit Vaccinia virus Infection by Preventing Viral Entry Through a Macropinocytosis-Dependent Mechanism. J. Biomed. Nanotechnol 2013, 9, 1624–1635. [Google Scholar]
- Lee, K.J.; Nallathamby, P.D.; Browning, L.M.; Osgood, C.J.; Xu, X.H.N. In vivo imaging of transport and biocompatibility of single silver nanoparticles in early development of zebrafish embryos. Acs Nano 2007, 1, 133–143. [Google Scholar]
- Wang, H.Y.; Wu, L.X.; Reinhard, B.M. Scavenger receptor mediated endocytosis of silver nanoparticles into J774A.1 macrophages is heterogeneous. Acs Nano 2012, 6, 7122–7132. [Google Scholar]
- Haberl, N.; Hirn, S.; Wenk, A.; Diendorf, J.; Epple, M.; Johnston, B.D.; Krombach, F.; Kreyling, W.G.; Schleh, C. Cytotoxic and proinflammatory effects of PVP-coated silver nanoparticles after intratracheal instillation in rats. Beilstein J. Nanotechnol 2013, 4, 933–940. [Google Scholar]
- Stern, S.T.; Adiseshaiah, P.P.; Crist, R.M. Autophagy and lysosomal dysfunction as emerging mechanisms of nanomaterial toxicity. Part. Fibre Toxicol 2012, 9. [Google Scholar] [CrossRef]
- Palomaki, J.; Valimaki, E.; Sund, J.; Vippola, M.; Clausen, P.A.; Jensen, K.A.; Savolainen, K.; Matikainen, S.; Alenius, H. Long, needle-like carbon nanotubes and asbestos activate the NLRP3 inflammasome through a similar mechanism. ACS Nano 2011, 5, 6861–6870. [Google Scholar]
- Hamilton, R.F.; Wu, N.; Porter, D.; Buford, M.; Wolfarth, M.; Holian, A. Particle length-dependent titanium dioxide nanomaterials toxicity and bioactivity. Part. Fibre Toxicol 2009, 6. [Google Scholar] [CrossRef]
- Hamilton, R.F.J.; Wu, Z.; Mitra, S.; Shaw, P.K.; Holian, A. Effect of MWCNT size, carboxylation, and purification on in vitro and in vivo toxicity, inflammation and lung pathology. Part. Fibre Toxicol 2013, 10. [Google Scholar] [CrossRef]
- Dostert, C.; Petrilli, V.; van Bruggen, R.; Steele, C.; Mossman, B.T.; Tschopp, J. Innate immune activation through Nalp3 inflammasome sensing of asbestos and silica. Science 2008, 320, 674–677. [Google Scholar]
- Xia, T.; Hamilton, R.F.; Bonner, J.C.; Crandall, E.D.; Elder, A.; Fazlollahi, F.; Girtsman, T.A.; Kim, K.; Mitra, S.; Ntim, S.A.; et al. Interlaboratory evaluation of in vitro cytotoxicity and inflammatory responses to engineered nanomaterials: The NIEHS Nano GO Consortium. Environ. Health Perspect 2013, 121, 683–690. [Google Scholar]
- Hamilton, R.F., Jr.; Thakur, S.A.; Mayfair, J.K.; Holian, A. MARCO mediates silica uptake and toxicity in alveolar macrophages from C57BL/6 mice. JBC 2006, 281, 34218–34226. [Google Scholar]
- Hamilton, R.F.; Girtsman, T.A.; Xiang, C.; Wu, N.; Holian, A. Nickel contamination on MWCNT is related to particle bioactivity but not toxicity in the THP-1 transformed macrophage model. IJBNN 2013, 3, 107–126. [Google Scholar]
- Hamilton, R.F., Jr.; Xiang, C.; Li, M.; Ka, I.; Yang, F.; Ma, D.; Porter, D.W.; Wu, N.; Holian, A. Purification and sidewall functionalization of multiwalled carbon nanotubes and resulting bioactivity in two macrophage models. Inhal. Toxicol 2013, 25, 199–210. [Google Scholar]
- Bouzas, V.; Haller, T.; Hobi, N.; Felder, E.; Pastoriza-Santos, I.; Perez-Gil, J. Nontoxic impact of PEG-coated gold nanospheres on functional pulmonary surfactant-secreting alveolar type II cells. Nanotoxicology 2014, 8, 813–823. [Google Scholar]




| Sample | 2% Serum | 10% Serum | 15% Serum |
|---|---|---|---|
| Media background no Ag * | 31.1 ± 24 nm | 30.9 ± 25.5 nm | 46.8 ± 23.9 nm |
| Media + 20 nm citrate Ag | 129.8 ± 78.0 nm | 131.6 ± 63.0 nm | 89.7 ± 47.5 nm |
| Media + 20 nm PVP Ag | 124.0 ± 95.0 nm | 136.6 ± 87.8 nm | 97.1 ± 62.0 nm |
| Media + 110 nm citrate Ag | 165.2 ± 44.2 nm | 164.2 ± 44.8 nm | 161.6 ± 44.5 nm |
| Media + 110 nm PVP Ag | 168.1 ± 42.9 nm | 171.9 ± 43.6 nm | 173.5 ± 48.5 nm |
| Nanosphere | Endotoxin (EU/mL) | Hydrodynamic Diameter by DLS (nm) | Core Diameter by TEM (nm) | Ag Concentration by ICP-MS (mg/g) | Zeta Potential (mV) | Medium Supplied In |
|---|---|---|---|---|---|---|
| 20 nm citrate stabilized Ag | <0.03 | 24.0 | 20.3 | 1.1 | −48 | 2 mM Citrate |
| 20 nm PVP stabilized Ag | <2.2 | 26.0 | 20.5 | 1.1 | −37 | H2O |
| 110 nm citrate stabilized Ag | <0.03 | 104.2 | 111.5 | 1.0 | −43 | 2 mM Citrate |
| 110 nm PVP stabilized Ag | <0.50 | 112.3 | 111.3 | 1.1 | −26 | H2O |
© 2014 by the authors; licensee MDPI, Basel, Switzerland This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Hamilton, R.F.; Buckingham, S.; Holian, A. The Effect of Size on Ag Nanosphere Toxicity in Macrophage Cell Models and Lung Epithelial Cell Lines Is Dependent on Particle Dissolution. Int. J. Mol. Sci. 2014, 15, 6815-6830. https://doi.org/10.3390/ijms15046815
Hamilton RF, Buckingham S, Holian A. The Effect of Size on Ag Nanosphere Toxicity in Macrophage Cell Models and Lung Epithelial Cell Lines Is Dependent on Particle Dissolution. International Journal of Molecular Sciences. 2014; 15(4):6815-6830. https://doi.org/10.3390/ijms15046815
Chicago/Turabian StyleHamilton, Raymond F., Sarah Buckingham, and Andrij Holian. 2014. "The Effect of Size on Ag Nanosphere Toxicity in Macrophage Cell Models and Lung Epithelial Cell Lines Is Dependent on Particle Dissolution" International Journal of Molecular Sciences 15, no. 4: 6815-6830. https://doi.org/10.3390/ijms15046815
APA StyleHamilton, R. F., Buckingham, S., & Holian, A. (2014). The Effect of Size on Ag Nanosphere Toxicity in Macrophage Cell Models and Lung Epithelial Cell Lines Is Dependent on Particle Dissolution. International Journal of Molecular Sciences, 15(4), 6815-6830. https://doi.org/10.3390/ijms15046815





