Melatonin Prevents Chemical-Induced Haemopoietic Cell Death
Abstract
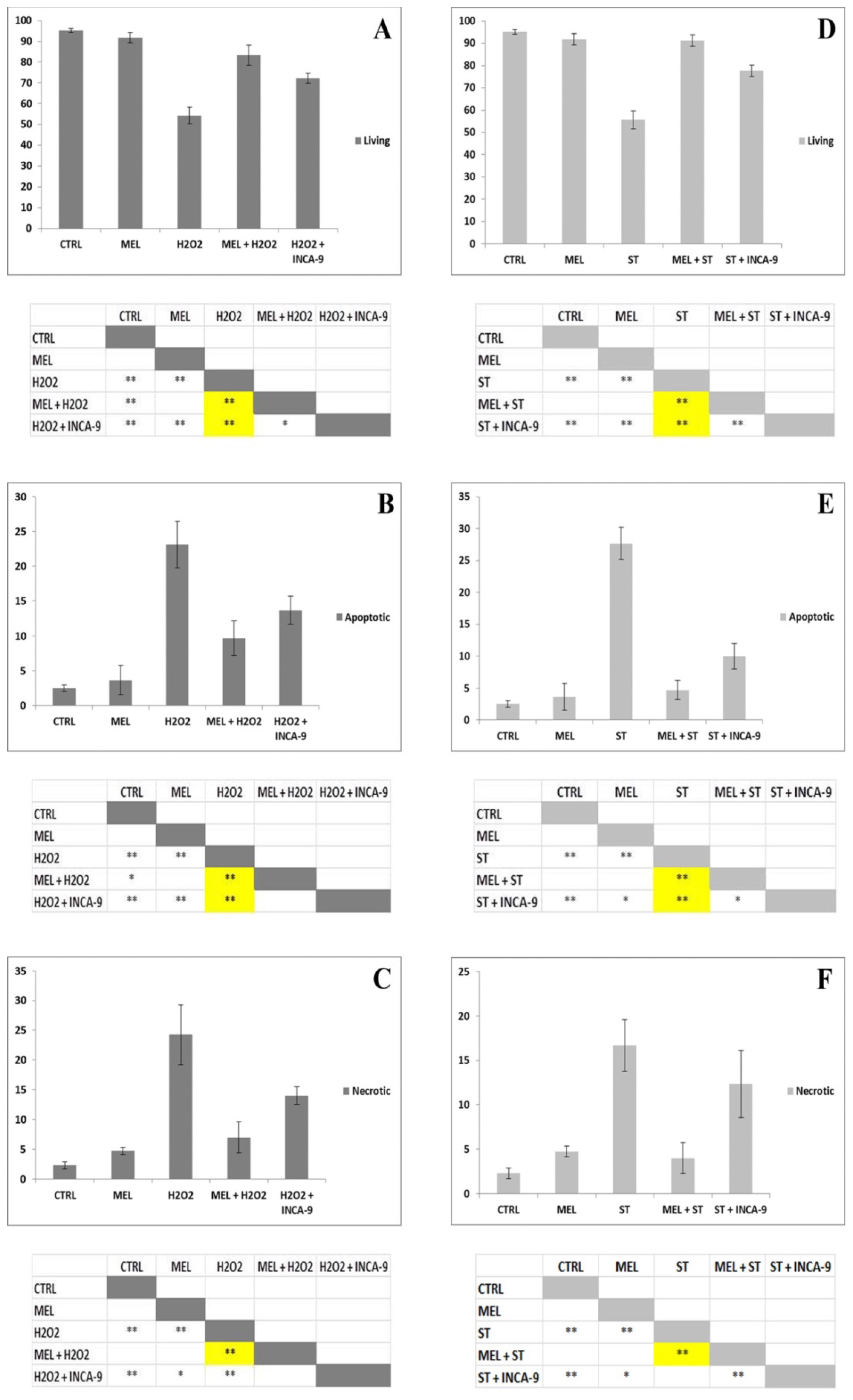
:1. Introduction
2. Results and Discussion
3. Experimental Section
3.1. Cell Culture
3.2. Tali® Image-Based Cytometer
3.2.1. Supravital Propidium Iodide (PI; Tali® Viability Kit; Life Technologies)
3.2.2. Annexin V and PI (Tali® Apoptosis Kit; Life Technologies)
3.2.3. Caspase-9 and −3 Inhibitor Evaluation with Annexin V and PI
3.2.4. Statistical Procedures
3.3. Scanning Electron Microscopy (SEM)
3.4. Transmission Electron Microscopy (TEM)
3.5. TUNEL
3.6. Acridine Orange (AO) and PI Nuclei Staining
4. Conclusions
Acknowledgments
Conflicts of Interest
References
- Reiter, R.J.; Tan, D.X. Melatonin: A novel protective agent against oxidative injury of the ischemic/reperfused heart. Cardiovasc. Res 2003, 58, 10–19. [Google Scholar]
- Tan, D.X.; Hardeland, R.; Manchester, L.C.; Poeggeler, B.; Lopez-Burillo, S.; Mayo, J.C.; Sainz, R.M.; Reiter, R.J. Mechanistic and comparative studies of melatonin and classic antioxidants in terms of their interactions with the ABTS cation radical. J. Pineal Res 2003, 34, 249–259. [Google Scholar]
- Hung, M.W.; Tipoe, G.L.; Poon, A.M.; Reiter, R.J.; Fung, M.L. Protective effect of melatonin against hippocampal injury of rats with intermittent hypoxia. J. Pineal Res 2008, 44, 214–221. [Google Scholar]
- Omurtag, G.Z.; Tozan, A.; Sehirli, A.O.; Sener, G. Melatonin protects against endosulfan-induced oxidative tissue damage in rats. J. Pineal Res 2008, 44, 432–438. [Google Scholar]
- Reiter, R.J.; Tan, D.X.; Rosales-Corral, S.; Manchester, L.C. The universal nature, unequal distribution and antioxidant functions of melatonin and its derivatives. Mini Rev. Med. Chem 2013, 13, 373–384. [Google Scholar]
- Luchetti, F.; Canonico, B.; Mannello, F.; Masoni, C.; D’Emilio, A.; Battistelli, M.; Papa, S.; Falcieri, E. Melatonin reduces early changes in intramitochondrial cardiolipin during apoptosis in U937 cell line. Toxicol. in Vitro 2007, 2, 293–301. [Google Scholar]
- Bai, J.; Dong, L.; Song, Z.; Ge, H.; Cai, X.; Wang, G.; Liu, P. The role of melatonin as an antioxidant in human lens epithelial cells. Free Radic. Res 2013, 47, 635–642. [Google Scholar]
- Rodriguez, C.; Mayo, J.C.; Sainz, R.M.; Antolín, I.; Herrera, F.; Martín, V.; Reiter, R.J. Regulation of antioxidant enzymes: A significant role for melatonin. J. Pineal Res 2004, 36, 1–9. [Google Scholar]
- Princ, F.G.; Maxit, A.G.; Cardalda, C.; Batlle, A.; Juknat, A.A. In vivo protection by melatonin against delta-aminolevulinic acid-induced oxidative damage and its antioxidant effect on the activity of haem enzymes. J. Pineal Res 1998, 24, 1–8. [Google Scholar]
- Allegra, M.; Reiter, R.J.; Tan, D.X.; Gentile, C.; Tesoriere, L.; Livrea, M.A. The chemistry of melatonin’s interaction with reactive species. J. Pineal Res 2003, 34, 1–10. [Google Scholar]
- Wölfler, A.; Caluba, H.C.; Abuja, P.M.; Dohr, G.; Schauenstein, K.; Liebmann, P.M. Prooxidant activity of melatonin promotes fas-induced cell death in human leukemic Jurkat cells. FEBS Lett 2001, 3, 127–131. [Google Scholar]
- Albertini, M.C.; Radogna, F.; Accorsi, A.; Uguccioni, F.; Paternoster, L.; Cerella, C.; de Nicola, M.; D’Alessio, M.; Bergamaschi, A.; Magrini, A.; et al. Intracellular pro-oxidant activity of melatonin deprives U937 cells of reduced glutathione without affecting glutathione peroxidase activity. Ann. N. Y. Acad. Sci 2006, 1091, 10–16. [Google Scholar]
- Perdomo, J.; Cabrera, J.; Estévez, F.; Loro, J.; Reiter, R.J.; Quintana, J. Melatonin induces apoptosis through a caspase-dependent but reactive oxygen species-independent mechanism in human leukemia Molt-3 cells. J. Pineal Res 2013, 55, 195–206. [Google Scholar]
- Casado-Zapico, S.; Rodriguez-Blanco, J.; García-Santos, G.; Martin, V.; Sánchez-Sánchez, A.M.; Antolin, I.; Rodriguez, C. Synergistic antitumor effect of melatonin with several chemotherapeutic drugs on human Ewing sarcoma cancer cells. Potentiation of the extrinsic apoptotic pathway. J. Pineal Res 2010, 48, 72–80. [Google Scholar]
- Martin, V.; García-Santos, G.; Rodriguez-Blanco, J.; Casado-Zapico, S.; Sanchez-Sanchez, A.; Antolin, I.; Medina, M.; Rodriguez, C. Melatonin sensitizes human malignant glioma cells against TRAIL-induced cell death. Cancer Lett 2010, 287, 216–223. [Google Scholar]
- Bejarano, I.; Espino, J.; Marchena, A.M.; Barriga, C.; Paredes, S.D.; Rodriguez, A.B.; Pariente, J.A. Melatonin enhances hydrogen peroxide-induced apoptosis in human promyelocytic leukemia HL-60 cells. Mol. Cell. Biochem 2011, 353, 167–176. [Google Scholar]
- Um, H.J.; Park, J.W.; Kwon, T.K. Melatonin sensitizes Caki renal cancer cells to kahweol-induced apoptosis through CHOP-mediated up-regulation of PUMA. J. Pineal Res 2011, 50, 359–366. [Google Scholar]
- Kim, J.H.; Jeong, S.J.; Kim, B.; Yun, S.M.; Choi, D.Y.; Kim, S.H. Melatonin synergistically enhances cisplatin-induced apoptosis via the dephosphorylation of ERK/p90 ribosomal S6 kinase/heat shock protein 27 in SK-OV-3 cells. J. Pineal Res 2012, 52, 244–252. [Google Scholar]
- Uguz, A.C.; Cig, B.; Espino, J.; Bejarano, I.; Naziroglu, M.; Rodriguez, A.B.; Pariente, J.A. Melatonin potentiates chemotherapy-induced cytotoxicity and apoptosis in rat pancreatic tumor cells. J. Pineal Res 2012, 53, 91–98. [Google Scholar]
- Anisimov, V.N.; Egormin, P.A.; Piskunova, T.S.; Popovich, I.G.; Tyndyk, M.L.; Yurova, M.N.; Zabezhinski, M.A.; Anikin, I.V.; Karkach, A.S.; Romanyukha, A.A. Metformin extends life span of HER-2/neu transgenic mice and in combination with melatonin inhibits growth of transplantable tumorsin vivo. Cell Cycle 2010, 9, 188–197. [Google Scholar]
- Padillo, F.J.; Ruiz-Rabelo, J.F.; Cruz, A.; Perea, M.D.; Tasset, I.; Montilla, P.; Tunez, I.; Muntané, J. Melatonin and celecoxib improve the outcomes in hamsters with experimental pancreatic cancer. J. Pineal Res 2010, 49, 264–270. [Google Scholar]
- Droge, W. Free radicals in the physiological control of cell function. Physiol. Rev 2002, 82, 47–95. [Google Scholar]
- Rodriguez, C.; Martín, V.; Herrera, F.; García-Santos, G.; Rodriguez-Blanco, J.; Casado-Zapico, S.; Sánchez-Sánchez, A.M.; Suárez, S.; Puente-Moncada, N.; Anítua, M.J.; et al. Mechanisms Involved in the Pro-Apoptotic Effect of Melatonin in Cancer Cells. Int. J. Mol. Sci 2013, 14, 6597–6613. [Google Scholar]
- Storz, P. Reactive oxygen species in tumor progression. Front. Biosci 2005, 10, 1881–1896. [Google Scholar]
- Valko, M. Free radicals and antioxidants in normal physiological functions and human disease. Int. J. Biochem. Cell Biol 2007, 39, 44–84. [Google Scholar]
- Lu, L.; Osmond, D.G. Apoptosis and its modulation during B lymphopoiesis in mouse bone marrow. Immunol. Rev 2000, 175, 158–174. [Google Scholar]
- Bolitho, P.; Voskoboinik, I.; Trapani, J.A.; Smyth, M.J. Apoptosis induced by the lymphocyte effector molecule perforin. Curr. Opin. Immunol 2007, 19, 339–347. [Google Scholar]
- Cubero, J.; Valero, V.; Narciso, D.; Rivero, M.; Marchena, J.M.; Rodriguez, A.B.; Barriga, C. l-tryptophan administered orally at night modifies the melatonin plasma levels, phagocytosis and oxidative metabolism of ringdove (Streptopelia roseogrisea) heterophils. Mol. Cell. Biochem 2006, 293, 79–85. [Google Scholar]
- Miller, S.C.; Pandi-Perumal, S.R.; Esquifino, A.I.; Cardinali, D.P.; Maestroni, G.J. The role of melatonin in immune enhancement: Potential application in cancer. Int. J. Exp. Pathol 2006, 87, 81–87. [Google Scholar]
- Wesche-Soldato, D.E.; Swan, R.Z.; Chung, C.S.; Ayala, A. The apoptotic pathway as a therapeutic target in sepsis. Curr. Drug Targets 2007, 8, 493–500. [Google Scholar]
- Radogna, F.; Paternoster, L.; Albertini, M.C.; Accorsi, A.; Cerella, C.; D’Alessio, M.; de Nicola, M.; Nuccitelli, S.; Magrini, A.; Bergamaschi, A.; et al. Melatonin as an apoptosis antagonist. Ann. N. Y. Acad. Sci 2006, 1090, 226–233. [Google Scholar]
- Radogna, F.; Paternoster, L.; Albertini, M.C.; Cerella, C.; Accorsi, A.; Bucchini, A.; Spadoni, G.; Diamantini, G.; Tarzia, G.; de Nicola, M.; et al. Melatonin antagonizes apoptosis via receptor interaction in U937 monocytic cells. J. Pineal Res 2007, 43, 154–162. [Google Scholar]
- Luchetti, F.; Betti, M.; Canonico, B.; Arcangeletti, M.; Ferri, P.; Galli, F.; Papa, S. ERK MAPK activation mediates the antiapoptotic signaling of melatonin in UVB-stressed U937 cells. Free Radic. Biol. Med 2009, 46, 339–351. [Google Scholar]
- Radogna, F.; Cristofanon, S.; Paternoster, L.; D’Alessio, M.; de Nicola, M.; Cerella, C.; Dicato, M.; Diederich, M.; Ghibelli, L. Melatonin antagonizes the intrinsic pathway of apoptosis via mitochondrial targeting of Bcl-2. J. Pineal Res. 2008, 44, 316–325. [Google Scholar]
- Luchetti, F.; Canonico, B.; Curci, R.; Battistelli, M.; Mannello, F.; Papa, S.; Tarzia, G.; Falcieri, E. Melatonin prevents apoptosis induced by UV-B treatment in U937 cell line. J. Pineal Res 2006, 40, 158–167. [Google Scholar]
- Salucci, S.; Battistelli, M.; Burattini, S.; Squillace, C.; Canonico, B.; Gobbi, P.; Papa, S.; Falcieri, E. C2C12 myoblast sensitivity to different apoptotic chemical triggers. Micron 2010, 41, 966–973. [Google Scholar]
- Salucci, S.; Burattini, S.; Baldassarri, V.; Battistelli, M.; Canonico, B.; Valmori, A.; Papa, S.; Falcieri, E. The peculiar apoptotic behavior of skeletal muscle cells. Histol. Histopathol 2013, 28, 1073–1087. [Google Scholar]
- Remple, K.; Stone, L. Assessment of GFP expression and viability using the tali image-based cytometer. J. Vis. Exp. 2011, 17, 3659. [Google Scholar]
- Zamai, L.; Canonico, B.; Luchetti, F.; Ferri, P.; Melloni, E.; Guidotti, L.; Cappellini, A.; Cutroneo, G.; Vitale, M.; Papa, S. Supravital exposure to propidium iodide identifies apoptosis on adherent cells. Cytometry 2001, 1, 57–64. [Google Scholar]
- D’Emilio, A.; Biagiotti, L.; Burattini, S.; Battistelli, M.; Canonico, B.; Evangelisti, C.; Ferri, P.; Papa, S.; Martelli, A.M.; Falcieri, E. Morphological and biochemical patterns in skeletal muscle apoptosis. Histol. Histopathol 2010, 25, 21–32. [Google Scholar]
- Burattini, S.; Salucci, S.; Baldassarri, V.; Accorsi, A.; Piatti, E.; Madrona, A.; Espartero, J.L.; Candiracci, M.; Zappia, G.; Falcieri, E. Anti-apoptotic activity of hydroxytyrosol and hydroxytyrosyl laurate. Food Chem. Toxicol 2013, 55, 248–256. [Google Scholar]
- Salucci, S.; Burattini, S.; Battistelli, M.; Baldassarri, V.; Maltarello, M.C.; Falcieri, E.; Ultraviolet, B. (UVB) Irradiation-Induced Apoptosis in Various Cell Lineages in Vitro. Int. J. Mol. Sci 2012, 14, 532–546. [Google Scholar]
- Mohan, S.; Bustamam, A.; Ibrahim, S.; Al-Zubairi, A.S.; Aspollah, M.; Abdullah, R.; Elhassan, M.M. In vitro ultramorphological assessment of apoptosis on CEMss induced by linoleic acid-rich fraction from typhonium flagelliforme tuber. Evid. Based Complement. Alternat. Med 2011, 2011, 421894. [Google Scholar]
- Manns, J.; Daubrawa, M.; Driessen, S.; Paasch, F.; Hoffmann, N.; Löffler, A.; Lauber, K.; Dieterle, A.; Alers, S.; Iftner, T.; et al. Triggering of a novel intrinsic apoptosis pathway by the kinase inhibitor staurosporine: Activation of caspase-9 in the absence of Apaf-1. FASEB J 2011, 25, 3250–3261. [Google Scholar]
- Dunai, Z.A.; Imre, G.; Barna, G.; Korcsmaros, T.; Petak, I.; Bauer, P.I.; Mihalik, R. Staurosporine induces necroptotic cell death under caspase-compromised conditions in U937 cells. PLoS One 2012, 7, e41945. [Google Scholar]
- Jou, M.J.; Peng, T.I.; Hsu, L.F.; Jou, S.B.; Reiter, R.J.; Yang, C.M.; Chiao, C.C.; Lin, Y.F.; Chen, C.C. Visualization of melatonin’s multiple mitochondrial levels of protection against mitochondrial Ca(2+)-mediated permeability transition and beyond in rat brain astrocytes. J. Pineal Res 2010, 48, 20–38. [Google Scholar]
- Paradies, G.; Petrosillo, G.; Paradies, V.; Reiter, R.J.; Ruggiero, F.M. Melatonin, cardiolipin and mitochondrial bioenergetics in health and disease. J. Pineal Res 2010, 48, 297–310. [Google Scholar]
- Dragicevic, N.; Delic, V.; Cao, C.; Copes, N.; Lin, X.; Mamcarz, M.; Wang, L.; Arendash, G.W.; Bradshaw, P.C. Caffeine increases mitochondrial function and blocks melatonin signaling to mitochondria in Alzheimer’s mice and cells. Neuropharmacology 2012, 63, 1368–1379. [Google Scholar]
- Martín, M.; Macías, M.; Escames, G.; León, J.; Acuña-Castroviejo, D. Melatonin but not vitamins C and E maintains glutathione homeostasis in t-butyl hydroperoxide-induced mitochondrial oxidative stress. FASEB J 2000, 14, 1677–1679. [Google Scholar]
- Tan, D.X.; Manchester, L.C.; Liu, X.; Rosales-Corral, S.A.; Acuna-Castroviejo, D.; Reiter, R.J. Mitochondria and chloroplasts as the original sites of melatonin synthesis: A hypothesis related to melatonin’s primary function and evolution in eukaryotes. J. Pineal Res 2013, 54, 127–138. [Google Scholar]
- Cheshchevik, V.T.; Dremza, I.K.; Lapshina, E.A.; Zabrodskaya, S.V.; Kujawa, J.; Zavodnik, I.B. Corrections by melatonin of liver mitochondrial disorders under diabetes and acute intoxication in rats. Cell Biochem. Funct 2011, 29, 481–488. [Google Scholar]
- Zavodnik, I.B.; Lapshina, E.A.; Cheshchevik, V.T.; Dremza, I.K.; Kujawa, J.; Zabrodskaya, S.V.; Reiter, R.J. Melatonin and succinate reduce rat liver mitochondrial dysfunction in diabetes. J. Physiol. Pharmacol 2011, 62, 421–427. [Google Scholar]
- Cheshchevik, V.T.; Lapshina, E.A.; Dremza, I.K.; Zabrodskaya, S.V.; Reiter, R.J.; Prokopchik, N.I.; Zavodnik, I.B. Rat liver mitochondrial damage under acute or chronic carbon tetrachloride-induced intoxication: Protection by melatonin and cranberry flavonoids. Toxicol. Appl. Pharmacol 2012, 261, 271–279. [Google Scholar]
- Jung, K.H.; Hong, S.W.; Zheng, H.M.; Lee, H.S.; Lee, H.; Lee, D.H.; Lee, S.Y.; Hong, S.S. Melatonin ameliorates cerulein-induced pancreatitis by the modulation of nuclear erythroid 2-related factor 2 and nuclear factor-kappaB in rats. J. Pineal Res 2010, 48, 239–250. [Google Scholar]
- Weinreb, O.; Mandel, S.; Youdim, M.B. cDNA gene expression profile homology of antioxidants and their antiapoptotic and proapoptotic activities in human neuroblastoma cells. FASEB J 2003, 7, 935–937. [Google Scholar]
- D’Alessio, M.; Cerella, C.; de Nicola, M.; Bergamaschi, A.; Magrini, A.; Gualandi, G.; Alfonsi, A.M.; Ghibelli, L. Apoptotic GSH extrusion is associated with free radical generation. Ann. N. Y. Acad. Sci 2003, 1010, 449–452. [Google Scholar]
- Andrabi, S.A.; Sayeed, I.; Siemen, D.; Wolf, G.; Horn, T.F. Direct inhibition of the mitochondrial permeability transition pore: A possible mechanism responsible for anti-apoptotic effects of melatonin. FASEB J 2004, 18, 69–71. [Google Scholar]
- Canonico, B.; Luchetti, F.; Ambrogini, P.; Arcangeletti, M.; Betti, M.; Cesarini, E.; Lattanzi, D.; Ciuffoli, S.; Palma, F.; Cuppini, R.; et al. Pharmacological doses of melatonin induce alterations in mitochondrial mass and potential, bcl-2 levels and K+ currents in UVB-exposed U937 cells. Cell Biol. Int. 2013, 37, 213–226. [Google Scholar]





© 2014 by the authors; licensee MDPI, Basel, Switzerland This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Salucci, S.; Burattini, S.; Battistelli, M.; Baldassarri, V.; Curzi, D.; Valmori, A.; Falcieri, E. Melatonin Prevents Chemical-Induced Haemopoietic Cell Death. Int. J. Mol. Sci. 2014, 15, 6625-6640. https://doi.org/10.3390/ijms15046625
Salucci S, Burattini S, Battistelli M, Baldassarri V, Curzi D, Valmori A, Falcieri E. Melatonin Prevents Chemical-Induced Haemopoietic Cell Death. International Journal of Molecular Sciences. 2014; 15(4):6625-6640. https://doi.org/10.3390/ijms15046625
Chicago/Turabian StyleSalucci, Sara, Sabrina Burattini, Michela Battistelli, Valentina Baldassarri, Davide Curzi, Aurelio Valmori, and Elisabetta Falcieri. 2014. "Melatonin Prevents Chemical-Induced Haemopoietic Cell Death" International Journal of Molecular Sciences 15, no. 4: 6625-6640. https://doi.org/10.3390/ijms15046625
APA StyleSalucci, S., Burattini, S., Battistelli, M., Baldassarri, V., Curzi, D., Valmori, A., & Falcieri, E. (2014). Melatonin Prevents Chemical-Induced Haemopoietic Cell Death. International Journal of Molecular Sciences, 15(4), 6625-6640. https://doi.org/10.3390/ijms15046625





