Importance of N-Glycosylation on CD147 for Its Biological Functions
Abstract
:1. Introduction
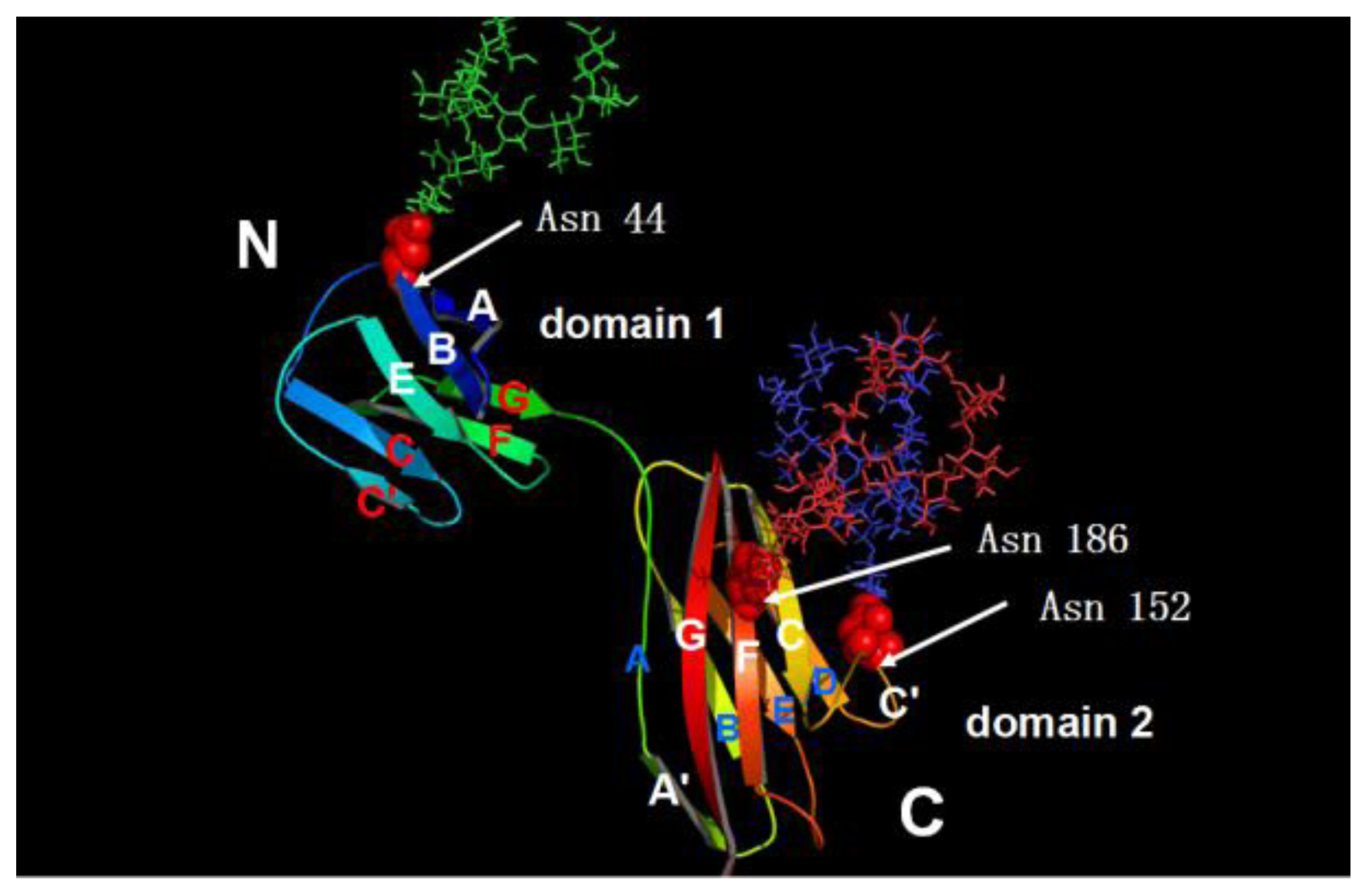
2. Structure of CD147
3. The Glycosylation Characteristic of CD147
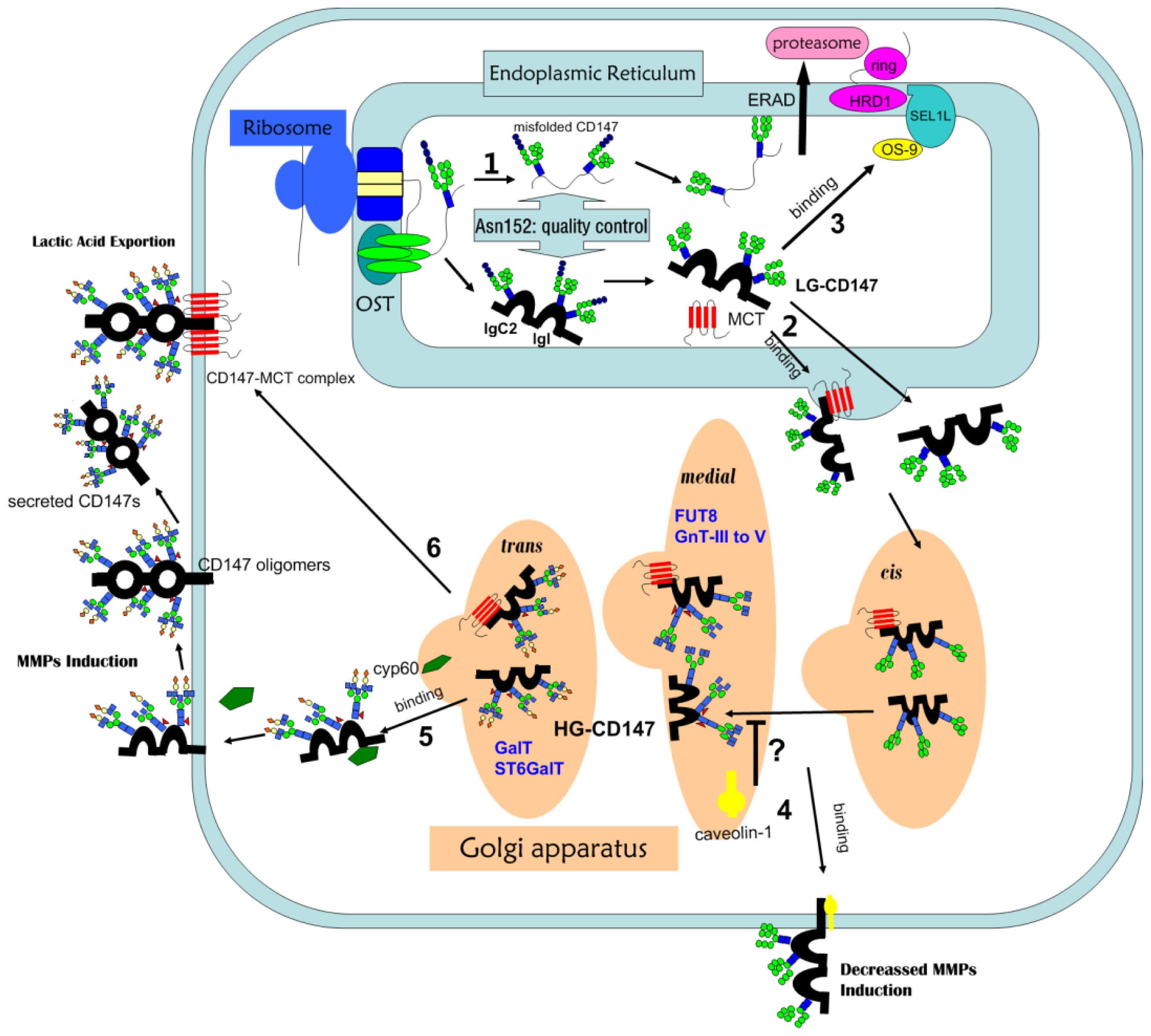
3.1. HG-CD147 and LG-CD147
3.2. The Structure of the Oligosaccharides of CD147
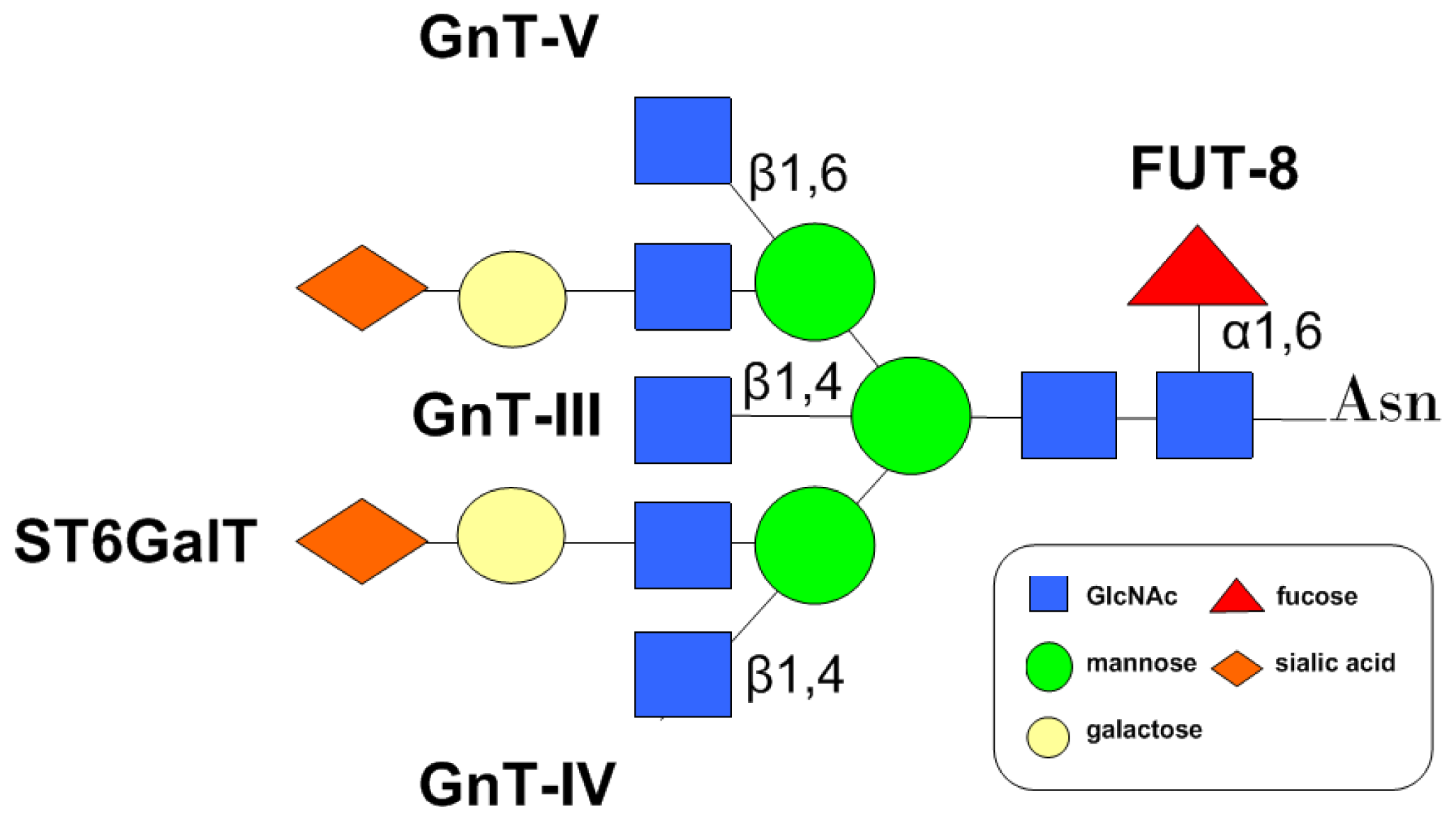
4. Glycosyltransferases Involved in the Modulation of CD147 N-Glycans
4.1. GnTs
4.2. FUT8
4.3. Sialyltransferase
5. Proteins Regulating the Glycosylation of CD147
6. Biological Role of CD147 Glycosylation
6.1. The Implication of HG/LG Ratio in Physiological and Pathological Processes
6.2. CD147 Glycosylation and MMPs Induction Activity
6.3. Role of N-Glycosylation in CD147 Maturation
6.4. Role of N-Glycosylation in the Interaction of CD147 and Other Proteins
7. Conclusions
Acknowledgments
Conflicts of Interest
- Author ContributionsY.B. and W.H. reviewed relevant literature and wrote the manuscript. L.-T.M. drew the figures and co-wrote the manuscript. J.-L.J. and Z.-N.C. supervised the review and co-wrote the manuscript.
References
- Biswas, C. Tumor cell stimulation of collagenase production by fibroblasts. Biochem. Biophys. Res. Commun 1982, 109, 1026–1034. [Google Scholar]
- Gabison, E.E.; Hoang-Xuan, T.; Mauviel, A.; Menashi, S. EMMPRIN/CD147, an MMP modulator in cancer, development and tissue repair. Biochimie 2005, 87, 361–368. [Google Scholar]
- Schmidt, R.; Bültmann, A.; Fischel, S.; Gillitzer, A.; Cullen, P.; Walch, A.; Jost, P.; Ungerer, M.; Tolley, N.D.; Lindemann, S.; et al. Extracellular matrix metalloproteinase inducer (CD147) is a novel receptor on platelets, activates platelets, and augments nuclear factor {kappa}B dependent inflammation in monocytes. Circ. Res 2008, 102, 302–309. [Google Scholar]
- Ruiz, S.; Castro, A.; Bustelo, X.R. CD147 inhibits the nuclear factor of activated T-cells by impairing vav1 and rac1 downstream signaling. J. Biol. Chem 2008, 283, 5554–5566. [Google Scholar]
- Biswas, C.; Zhang, Y.; DeCastro, R.; Guo, H.; Nakamura, T.; Kataoka, H.; Nabeshima, K. The human tumor cell-derived collagenase stimulatory factory factor (renamed EMMPRIN) is a member of the immunoglobulin superfamily. Cancer. Res 1995, 55, 434–439. [Google Scholar]
- Miyauchi, T.; Masuzawa, Y.; Muramatsu, T. The basigin group of the immunoglobulin superfamily: Complete conservation of a segment in and around transmembrane domains of human and mouse basigin and chicken HT7 antigen. J. Biochem 1991, 110, 770–774. [Google Scholar]
- Kasinrerk, W.; Fiebiger, E.; Stefanova, I.; Baumruker, T.; Knapp, W.; Stockinger, H. Human leukocyte activation antigen M6, a member of the Ig superfamily, is the species homologue of rat OX-47, mouse basigin, and chicken HT7 molecule. J. Immunol 1992, 149, 847–854. [Google Scholar]
- Jiang, J.L.; Zhou, Q.; Yu, M.K.; Ho, L.S.; Chen, Z.N.; Chan, H.C. The involvement of HAb18G/CD147 in regulation of store-operated calcium entry and metastasis of human hepatoma cells. J. Biochem 2001, 276, 46870–46877. [Google Scholar]
- Fossum, S.; Mallett, S.; Barclay, A.N. The MRC OX-47 antigen is a member of the immunoglobulin superfamily with an unusual transmembrane sequence. Eur. J. Immunol 1991, 21, 671–679. [Google Scholar]
- Nehme, C.L.; Cesario, M.M.; Myles, D.G.; Koppel, D.E.; Bartles, J.R. Breaching the diffusion barrier that compartmentalizes the transmembrane glycoprotein CE9 to the posterior-tail plasma membrane domain of the rat spermatozoon. J. Cell Biol 1993, 120, 687–694. [Google Scholar]
- Altruda, F.; Cervella, P.; Gaeta, M.L.; Daniele, A.; Giancotti, F.; Tarone, G.; Stefanuto, G.; Silengo, L. Cloning of cDNA for a novel mousemembrane glycoprotein (gp42): Shared identity to histocompatibility antigens, immunoglobulins and neural-cell adhesionmolecules. Gene 1989, 85, 445–451. [Google Scholar]
- Ochrietor, J.D.; Moroz, T.P.; van Ekeris, L.; Clamp, M.F.; Jefferson, S.C.; deCarvalho, A.C.; Fadool, J.M.; Wistow, G.; Muramatsu, T.; Linser, P.J. Retina-specific expression of 5A11/Basigin-2, a member of the immunoglobulin gene superfamily. Investig. Ophthalmol. Vis. Sci 2003, 44, 4086–4096. [Google Scholar]
- Fadool, J.M.; Linser, P.J. Differential glycosylation of the 5A11/HT7 antigen by neural retina and epithelial tissues in the chicken. J. Neurochem 1993, 60, 1354–1364. [Google Scholar]
- Schlosshauer, B.; Herzog, K.H. Neurothelin: An inducible cell surface glycoprotein of blood-brain barrier-specific endothelial cells and distinct neurons. J. Cell Biol 1990, 110, 1261–1274. [Google Scholar]
- Fadool, J.M.; Linser, P.J. 5A11 antigen is a cell recognition molecule which is involved in neuronal-glial interactions in avian neural retina. Dev. Dyn 1993, 196, 252–262. [Google Scholar]
- Agrawal, S.M.; Yong, V.W. The many faces of EMMPRIN—Roles in neuroinflammation. Biochim. Biophys. Acta 2011, 1812, 213–219. [Google Scholar]
- Bi, J.J.; Li, Y.F.; Sun, F.Y.; Saalbach, A.; Klein, C.; Miller, D.J.; Hess, R.; Nowak, R.A. Basigin null mutant male mice are sterile and exhibit impaired interactions between germ cells and Sertoli cells. Dev. Biol 2013, 380, 145–156. [Google Scholar]
- Iacono, K.T.; Brown, A.L.; Greene, M.I.; Saouaf, S.J. CD147 immunoglobulin superfamily receptor function and role in pathology. Exp. Mol. Pathol 2007, 83, 283–295. [Google Scholar]
- Jia, J.F.; Wang, C.H.; Shi, Z.G.; Zhao, J.K.; Jia, Y.; Zhao-Hui, Z.; Li, X.Y.; Chen, Z.N.; Zhu, P. Inhibitory effect of CD147/HAb18G monoclonal antibody on cartilage erosion and synovitis in the SCID mouse model for rheumatoid arthritis. Rheumatology (Oxford) 2009, 48, 721–726. [Google Scholar]
- Joghetaei, N.; Stein, A.; Byrne, R.A.; Schulz, C.; King, L.; May, A.E.; May, A.E.; Schmidt, R. The Extracellular Matrix Metalloproteinase Inducer (EMMPRIN, CD147)—A potential novel target in atherothrombosis prevention? Thromb. Res 2013, 131, 474–480. [Google Scholar]
- Crosnier, C.; Bustamante, L.Y.; Bartholdson, S.J.; Bei, A.K.; Theron, M.; Uchikawa, M.; Mboup, S.; Ndir, O.; Kwiatkowski, D.P.; Duraisingh, M.T.; et al. Basigin is a receptor essential for erythrocyte invasion by Plasmodium falciparum. Nature 2011, 480, 534–537. [Google Scholar]
- Watanabe, A.; Yoneda, M.; Ikeda, F.; Terao-Muto, Y.; Sato, H.; Kai, C. CD147/EMMPRIN acts as a functional entry receptor for measles virus on epithelial cells. J. Virol 2010, 84, 4183–4193. [Google Scholar]
- Tang, Y.; Nakada, M.T.; Kesavan, P.; McCabe, F.; Millar, H.; Rafferty, P.; Bugelski, P.; Yan, L. Extracellular matrix metalloproteinase inducer stimulates tumor angiogenesis by elevating vascular endothelial cell growth factor and matrix metalloproteinases. Cancer Res 2005, 65, 3193–3199. [Google Scholar]
- Dai, J.Y.; Dou, K.F.; Wang, C.H.; Zhao, P.; Lau, W.B.; Tao, L.; Wu, Y.M.; Tang, J.; Jiang, J.L.; Chen, Z.N. The interaction of HAb18G/CD147 with integrin alpha6beta1 and its implications for the invasion potential of human hepatoma cells. BMC Cancer 2009, 9, 337. [Google Scholar]
- Chen, H.M.; Wang, L.; Beretov, J.; Hao, J.L.; Xiao, W.W.; Li, Y. Coexpression of CD147/EMMPRIN with monocarboxylate transporters and multiple drug resistance proteins is associated with epithelial ovarian cancer progression. Clin. Exp. Metastasis 2010, 27, 557–569. [Google Scholar]
- Yurchenko, V.; Constant, S.; Bukrinsky, M. Dealing with the family: CD147 interactions with cyclophilins. Immunology 2006, 117, 301–309. [Google Scholar]
- Kato, N.; Yuzawa, Y.; Kosugi, T.; Hobo, A.; Sato, W.; Miwa, Y.; Sakamoto, K.; Matsuo, S.; Kadomatsu, K. The E-selectin ligand basigin/CD147 is responsible for neutrophil recruitment in renal ischemia/reperfusion. J. Am. Soc. Nephrol 2009, 20, 1565–1576. [Google Scholar]
- Gruszewska, E.; Chrostek, L. The alterations of glycosylation in malignant diseases. Pol. Merkur. Lekarski 2013, 34, 58–61. [Google Scholar]
- Miyauchi, T.; Kanekura, T.; Yamaoka, A.; Ozawa, M.; Miyazawa, S.; Muramatsu, T. Basigin, a new broadly distributed member of the immunoglobulin superfamily, has strong homology with both the immunoglobulin V domain and the beta-chain of major histocompatibility complex class II antigen. J. Biochem 1990, 107, 316–323. [Google Scholar]
- Yoshida, S.; Shibata, M.; Yamamoto, S.; Hagihara, M.; Asai, N.; Takahashi, M.; Mizutani, S.; Muramatsu, T.; Kadomatsu, K. Homo-oligomer formation by basigin, an immunoglobulin superfamily member, via its N-terminal immunoglobulin domain. Eur. J. Biochem 2000, 267, 4372–4380. [Google Scholar]
- Sun, J.; Hemler, M.E. Regulation of MMP-1 and MMP-2 production through CD147/extracellular matrix metalloproteinase inducer interactions. Cancer Res 2001, 61, 2276–2281. [Google Scholar]
- Tang, W.; Chang, S.B.; Hemler, M.E. Links between CD147 function, glycosylation, and caveolin-1. Mol. Biol. Cell 2004, 15, 4043–4050. [Google Scholar]
- Li, Y.; Wu, J.; Song, F.; Tang, J.; Wang, S.J.; Yu, X.L.; Chen, Z.N.; Jiang, J.L. Extracellular membrane-proximal domain of HAb18G/CD147 binds to metal ion-dependent adhesion site (MIDAS) motif of integrin β1 to modulate malignant properties of hepatoma cells. J. Biol. Chem 2012, 287, 4759–4772. [Google Scholar]
- Zhao, P.; Zhang, W.; Wang, S.J.; Yu, X.L.; Tang, J.; Huang, W.; Li, Y.; Cui, H.Y.; Guo, Y.S.; Tavernier, J.; et al. HAb18G/CD147 promotes cell motility by regulating annexin II-activated RhoA and Rac1 signaling pathways in hepatocellular carcinoma cells. Hepatology 2011, 54, 2012–2024. [Google Scholar]
- Pushkarsky, T.; Yurchenko, V.; Vanpouille, C.; Brichacek, B.; Vaisman, I.; Hatakeyama, S.; Nakayama, K.I.; Sherry, B.; Bukrinsky, M.I. Cell surface expression of CD147/EMMPRIN is regulated by cyclophilin 60. J. Biol. Chem 2005, 280, 27866–27871. [Google Scholar]
- Khunkaewla, P.; Schiller, H.B.; Paster, W.; Leksa, V.; Čermák, L.; Anděra, L.; Horejsí, V.; Stockinger, H. LFA-1-mediated leukocyte adhesion regulated by interaction of CD43 with LFA-1 and CD147. Mol. Immunol 2008, 45, 1703–1711. [Google Scholar]
- Pakula, R.; Melchior, A.; Denys, A.; Vanpouille, C.; Mazurier, J.; Allain, F. Syndecan-1/CD147 association is essential for cyclophilin B-induced activation of p44/42 mitogen-activated protein kinases and promotion of cell adhesion and chemotaxis. Glycobiology 2007, 17, 492–503. [Google Scholar]
- Wilson, M.C.; Meredith, D.; Halestrap, A.P. Fluorescenceresonance energy transfer studies on the interaction between the lactate transporter MCT1 and CD147 provide information on the topology and stoichiometry of the complex in situ. J. Biol. Chem. 2002, 277, 3666–3672. [Google Scholar]
- Hanna, S.M.; Kirk, P.; Holt, O.J.; Puklavec, M.J.; Brown, M.H.; Barclay, A.N. A novel form of the membrane protein CD147 that contains an extra Ig-like domain and interacts homophilically. BMC Biochem 2003, 4. [Google Scholar] [CrossRef] [Green Version]
- Redzic, J.S.; Armstrong, G.S.; Isern, N.G.; Jones, D.N.; Kieft, J.S.; Eisenmesser, E.Z. The retinal specific CD147 Ig0 domain: From molecular structure to biological activity. J. Mol. Biol 2011, 411, 68–82. [Google Scholar]
- Liao, C.G.; Kong, L.M.; Song, F.; Xing, J.L.; Wang, L.X.; Sun, Z.J.; Tang, H.; Yao, H.; Zhang, Y.; Wang, L.; et al. Characterization of Basigin isoforms and the inhibitory function of Basigin-3 in human hepatocellular carcinoma proliferation and invasion. Mol. Cell Biol 2011, 31, 2591–2604. [Google Scholar]
- Yu, X.L.; Hu, T.C.; Du, J.M.; Ding, J.P.; Yang, X.M.; Zhang, J.; Yang, B.; Shen, X.; Zhang, Z.; Zhong, W.D.; et al. Crystal structure of HAb18G/CD147: Implications for immunoglobulin superfamily homophilic adhesion. J. Biol. Chem 2008, 283, 18056–18065. [Google Scholar]
- Luo, J.; Teplyakov, A.; Obmolova, G.; Malia, T.; Wu, S.J.; Beil, E.; Baker, A.; Swencki-Underwood, B.; Zhao, Y.; Sprenkle, J.; et al. Structure of the EMMPRIN N-terminal domain 1: Dimerization via beta-strand swapping. Proteins 2009, 77, 1009–1014. [Google Scholar]
- Fadool, J.M.; Linser, P.J. Evidence for the formation of multimeric forms of the 5A11/HT7 antigen. Biochem. Biophys. Res. Commun 1996, 229, 280–286. [Google Scholar]
- Cui, H.Y.; Guo, T.; Wang, S.J.; Zhao, P.; Dong, Z.S.; Zhang, Y.; Jiang, J.L.; Chen, Z.N.; Yu, X.L. Dimerization is essential for HAb18G/CD147 promoting tumor invasion via MAPK pathway. Biochem. Biophys. Res. Commun 2012, 419, 517–522. [Google Scholar]
- Bohne-Lang, A.; von der Lieth, C.W. GlyProt: In silico glycosylation of proteins. Nucleic. Acids Res 2005, 33, 214–219. [Google Scholar]
- GlyProt. Available online: http://www.glycosciences.de (accessed on 9 October 2013).
- Kanekura, T.; Miyauchi, T.; Tashiro, M.; Muramatsu, T. Basigin, a new member of the immunoglobulin superfamily: Genes in different mammalian species, glycosylation changes in the molecule from adult organs and possible variation in the N-terminal sequences. Cell Struct. Funct 1991, 16, 23–30. [Google Scholar]
- Li, R.; Huang, L.; Guo, H.; Toole, B.P. Basigin (murine EMMPRIN) stimulates matrix metalloproteinase production by fibroblasts. J. Cell Physiol 2001, 186, 371–379. [Google Scholar]
- Nehme, C.L.; Fayos, B.E.; Bartles, J.R. Distribution of the integral plasma membrane glycoprotein CE9 (MRC OX-47) among rat tissues and its induction by diverse stimuli of metabolic activation. Biochem. J 1995, 310, 693–698. [Google Scholar]
- Yu, X.L.; Jiang, J.L.; Li, L.; Feng, Q.; Xu, J.; Chen, Z.N. The glycosylation characteristic of hepatoma-associated antigen HAb18G/CD147 in human hepatoma cells. Int. J. Biochem. Cell Biol 2006, 38, 1939–1945. [Google Scholar]
- Varki, A.; Cummings, R.; Esko, J.; Frecze, H.; Gerald, H.; Marth, J. Essentials of Glycobiology; Coldspring Harbor Laboratory Press: New York, NY, USA, 1999. [Google Scholar]
- Huang, W.; Luo, W.J.; Zhu, P.; Tang, J.; Yu, X.L.; Cui, H.Y.; Wang, B.; Zhang, Y.; Jiang, J.L.; Chen, Z.N. Modulation of CD147-induced matrix metalloproteinase activity: Role of CD147 N-glycosylation. Biochem. J 2013, 449, 437–448. [Google Scholar]
- Tyler, R.E.; Pearce, M.M.; Shaler, T.A.; Olzmann, J.A.; Greenblatt, E.J.; Kopito, R.R. Unassembled CD147 is an endogenous endoplasmic reticulum-associated degradation substrate. Mol. Biol. Cell 2012, 23, 4668–4678. [Google Scholar]
- Fan, J.H.; Wang, S.J.; Yu, S.J.; He, J.N.; Zheng, W.L.; Zhang, J.N. N-acetylglucosaminyltransferase IVa regulates metastatic potential of mouse hepatocarcinoma cells through glycosylation of CD147. Glycoconj. J 2012, 29, 323–334. [Google Scholar]
- Yang, L.; Nyalwidhe, J.O.; Guo, S.; Drake, R.R.; Semmes, O.J. Targeted identification of metastasis-associated cell-surface sialoglycoproteins in prostate cancer. Mol. Cell. Proteomics 2011, 10, M110. [Google Scholar]
- Jia, L.; Wang, S.J.; Zhou, H.M.; Cao, J.; Hu, Y.C.; Zhang, J.N. Caveolin-1 up-regulates CD147 glycosylation and the invasive capability of murine hepatocarcinoma cell lines. Int. J. Biochem. Cell Biol 2006, 38, 1584–1593. [Google Scholar]
- Deora, A.A.; Philp, N.; Hu, J.; Bok, D.; Rodriguez-Boulan, E. Mechanisms regulating tissue-specific polarity of monocarboxylate transporters and their chaperone CD147 inkidney and retinal epithelia. Proc. Natl. Acad. Sci. USA 2005, 102, 16245–16250. [Google Scholar]
- Gallagher, S.M.; Castorino, J.J.; Wang, D.; Philp, N.J. Monocarboxylate transporter 4 regulates maturation and trafficking of CD147 to the plasma membrane in the metastatic breast cancer cell line MDA-MB-231. Cancer Res 2007, 67, 4182–4189. [Google Scholar]
- Yan, A.; Lennarz, W.J. Unraveling the mechanism of protein N-glycosylation. J. Biol. Chem 2005, 280, 3121–3124. [Google Scholar]
- Zheng, H.C.; Wang, W.; Xu, X.Y.; Xia, P.; Yu, M.; Sugiyama, T.; Takano, Y. Up-regulated EMMPRIN/CD147 protein expression might play a role in colorectal carcinogenesis and its subsequent progression without an alteration of its glycosylation and mRNA level. J. Cancer Res. Clin. Oncol 2011, 137, 585–596. [Google Scholar]
- Beesley, A.H.; Weller, R.E.; Kees, U.R. The role of BSG (CD147) in acute lymphoblastic leukaemia and relapse. Br. J. Haematol 2008, 142, 1000–1002. [Google Scholar]
- Riethdorf, S.; Reimers, N.; Assmann, V.; Kornfeld, J.W.; Terracciano, L.; Sauter, G.; Pantel, K. High incidence of EMMPRIN expression in human tumors. Int. J. Cancer 2006, 119, 1800–1810. [Google Scholar]
- Vigneswaran, N.; Beckers, S.; Waigel, S.; Mensah, J.; Wu, J.; Mo, J.; Fleisher, K.E.; Bouquot, J.; Sacks, P.G.; Zacharias, W. Increased EMMPRIN (CD147) expression during oral carcinogenesis. Exp. Mol. Pathol 2006, 80, 147–159. [Google Scholar]
- Yarema, K.J.; Bertozzi, C.R. Characterizing glycosylation pathways. Genome Biol 2001, 2, S4. [Google Scholar]
- Brockhausen, I.; Narasimhan, S.; Schachter, H. The biosynthesis of highly branched N-glycans: Studies on the sequential pathway and functional role of N-acetylglucosaminyltransferases I, II, III, IV, V and VI. Biochimie 1988, 70, 1521–1533. [Google Scholar]
- Granovsky, M.; Fata, J.; Pawling, J.; Muller, W.J.; Khokha, R.; Dennis, J.W. Suppression of tumor growth and metastasis in Mgat5-deficient mice. Nat. Med 2000, 6, 306–312. [Google Scholar]
- Pinho, S.S.; Seruca, R.; Gärtner, F.; Yamaguchi, Y.; Gu, J.; Taniguchi, N.; Reis, C.A. Modulation of E-cadherin function and dysfunction by N-glycosylation. Cell Mol. Life Sci 2011, 68, 1011–1020. [Google Scholar]
- Gu, J.; Isaji, T.; Sato, Y.; Kariya, Y.; Fukuda, T. Importance of N-glycosylation on alpha5beta1 integrin for its biological functions. Biol. Pharm. Bull 2009, 32, 780–785. [Google Scholar]
- Ihara, S.; Miyoshi, E.; Ko, J.H.; Murata, K.; Nakahara, S.; Honke, K.; Dickson, R.B.; Lin, C.Y.; Taniguchi, N. Prometastatic effect of N-acetylglucosaminyltransferase V is due to modification and stabilization of active matriptase by adding beta 1–6 GlcNAc branching. J. Biol. Chem 2002, 277, 16960–16967. [Google Scholar]
- Kim, Y.S.; Hwang, S.Y.; Kang, H.Y.; Sohn, H.; Oh, S.; Kim, J.Y.; Yoo, J.S.; Kim, Y.H.; Kim, C.H.; Jeon, J.H.; et al. Functional proteomics study reveals that N-acetylglucosaminyltransferase V reinforces the invasive/metastatic potential of colon cancer through aberrant glycosylation on tissue inhibitor of metalloproteinase-1. Mol. Cell. Proteomics 2008, 7, 1–14. [Google Scholar]
- Zhao, Y.; Nakagawa, T.; Itoh, S.; Inamori, K.; Isaji, T.; Kariya, Y.; Kondo, A.; Miyoshi, E.; Miyazaki, K.; Kawasaki, N.; et al. N-acetylglucosaminyltransferase III antagonizes the effect of N-acetylglucosaminyltransferase V on alpha3beta1 integrin-mediated cell migration. J. Biol. Chem 2006, 281, 32122–32130. [Google Scholar]
- Minowa, M.T.; Oguri, S.; Yoshida, A.; Hara, T.; Iwamatsu, A.; Ikenaga, H.; Takeuchi, M. cDNA cloning and expression of bovine UDP-N-acetylglucosamine: Alpha1, 3-d-mannoside beta1,4-N-acetylglucosaminyltransferase IV. J. Biol. Chem 1998, 273, 11556–11562. [Google Scholar]
- Yoshida, A.; Minowa, M.T.; Takamatsu, S.; Hara, T.; Ikenaga, H.; Takeuchi, M. A novel second isoenzyme of the human UDP-Nacetylglucosamine: Alpha1,3-d-mannoside beta1, 4-N-acetylglucosaminyltransferasefamily: cDNA cloning, expression, and chromosomalassignment. Glycoconj. J 1998, 15, 1115–1123. [Google Scholar]
- Mizuochi, T.; Nishimura, R.; Derappe, C.; Taniguchi, T.; Hamamoto, T.; Mochizuki, M.; Kobata, A. Structures of the asparagine-linked sugar chains of human chorionic gonadotropin produced in choriocarcinoma. Appearance of triantennary sugarchains and unique biantennary sugar chains. J. Biol. Chem 1983, 258, 14126–14129. [Google Scholar]
- Niimi, K.; Yamamoto, E.; Fujiwara, S.; Shinjo, K.; Kotani, T.; Umezu, T.; Kajiyama, H.; Shibata, K.; Ino, K.; Kikkawa, F. High expression of N-acetylglucosaminyltransferase IVa promotes invasion of choriocarcinoma. Br. J. Cancer 2012, 107, 1969–1977. [Google Scholar]
- Yamashita, K.; Totani, K.; Iwaki, Y.; Takamisawa, I.; Tateishi, N.; Higashi, T.; Sakamoto, Y.; Kobata, A. Comparative study of the sugar chains of gamma-glutamyltranspeptidases purified from human hepatocellular carcinoma and from human liver. J. Biochem 1989, 105, 728–735. [Google Scholar]
- Miyoshi, E.; Uozumi, N.; Noda, K.; Hayashi, N.; Hori, M.; Taniguchi, N. Expression of alpha1–6 fucosyltransferase in rat tissues and human cancer cell lines. Int. J. Cancer 1997, 72, 1117–1121. [Google Scholar]
- Miyoshi, E.; Noda, K.; Yamaguchi, Y.; Inoue, S.; Ikeda, Y.; Wang, W.; Ko, J.H.; Uozumi, N.; Li, W.; Taniguchi, N. The alpha1–6-fucosyltransferase gene and its biological significance. Biochim. Biophys. Acta 1999, 1473, 9–20. [Google Scholar]
- Zhao, Y.; Itoh, S.; Wang, X.; Isaji, T.; Miyoshi, E.; Kariya, Y.; Miyazaki, K.; Kawasaki, N.; Taniguchi, N.; Gu, J. Deletion of core fucosylation on alpha3beta1 integrin down-regulates its functions. J. Biol. Chem 2006, 281, 38343–38350. [Google Scholar]
- Geng, F.; Shi, B.Z.; Yuan, Y.F.; Wu, X.Z. The expression of core fucosylated E-cadherin in cancer cells and lung cancer patients: Prognostic implications. Cell Res 2004, 14, 423–433. [Google Scholar]
- Liu, Y.C.; Yen, H.Y.; Chen, C.Y.; Chen, C.H.; Cheng, P.F.; Juan, Y.H.; Chen, C.H.; Khoo, K.H.; Yu, C.J.; Yang, P.C.; et al. Sialylation and fucosylation of epidermal growth factor receptor suppress its dimerization and activation in lung cancer cells. Proc. Natl. Acad. Sci. USA 2011, 108, 11332–11337. [Google Scholar]
- Dai, Z.; Zhou, J.; Qiu, S.J.; Liu, Y.K.; Fan, J. Lectin-based glycoproteomics to explore and analyze hepatocellular carcinoma-related glycoprotein markers. Electrophoresis 2009, 30, 2957–2966. [Google Scholar]
- Ogata, S.; Ho, I.; Chen, A.; Dubois, D.; Maklansky, J.; Singhal, A.; Hakomori, S.; Itzkowitz, S.H. Tumor-associated sialylated antigens are constitutively expressed in normal human colonic mucosa. Cancer Res 1995, 55, 1869–1874. [Google Scholar]
- Tanaka, F.; Otake, Y.; Nakagawa, T.; Kawano, Y.; Miyahara, R.; Li, M.; Yanagihara, K.; Inui, K.; Oyanagi, H.; Yamada, T.; et al. Prognostic significance of polysialic acid expression in resected non-small cell lung cancer. Cancer. Res 2001, 61, 1666–1670. [Google Scholar]
- Delmotte, P.; Degroote, S.; Lafitte, J.J.; Lamblin, G.; Perini, J.M.; Roussel, P. Tumor necrosis factor alpha increases the expression of glycosyltransferases and sulfotransferases responsible for the biosynthesis of sialylated and/or sulfated Lewis x epitopes in the human bronchial mucosa. J. Biol. Chem 2002, 277, 424–431. [Google Scholar]
- Varki, A. Glycan-based interactions involving vertebrate sialic-acid-recognizing proteins. Nature 2007, 446, 1023–1029. [Google Scholar]
- Yu, S.J.; Fan, J.H.; Liu, L.H.; Zhang, L.J.; Wang, S.J.; Zhang, J.N. Caveolin-1 up-regulates integrin α2,6-sialylation to promote integrin α5β1-dependent hepatocarcinoma cell adhesion. FEBS Lett 2013, 587, 782–787. [Google Scholar]
- Tang, W.; Hemler, M.E. Caveolin-1 regulates matrix metalloproteinases-1 induction and CD147/EMMPRIN cell surface clustering. J. Biol. Chem 2004, 279, 11112–11118. [Google Scholar]
- Sasai, K.; Ikeda, Y.; Ihara, H.; Honke, K.; Taniguchi, N. Caveolin-1 regulates the functional localization of N-acetylglucosaminyltransferase III within the Golgi apparatus. J. Biol. Chem 2003, 278, 25295–27301. [Google Scholar]
- Barth, K.; Bläche, R.; Kasper, M. Lack of evidence for caveolin-1 and CD147 interaction before and after bleomycin-induced lung injury. Histochem. Cell Biol 2006, 126, 563–573. [Google Scholar]
- Yu, S.J.; Zhang, L.J.; Li, N.Y.; Fan, J.H.; Liu, L.H.; Zhang, J.N.; Wang, S.J. Caveolin-1 up-regulates ST6Gal-I to promote the adhesive capability of mouse hepatocarcinoma cells to fibronectin via FAK-mediated adhesion signaling. Biochem. Biophys. Res. Commun 2012, 427, 506–512. [Google Scholar]
- Kato, Y.; Ozawa, S.; Miyamoto, C.; Maehata, Y.; Suzuki, A.; Maeda, T.; Baba, Y. Acidic extracellular microenvironment and cancer. Cancer Cell Int 2013, 13, 89. [Google Scholar]
- Kirk, P.; Wilson, M.C.; Heddle, C.; Brown, M.H.; Barclay, A.N.; Halestrap, A.P. CD147 is tightly associated with lactate transporters MCT1 and MCT4 and facilitates their cell surface expression. EMBO J 2000, 19, 3896–3904. [Google Scholar]
- Walters, D.K.; Arendt, B.K.; Jelinek, D.F. CD147 regulates the expression of MCT1 and lactate export in multiple myeloma cells. Cell Cycle 2013, 12, 3175–3183. [Google Scholar]
- Philp, N.J.; Ochrietor, J.D.; Rudoy, C.; Muramatsu, T.; Linser, P.J. Loss of MCT1, MCT3, and MCT4 expression in the retinal pigment epithelium epithelium and neural retina of the 5A11/basigin-null mouse. Investig. Ophthalmol. Vis. Sci 2003, 44, 1305–1311. [Google Scholar]
- Yurchenko, V.; Pushkarsky, T.; Li, J.H.; Dai, W.W.; Sherry, B.; Bukrinsky, M. Regulation of CD147 cell surface expression: Involvement of the proline residue in the CD147 transmembrane domain. J. Biol. Chem 2005, 280, 17013–17019. [Google Scholar]
- Suh, Y.H.; Checler, F. Amyloid precursor protein, presenilins, and alpha-synuclein: Molecular pathogenesis and pharmacological applications in Alzheimer’s disease. Pharmacol. Rev 2002, 54, 469–525. [Google Scholar]
- Zhou, S.; Zhou, H.; Walian, P.J.; Jap, B.K. CD147 is a regulatory subunit of the gamma-secretase complex in Alzheimer’s disease amyloid beta-peptide production. Proc. Natl. Acad. Sci. USA 2005, 102, 7499–7504. [Google Scholar]
- Nahalkova, J.; Volkmann, I.; Aoki, M.; Winblad, B.; Bogdanovic, N.; Tjernberg, L.O.; Behbahani, H. CD147, a gamma-secretase associated protein is upregulated in Alzheimer’s disease brain and its cellular trafficking is affected by presenilin-2. Neurochem. Int 2010, 56, 67–76. [Google Scholar]
- Li, W.; Alfaidy, N.; Challis, J.R. Expression of extracellular matrix metalloproteinase inducer in human placenta and fetal membranes at term labor. J. Clin. Endocrinol. Metab 2004, 89, 2897–2904. [Google Scholar]
- Noguchi, Y.; Sato, T.; Hirata, M.; Hara, T.; Ohama, K.; Ito, A. Identification and characterization of extracellular matrix metalloproteinase inducer in human endometrium during the menstrual cycle in vivo and in vitro. J. Clin. Endocrinol. Metab. 2003, 88, 6063–6072. [Google Scholar]
- Wadsworth, S.J.; Yang, J.; Singhera, G.K.; Dorscheid, D. EMMPRIN/CD147 regulates cytokine-induced airway epithelial MMP expression via interaction with caveolin-1. Proceeding of the American Thoracic Society International Conference, Denver, CO, USA, 13–18 May 2011; pp. 13–18.
- Sluijter, J.P.; Pulskens, W.P.; Schoneveld, A.H.; Velema, E.; Strijder, C.F.; Moll, F.; de Vries, J.P.; Verheijen, J.; Hanemaaijer, R.; de Kleijn, D.P.; et al. Matrix metalloproteinase 2 is associated with stable and matrix metalloproteinases 8 and 9 with vulnerable carotidatherosclerotic lesions: A study in human endarterectomy specimen pointing to a role for different extracellularmatrix metalloproteinase inducer glycosylation forms. Stroke 2006, 37, 235–239. [Google Scholar]
- Wang, J.; Yang, D.; Li, C.; Shang, S.; Xiang, J. Expression of extracellular matrix metalloproteinase inducer glycosylation and caveolin-1 in healthy and inflamed human gingiva. J. Periodontal Res 2014, 49, 197–204. [Google Scholar]
- Jia, L.; Zhou, H.M.; Wang, S.J.; Cao, J.; Wei, W.; Zhang, J.N. Deglycosylation of CD147 down-regulates Matrix Metalloproteinase-11 expression and the adhesive capability of murine hepatocarcinoma cell HcaFin vitro. IUBMB Life 2006, 58, 209–216. [Google Scholar]
- Zhang, Z.H.; Zhao, Y.F.; Jiang, L.L.; Miao, X.Y.; Zhou, H.M.; Jia, L. Glycomic alterations are associated with multidrug resistance in human leukemia. Int. J. Biochem. Cell Biol 2012, 44, 1244–1253. [Google Scholar]
- Guo, H.; Zucker, S.; Gordon, M.K.; Toole, B.P.; Biswas, C. Stimulation of matrix metalloproteinase production by recombinant extracellular matrix metalloproteinase inducer from transfected Chinese hamster ovary cells. J. Biol. Chem 1997, 272, 24–27. [Google Scholar]
- Belton, R.J.; Chen, L.; Mesquita, F.S.; Nowak, R.A. Basigin-2 is a cell surface receptor for soluble basigin ligand. J. Biol. Chem 2008, 283, 17805–17814. [Google Scholar]
- Hojo, H.; Haginoya, E.; Matsumoto, Y.; Nakahara, Y.; Nabeshima, K.; Toole, B.P.; Watanabe, Y. The first synthesis of peptide thioester carrying N-linked core pentasaccharide through modified Fmoc thioester preparation: Synthesis of an N-glycosylated Ig domain of emmprin. Tetrahedron Lett 2003, 44, 2961–2964. [Google Scholar]
- Hojo, H.; Watabe, J.; Nakahara, Y.; Ito, Y.; Nabeshima, K.; Toole, B.P. Synthesis of the extracellular Ig domain I of Emmprin carrying a chitobiose unit. Tetrahedron Lett 2001, 42, 3001–3004. [Google Scholar]
- Kawakami, T.; Sameshima, T.; Hojo, H.; Koga, K.; Nakahara, Y.; Toole, B.P.; Suzumiya, J.; Okada, Y.; Iwasaki, A.; Nabeshima, K. Synthetic emmprin peptides with chitobiose substitution stimulate MMP-2 production by Fibroblasts. BMC Cancer 2011, 11. [Google Scholar] [CrossRef]
- Toole, B.P. Emmprin (CD147), a cell surface regulator of matrix metalloproteinase production and function. Curr. Top. Dev. Biol 2003, 54, 371–389. [Google Scholar]
- Papadimitropoulou, A.; Mamalaki, A. The glycosylated IgII extracellular domain of EMMPRIN is implicated in the induction of MMP-2. Mol. Cell Biochem 2013, 379, 107–113. [Google Scholar]
- Schlegel, J.; Redzic, J.S.; Porter, C.C.; Yurchenko, V.; Bukrinsky, M.; Labeikovsky, W.; Armstrong, G.S.; Zhang, F.; Isern, N.G.; DeGregori, J.; et al. Solution characterization of the extracellular region of CD147 and its interaction with its enzyme ligand cyclophilin A. J. Mol. Biol 2009, 391, 518–535. [Google Scholar]
- Gupta, G.; Sinha, S.; Mitra, N.; Surolia, A. Probing into the role of conserved N-glycosylation sites in the Tyrosinase glycoprotein family. Glycoconj. J 2009, 26, 691–695. [Google Scholar]
- Taylor, P.M.; Woodfield, R.J.; Hodgkin, M.N.; Pettitt, T.R.; Martin, A.; Kerr, D.J.; Wakelam, M.J. Breast cancer cell-derived EMMPRIN stimulates fibroblast MMP2 release through a phospholipase A(2) and 5-lipoxygenase catalyzed pathway. Oncogene 2002, 21, 5765–5772. [Google Scholar]
- Egawa, N.; Koshikawa, N.; Tomari, T.; Nabeshima, K.; Isobe, T.; Seiki, M. Membrane type I matrix metanoproteinase (MT1-MMP/MMP-14) cleaves and releases a 22-kDa extracellular matrix metanoproteinase inducer (EMMPRIN) fragment from tumor cells. J. Biol. Chem 2006, 281, 37576–37585. [Google Scholar]
- Millimaggi, D.; Mari, M.; D’Ascenzo, S.; Carosa, E.; Jannini, E.A.; Zucker, S.; Carta, G.; Pavan, A.; Dolo, V. Tumor vesicle associated CD147 modulates the angiogenic capability of endothelial cells. Neoplasia 2007, 9, 349–357. [Google Scholar]
- Sidhu, S.S.; Mengistab, A.T.; Tauscher, A.N.; LaVail, J.; Basbaum, C. The microvesicle as a vehicle for EMMPRIN in tumor-stromal interactions. Oncogene 2004, 23, 956–963. [Google Scholar]
- Fanelli, A.; Grollman, E.F.; Wang, D.; Philp, N.J. MCT1 and its accessory protein CD147 are differentially regulated by TSH in rat thyroid cells. Am. J. Physiol. Endocrinol. Metab 2003, 285, E1223–E1229. [Google Scholar]
- Lowe, J.B. Glycan-dependent leukocyte adhesion and recruitment in inflammation. Curr. Opin. Cell Biol 2003, 15, 531–538. [Google Scholar]
- Katayama, Y.; Hidalgo, A.; Chang, J.; Peired, A.; Frenette, P.S. CD44 is a physiological E-selectin ligand on neutrophils. J. Exp. Med 2005, 201, 1183–1189. [Google Scholar]
- Chen, Z.N.; Mi, L.; Xu, J.; Song, F.; Zhang, Q.; Zhang, Z.; Xing, J.L.; Bian, H.J.; Jiang, J.L.; Wang, X.H.; et al. Targeting radioimmunotherapy of hepatocellular carcinoma with iodine (131I) Metuximab injection: Clinical phase I/II trials. Int. J. Radiat. Oncol. Biol. Phys 2006, 65, 435–444. [Google Scholar]
- Xu, J.; Shen, Z.Y.; Chen, X.G.; Zhang, Q.; Bian, H.J.; Zhu, P.; Xu, H.Y.; Song, F.; Yang, X.M.; Mi, L.; et al. A randomized controlled trial of Licartin for preventing hepatoma recurrence after liver transplantation. Hepatology 2007, 45, 269–276. [Google Scholar]
- Gildersleeve, J.C.; Wang, B.; Achilefu, S.; Tu, Z.; Xu, M. Glycan array analysis of the antigen repertoire targeted by tumor-binding antibodies. Bioorgan. Med. Chem. Lett 2012, 22, 6839–6843. [Google Scholar]
© 2014 by the authors; licensee MDPI, Basel, Switzerland This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Bai, Y.; Huang, W.; Ma, L.-T.; Jiang, J.-L.; Chen, Z.-N. Importance of N-Glycosylation on CD147 for Its Biological Functions. Int. J. Mol. Sci. 2014, 15, 6356-6377. https://doi.org/10.3390/ijms15046356
Bai Y, Huang W, Ma L-T, Jiang J-L, Chen Z-N. Importance of N-Glycosylation on CD147 for Its Biological Functions. International Journal of Molecular Sciences. 2014; 15(4):6356-6377. https://doi.org/10.3390/ijms15046356
Chicago/Turabian StyleBai, Yang, Wan Huang, Li-Tian Ma, Jian-Li Jiang, and Zhi-Nan Chen. 2014. "Importance of N-Glycosylation on CD147 for Its Biological Functions" International Journal of Molecular Sciences 15, no. 4: 6356-6377. https://doi.org/10.3390/ijms15046356
APA StyleBai, Y., Huang, W., Ma, L.-T., Jiang, J.-L., & Chen, Z.-N. (2014). Importance of N-Glycosylation on CD147 for Its Biological Functions. International Journal of Molecular Sciences, 15(4), 6356-6377. https://doi.org/10.3390/ijms15046356




