Toxicity and Metabolism of Layered Double Hydroxide Intercalated with Levodopa in a Parkinson’s Disease Model
Abstract
:1. Introduction
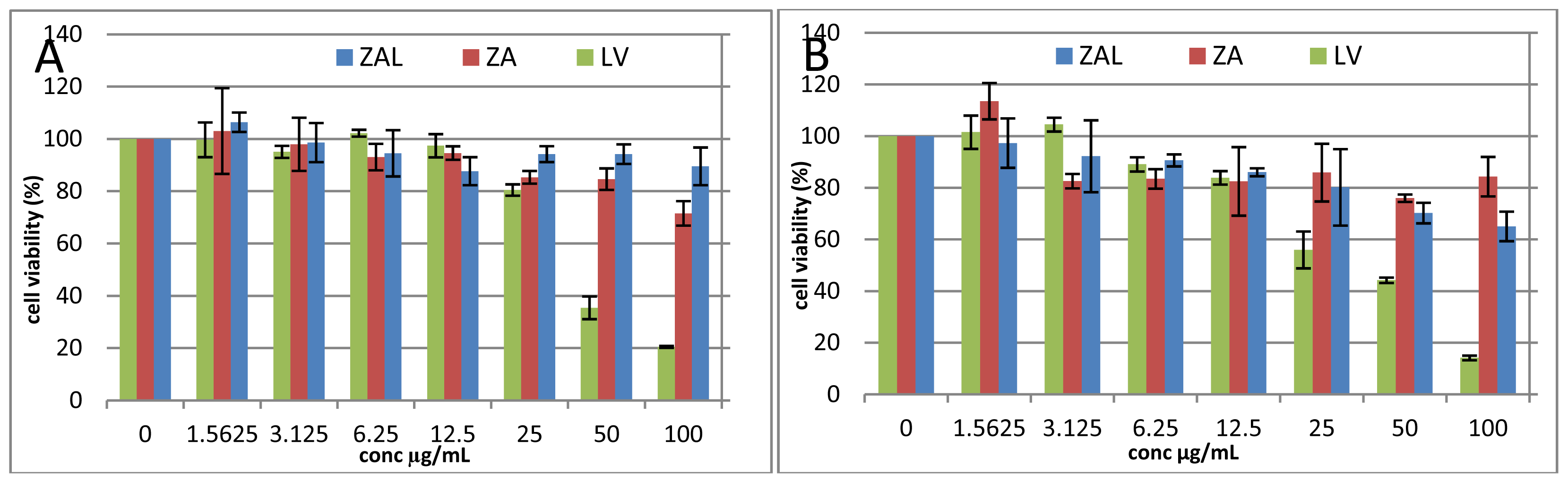
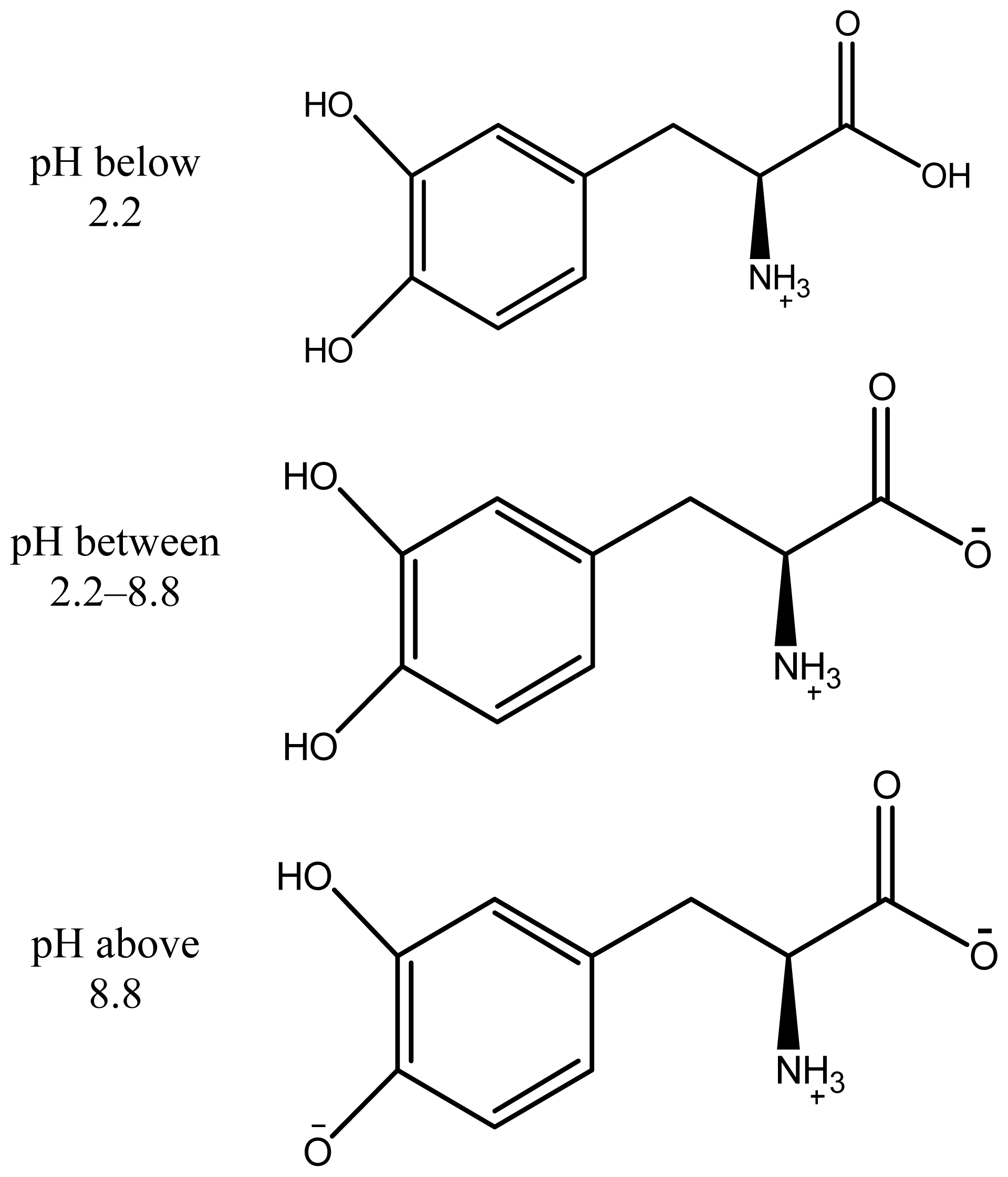
2. Results and Discussion
2.1. Cell Viability Study
3. Materials and Methods
3.1. Cell Culture
3.2. Preparation of Nanoparticles for Viability Assay
3.3. Cell Viability Study
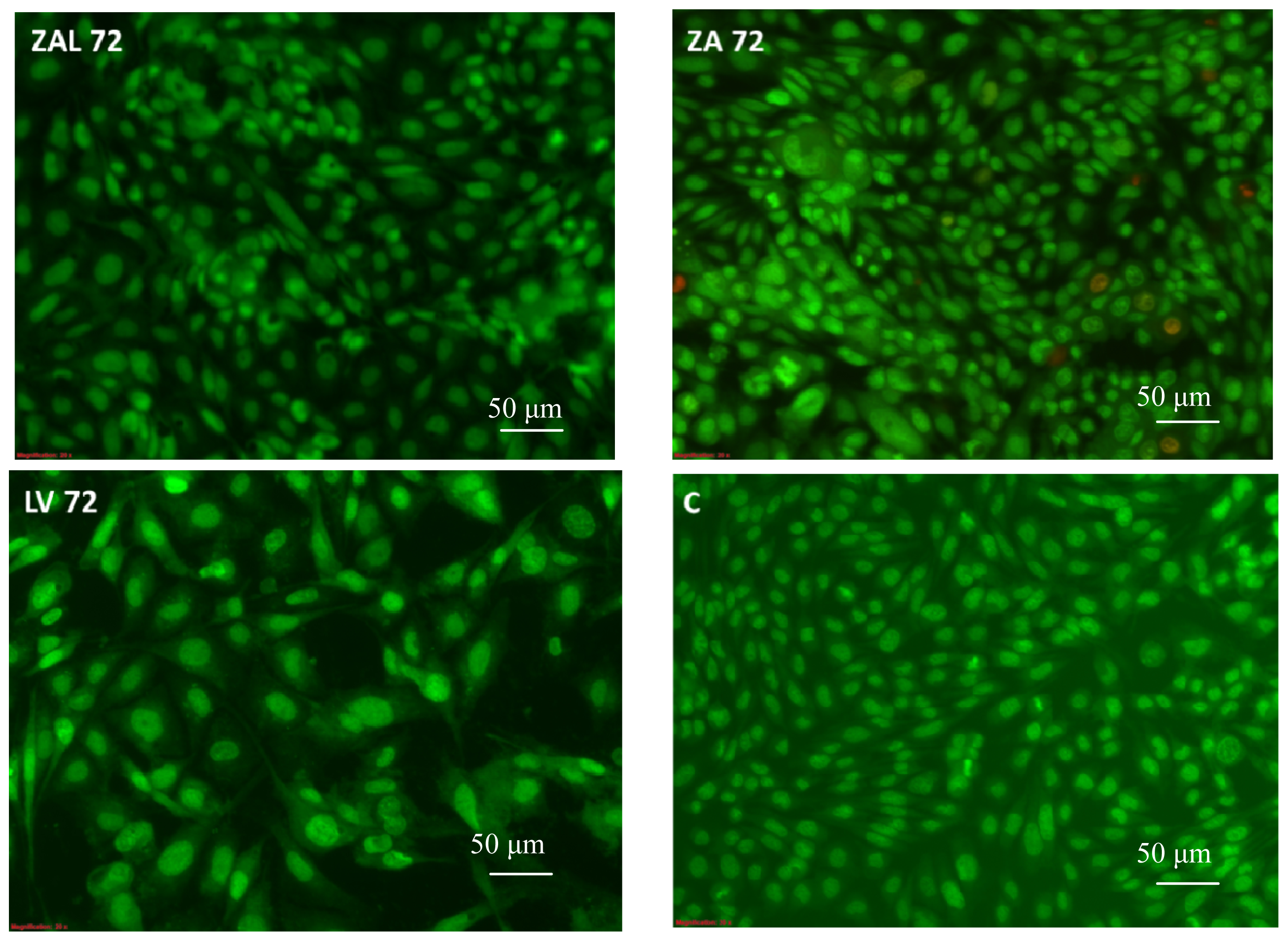
3.4. Surface Morphological Changes of PC12 Cells Due to Exposure to Nanocomposites or Pure Levodopa
3.4.1. Microscopic Study
3.4.2. Scanning Electron Microscope (SEM) Analysis
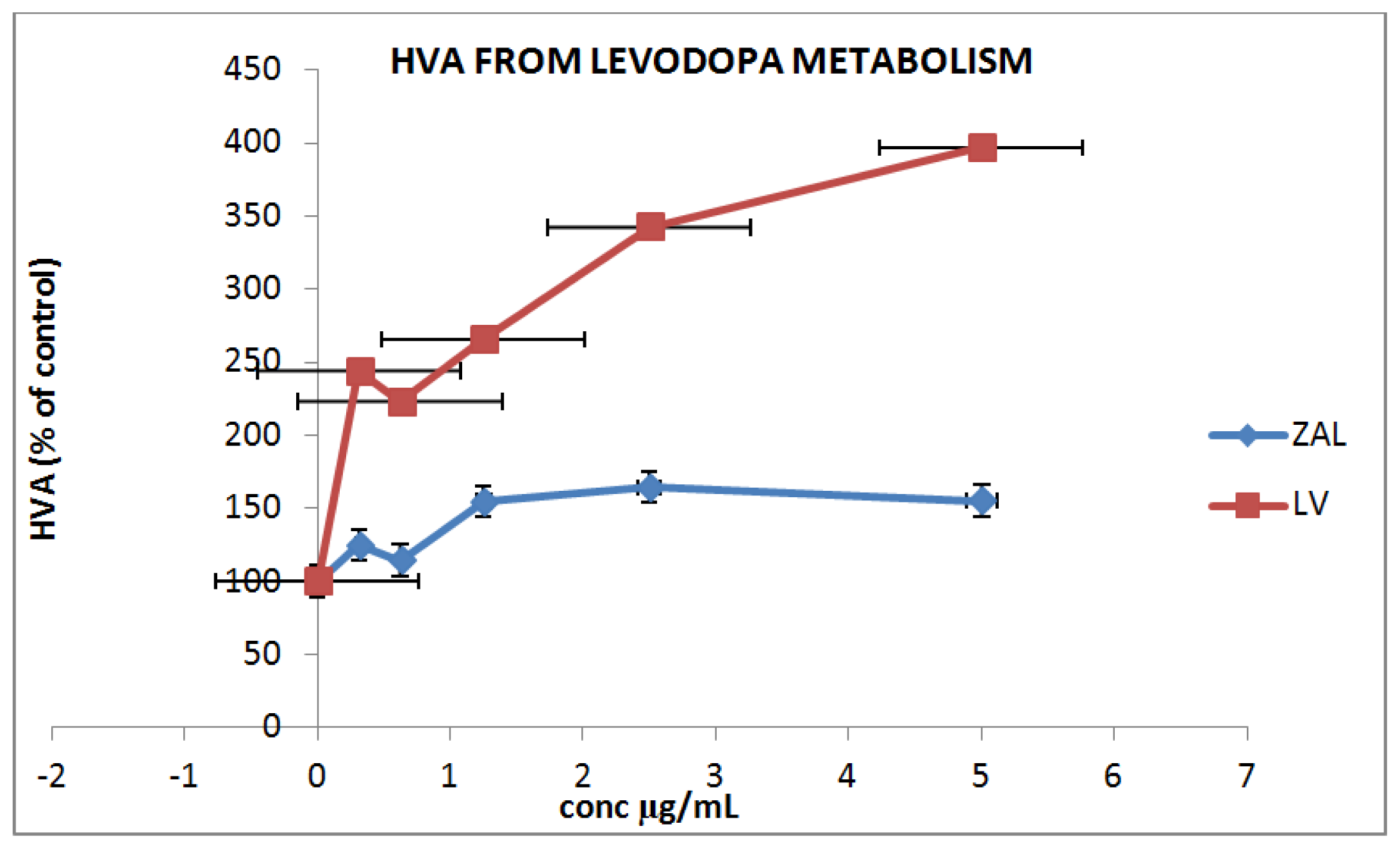
3.4.3. In Vitro Drug Metabolism and Uptake
4. Conclusions
Acknowledgments
Conflicts of Interest
References
- Zhu, X.; Zhu, L.; Chen, Y.; Tian, S. Acute toxicities of six manufactured nanomaterial suspensions toDaphnia magna. J. Nanopat. Res 2009, 11, 67–75. [Google Scholar]
- Donaldson, K.; Stone, V.; Tran, C.L.; Kreyling, W.; Borm, P.J.A. Nanotoxicology. Occup. Environ. Med 2004, 61, 727–728. [Google Scholar]
- Xu, Z.P.; Lu, G.Q. Layered double hydroxide nanomaterials as potential cellular drug delivery agents. Pure Appl. Chem 2006, 78, 1771–1779. [Google Scholar]
- Olivier, J.-C. Drug transport to brain with targeted nanoparticles. J. Am. Soc. Exp 2005, 2, 108–119. [Google Scholar]
- Pinto, R.C.; Neufeld, R.J.; Ribeiro, A.J.; Veiga, F.; Nanoencapsulation, I. Methods for preparation of drug-loaded polymeric nanoparticles. Nanomedicine 2006, 2, 8–21. [Google Scholar]
- Nalawade, P.; Aware, B.; Kadam, V.J.; Hirlekar, R.S. Bharati Vidyapeeths. Layered double hydroxide: A review. J. Sci. Ind. Res 2009, 68, 267–272. [Google Scholar]
- Parkkinen, L.; O’Sullivan, S.S.; Kuoppamäki, M.; Collins, C.; Kallis, C.; Holton, J.L.; Williams, D.R.; Revesz, T.; Lees, A.J. Does levodopa accelerate the pathologic process in Parkinson disease brain? Neurology 2011, 77, 1420–1426. [Google Scholar]
- Kura, A.U.; Ali, S.H.H.A.; Hussein, M.Z.; Fakurazi, S.; Arulselvan, P. Development of a controlled-release anti-Parkinsonian nano delivery system using levodopa as the active agent. Int. J. Nanomed 2013, 8, 1103–1110. [Google Scholar]
- Jaber, M.; Bouchoucha, M.; Delmotte, L.; Méthivier, C.; Lambert, J.-F. Fate of L-DOPA in the presence of inorganic matrices: Vectorization or composite material formation? J. Phys. Chem. C 2011, 115, 19216–19225. [Google Scholar]
- Wei, M.; Pu, M.; Guo, J.; Han, J.; Li, F.; He, J.; Evans, D.G.; Duan, X. The intercalation of L-Dopa into layered double hydroxides: Enhancement of both chemical and stereo chemical stabilities of a drug through host-guest interactions. Chem. Mater 2008, 20, 5169–5180. [Google Scholar]
- Juan, S.-A. Catecholaminergic cell lines for the study of dopamine metabolism and neurotoxicity. In Cell Culture Techniques, Neuromethods; Humana Press: New York, NY, USA, 2011; Volume 56, pp. 383–402. [Google Scholar]
- Ju, M.S.; Kim, H.G.; Choi, J.G.; Ryu, J.H.; Hur, J.; Kim, Y.J.; Oha, M.S. Cassiae semen, a seed of Cassia obtusifolia, has neuroprotective effects in Parkinson’s disease models. Food Chem. Toxicol 2010, 48, 2037–2044. [Google Scholar]
- Baea, N.; Ahnb, T.; Chungc, S.; Ohd, M.S.; Hyeonseok, K.; Oh, H.; Park, G.; Yang, H.O. The neuroprotective effect of modified Yeoldahanso-tang via autophagy enhancement in models of Parkinson’s disease. J. Ethnopharmacol 2011, 134, 313–322. [Google Scholar]
- Basma, A.N.; Morris, E.J.; Nicklas, W.J.; Geller, H.M. l-Dopa cytotoxicity to PC12 cells in culture is via its autoxidation. J. Neurochem 1995, 64, 825–832. [Google Scholar]
- Hasan, S.; Hussein, A.A.; Mothanna, A.-Q.; Mohd, Z.H.; Maznah, I.; Zainal, Z.; Muhammad, N.H. Controlled-release formulation of antihistamine based on cetirizine zinc-layered hydroxide nanocomposites and its effect on histamine release from basophilic leukemia (RBL-2H3) cells. Int. J. Nanomed 2012, 7, 3351–3363. [Google Scholar]
- Choi, S.-J.; Oh, J.-M.; Choy, J.-H. Human-related application and nanotoxicology of inorganic particles: complementary aspects. J. Mater. Chem 2008, 18, 615–620. [Google Scholar]
- Pisanic, T.R.; Blackwella, J.D.; Shubayev, V.I.; Fiñones, R.R.; Jin, S. Nanotoxicity of iron oxide nanoparticle internalization in growing neurons. Biomaterials 2007, 28, 2572–2581. [Google Scholar]
- Das, K.P.; Freudenrich, T.M.; Mundy, W.R. Assessment of PC12 cell differentiation and neurite growth: A comparison of morphological and neurochemical measures. Neurotoxicol. Teratol 2004, 26, 397–406. [Google Scholar]
- Miller, M.R.; Wentz, E.; Blair, J.B.; Pack, D.; Hinton, D.E. Acetaminophen toxicity in cultured trout liver cells morphological alterations and effects on cytochromeP450. Exp. Mol. Pathol 1993, 58, 114–126. [Google Scholar]
- Wong, Y.; Markham, K.; Xu, Z.P.; Chen, M.; Lu, G.Q.; Bartlett, P.F.; Cooper, H.M. Efficient delivery of siRNA to cortical neurons using layered double hydroxide nanoparticles. Biomaterials 2010, 31. [Google Scholar] [CrossRef]
- Choi1, S.-J.; Oh1, J.-M.; Choy, J.-H. Biocompatible nanoparticles intercalated withanticancer drug for target delivery: Pharmacokinetics and biodistribution study. J. Nanosci. Nanotechnol 2010, 10, 2913–2916. [Google Scholar]
- Lee, N.; Hummer, D.R.; Sverjensky, D.A.; Rajh, T.; Hazen, R.M.; Steele, A.; Cody, G.D. Speciation of l-DOPA on nanorutile as a function of pH and surface coverage using Surface-Enhanced Raman Spectroscopy (SERS). Langmuir 2012, 28, 17322–17330. [Google Scholar]
- Sanaie, N.; Haynes, C.A. Modeling l-dopa purification by chiral ligand-exchange chromatography. AIChE J 2007, 53, 617–626. [Google Scholar]
- Xia, S.-J.; Ni, Z.-M.; Xu, Q.; Hu, B.-X.; Hu, J. Layered double hydroxides as support for intercalation and sustained release of antihypertensive drugs. J. Solid State Chem 2008, 181, 2610–2619. [Google Scholar]
- Estes, L. Review of pharmacokinetics and pharmacodynamics of antimicrobial agents. Mayo Clin. Proc 1998, 73, 1114–1122. [Google Scholar]
- Mosmann, T. Rapid colorimetric assay for cellular growth and survival: Application to proliferation and cytotoxicity assays. J. Immunol. Methods 1983, 65, 55–63. [Google Scholar]
- Eom, H.-J.; Choi, J. SiO2 nanoparticles induced cytotoxicity by oxidative stress in human bronchial epithelial cell, beas-2B. Environ. Health Toxicol 2011, 26, e2011013. [Google Scholar]


© 2014 by the authors; licensee MDPI, Basel, Switzerland This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Kura, A.U.; Ain, N.M.; Hussein, M.Z.; Fakurazi, S.; Hussein-Al-Ali, S.H. Toxicity and Metabolism of Layered Double Hydroxide Intercalated with Levodopa in a Parkinson’s Disease Model. Int. J. Mol. Sci. 2014, 15, 5916-5927. https://doi.org/10.3390/ijms15045916
Kura AU, Ain NM, Hussein MZ, Fakurazi S, Hussein-Al-Ali SH. Toxicity and Metabolism of Layered Double Hydroxide Intercalated with Levodopa in a Parkinson’s Disease Model. International Journal of Molecular Sciences. 2014; 15(4):5916-5927. https://doi.org/10.3390/ijms15045916
Chicago/Turabian StyleKura, Aminu Umar, Nooraini Mohd Ain, Mohd Zobir Hussein, Sharida Fakurazi, and Samer Hasan Hussein-Al-Ali. 2014. "Toxicity and Metabolism of Layered Double Hydroxide Intercalated with Levodopa in a Parkinson’s Disease Model" International Journal of Molecular Sciences 15, no. 4: 5916-5927. https://doi.org/10.3390/ijms15045916
APA StyleKura, A. U., Ain, N. M., Hussein, M. Z., Fakurazi, S., & Hussein-Al-Ali, S. H. (2014). Toxicity and Metabolism of Layered Double Hydroxide Intercalated with Levodopa in a Parkinson’s Disease Model. International Journal of Molecular Sciences, 15(4), 5916-5927. https://doi.org/10.3390/ijms15045916



