Exposure to Atrazine during Gestation and Lactation Periods: Toxicity Effects on Dopaminergic Neurons in Offspring by Downregulation of Nurr1 and VMAT2
Abstract
:1. Introduction
2. Results
2.1. General Status of Rats
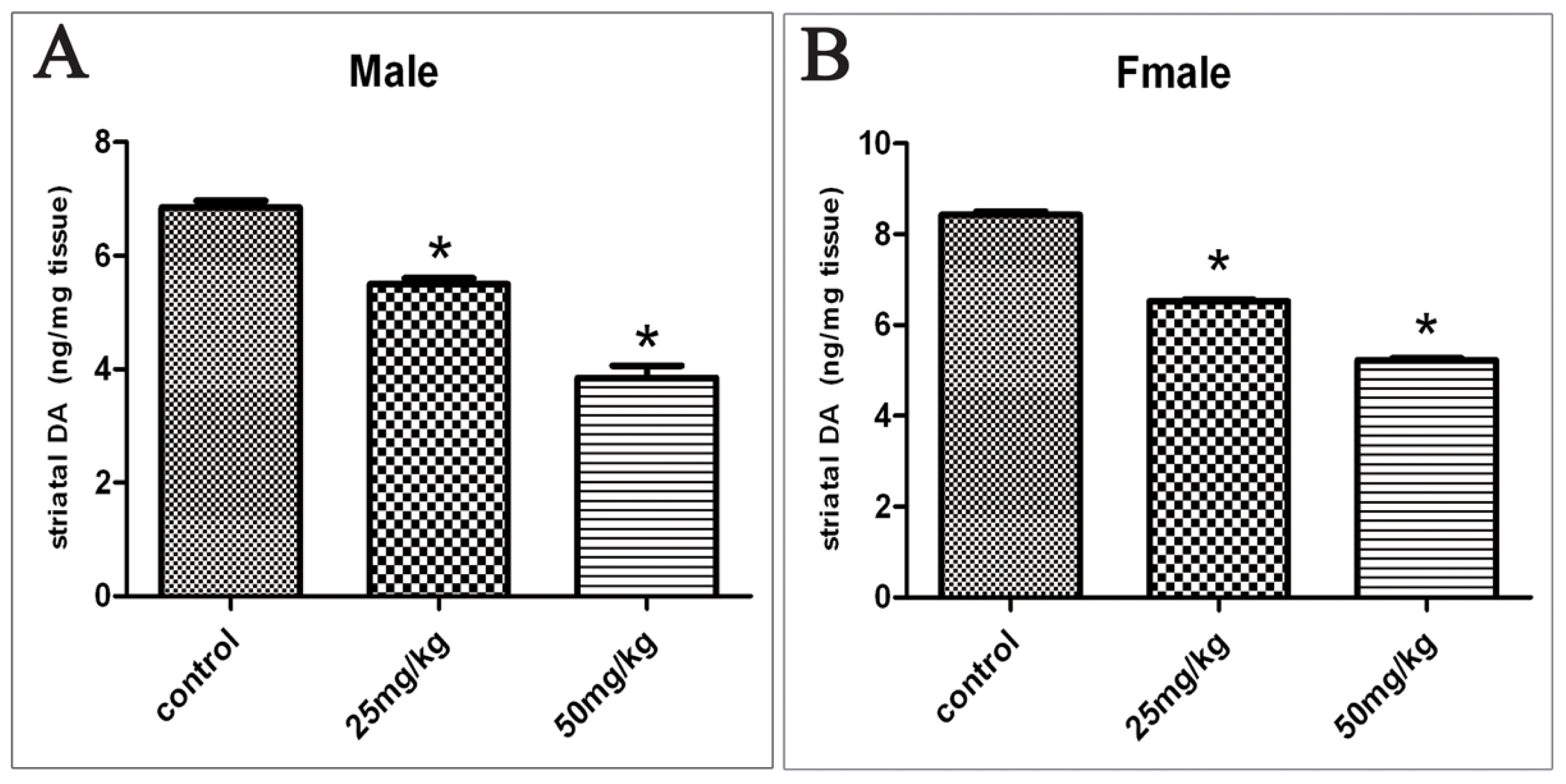
2.2. Changes in DA Levels
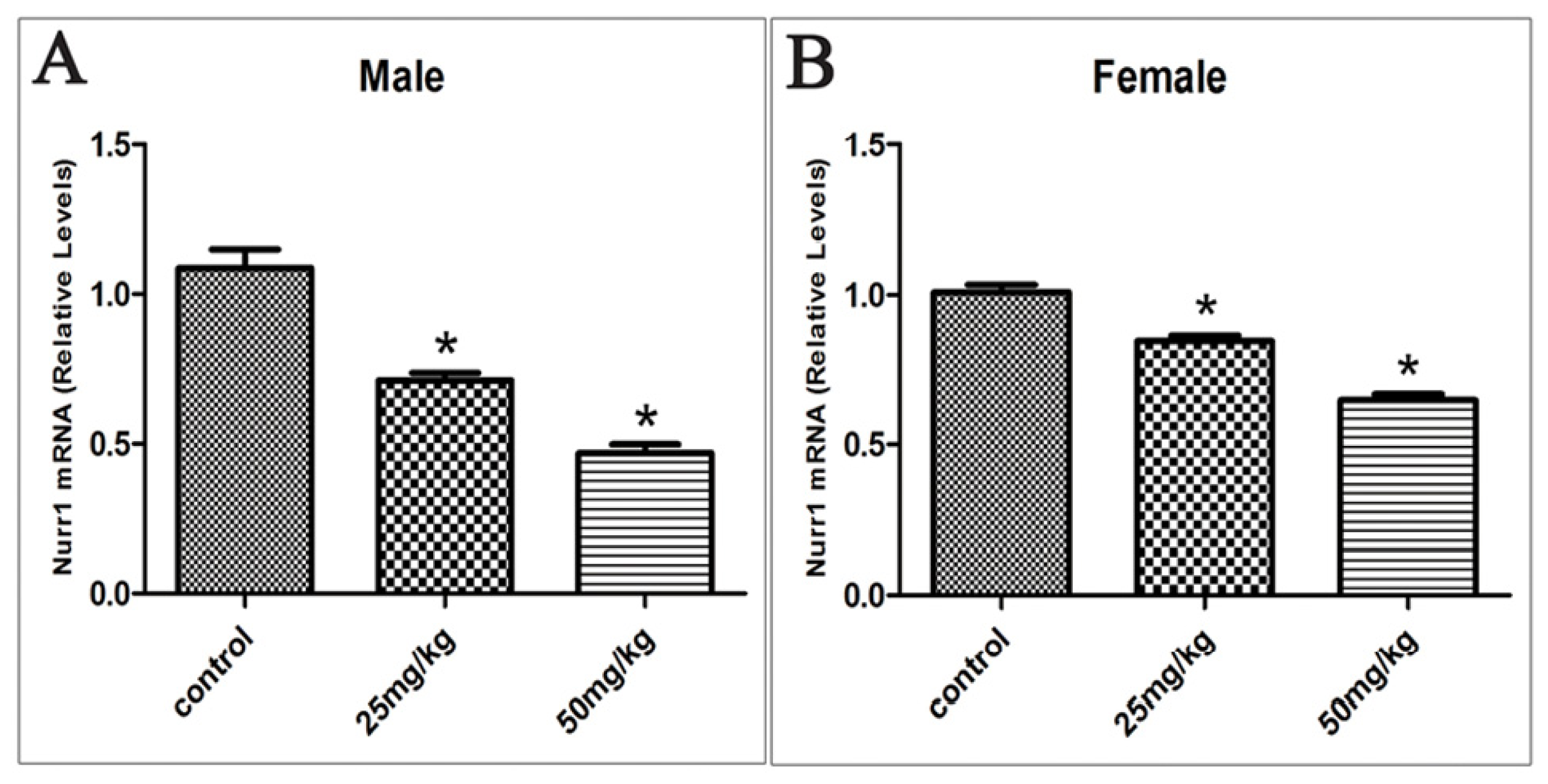
2.3. Effects of Developmental ATR Exposure on Nurr1 mRNA Levels
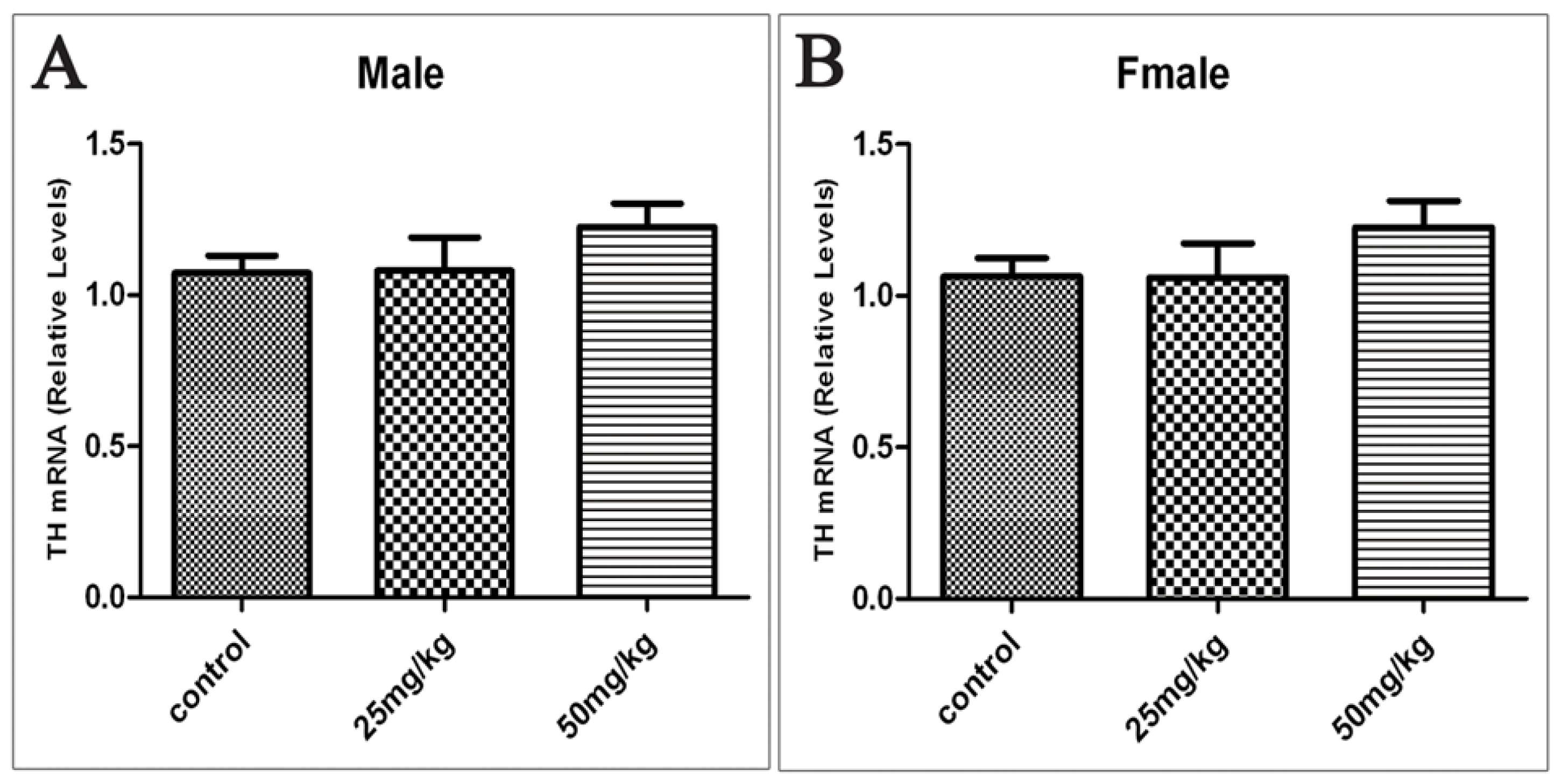
2.4. Effects of Developmental ATR Exposure on the mRNA Levels of TH
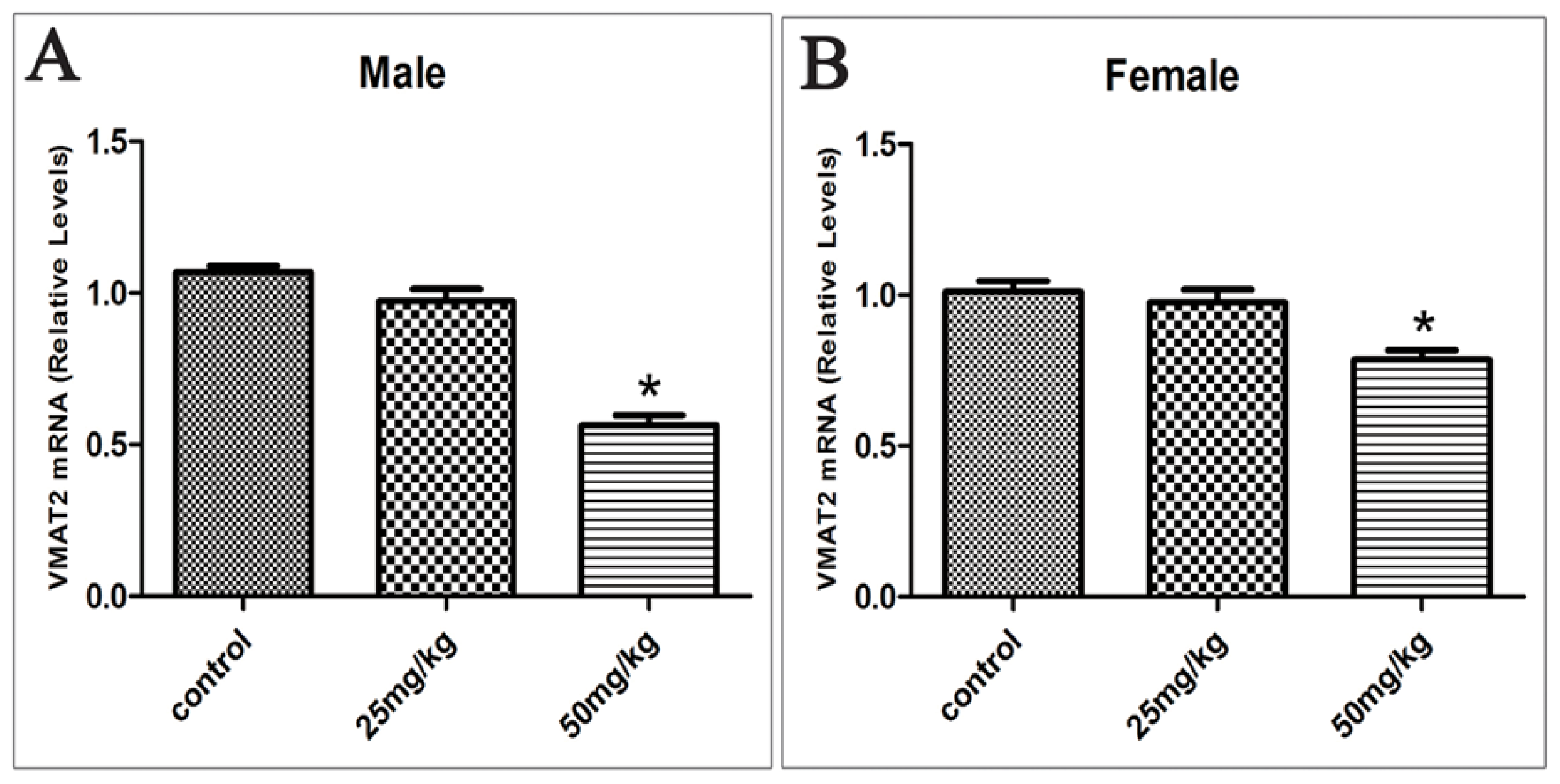
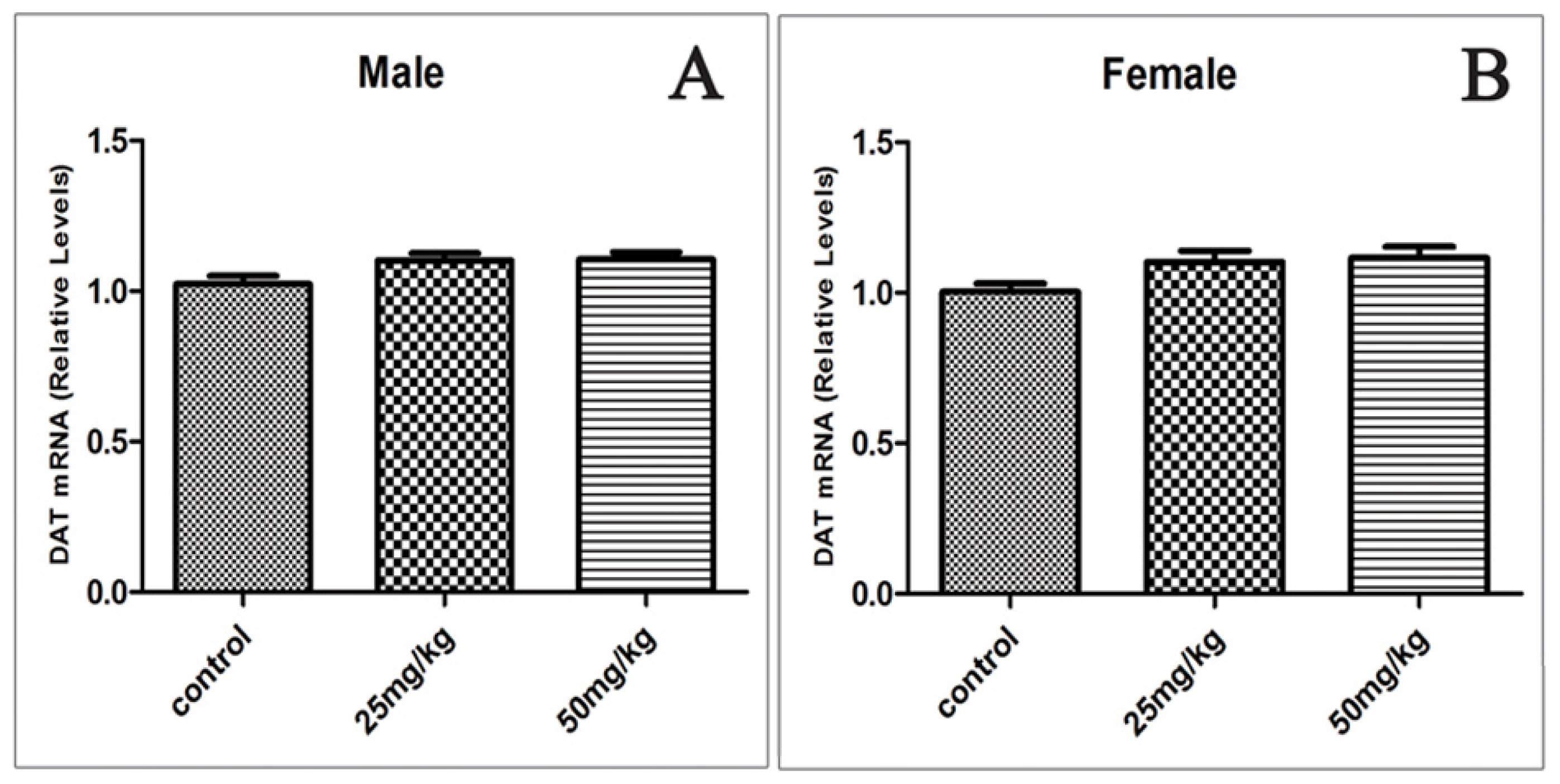
2.5. Effects of Developmental ATR Exposure on the mRNA Levels of DAT and VMAT2
3. Discussion
4. Experimental Section
4.1. Chemicals and Reagents
4.2. Animals and Treatment
4.3. Body Weight and Food Consumption
4.4. HPLC-FL Determination of DA
4.5. Total RNA Extraction and Real-Time PCR
4.6. Western Blotting
4.7. Statistical Analysis
5. Conclusions
Acknowledgments
Conflicts of Interest
Abbreviations
| ATR | Atrazine |
| DA | Dopamine |
| HPLC-FL | high-pressure liquid chromatography with a fluorescence detector |
| TH | tyrosine hydroxylase |
| Nurr1 | orphan nuclear hormone |
| DAT | dopamine transporter |
| VMAT2 | vesicular monoaminetransporter 2 |
| PD | Parkinson’s disease |
| SN | substantia nigra |
| GD | pregnancy day |
| PND | postnatal day |
| Ct | cycle number |
| ADD | absorbed daily dose |
| L-DOPA | levodopa |
| AAAD | aromatic amino acid decarboxylase |
| DR | dopamine receptors |
| DAT | dopamine transporter |
| HVA | homovanillic acid |
| COMT | catechol-O-methyl transferase |
| MAO | monoamine oxidase |
| DOPAC | 3, 4-dihydroxyphenylacetic acid |
| ROS | reactive oxygen species |
References
- Tanner, C.M.; Chen, B.; Wang, W.; Peng, M.; Liu, Z.; Liang, X.; Kao, L.C.; Gilley, D.W.; Goetz, C.G.; Schoenberg, B.S. Environmental factors and Parkinson’s disease: A case-control study in China. Neurology 1989, 39, 660–664. [Google Scholar]
- Cory-Slechta, D.A.; Thiruchelvam, M.; Barlow, B.K.; Richfield, E.K. Developmental pesticide models of the Parkinson disease phenotype. Environ. Health Perspect 2005, 113, 1263–1270. [Google Scholar]
- Brown, T.P.; Rumsby, P.C.; Capleton, A.C.; Rushton, L.; Levy, L.S. Pesticides and Parkinson’s disease-is there a Link? Environ. Health Perspect 2006, 114, 156–164. [Google Scholar]
- McCormack, A.L.; Thiruchelvam, M.; Manning-Bog, A.B.; Thiffault, C.; Langston, J.W.; Cory-Slechta, D.A.; di Monte, D.A. Environmental risk factors and Parkinson’s disease: Selective degeneration of nigral dopaminergic neurons caused by the herbicide paraquat. Neurobiol. Dis 2002, 10, 119–127. [Google Scholar]
- Drechsel, D.A.; Patel, M. Role of reactive oxygen species in the neurotoxicity of environmental agents implicated in Parkinson’s disease. Free Radic. Biol. Med 2008, 44, 1873–1886. [Google Scholar]
- Gao, H.M.; Hong, J.S.; Zhang, W.; Liu, B. Synergistic dopaminergic neurotoxicity of the pesticide rotenone and inflammogen lipopolysaccharide: Relevance to the etiology of Parkinson’s disease. J. Neurosci 2003, 23, 1228–1236. [Google Scholar]
- Hines, C.J.; Deddens, J.A.; Lu, C.; Fenske, R.; Striley, C.A. Mixed-effect models for evaluating multiple measures of atrazine exposure among custom applicators. J. Occup. Environ Hyg 2006, 3, 274–283. [Google Scholar]
- Cooper, R.L.; Stoker, T.E.; Tyrey, L.; Goldman, J.M.; McElroy, W.K. Atrazine disrupts the hypothalamic control of pituitary-ovarian function. Toxicol. Sci 2000, 53, 297–307. [Google Scholar]
- Narotsky, M.G.; Best, D.S.; Guidici, D.L.; Cooper, R.L. Strain comparisons of atrazine-induced pregnancy loss in the rat. Reprod. Toxicol 2001, 15, 61–69. [Google Scholar]
- Stoker, T.E.; Guidici, D.L.; Laws, S.C.; Cooper, R.L. The effects of atrazine metabolites on puberty and thyroid function in the male Wistar rat. Toxicol. Sci 2002, 67, 198–206. [Google Scholar]
- Rodriguez, V.M.; Thiruchelvam, M.; Cory-Slechta, D.A. Sustained exposure to the widely used herbicide atrazine: Altered function and loss of neurons in brain monoamine systems. Environ. Health Perspect 2005, 113, 708–715. [Google Scholar]
- Coban, A.; Filipov, N.M. Dopaminergic toxicity associated with oral exposure to the herbicide atrazine in juvenile male C57BL/6 mice. J. Neurochem 2007, 100, 1177–1187. [Google Scholar]
- Rosenberg, B.G.; Chen, H.; Folmer, J.; Liu, J.; Papadopoulos, V.; Zirkin, B.R. Gestational exposure to atrazine: Effects on the postnatal development of male offspring. J. Androl 2008, 29, 304–311. [Google Scholar]
- Richardson, J.R.; Caudle, W.M.; Wang, M.Z.; Dean, E.D.; Pennell, K.D.; Miller, G.W. Developmental heptachlor exposure increases susceptibility of dopamine neurons to N-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP) in a gender-specific manner. Neurotoxicology 2008, 29, 855–863. [Google Scholar]
- Tanner, C.M.; Ottman, R.; Goldman, S.M.; Ellenberg, J.; Chan, P.; Mayeux, R.; Langston, J.W. Parkinson disease in twins: An etiologic study. JAMA 1999, 281, 341–346. [Google Scholar]
- Federoff, H.J. Nur(R1) turing a notion on the etiopathogenesis of Parkinson’s disease. Neurotox. Res 2009, 16, 261–270. [Google Scholar]
- Katunar, M.R.; Saez, T.; Brusco, A.; Antonelli, M.C. Immunocytochemical expression of dopamine-related transcription factors Pitx3 and Nurr1 in prenatally stressed adult rats. J. Neurosci. Res 2009, 87, 1014–1022. [Google Scholar]
- Decressac, M.; Kadkhodaei, B.; Mattsson, B.; Laguna, A.; Perlmann, T.; Björklund, A. Alpha-synuclein-induced down-regulation of nurr1 disrupts GDNF signaling in nigral dopamine neurons. Sci. Transl. Med 2012, 4, 163ra156. [Google Scholar]
- Short, P.; Colborn, T. Pesticide use in the U.S. and policy implications: A focus on herbicides. Toxicol. Ind. Health 1999, 15, 240–275. [Google Scholar]
- Gilliom, R.J.; Barbash, J.E.; Crawford, C.G.; Hamilton, P.A.; Martin, J.D.; Nakagaki, N.; Nowell, L.H.; Scott, J.C. Pesticides in the Nation’s Streams and Groundwater, 1992–2001; U.S. Geological Survey: Reston, VA, USA, 2006; pp. 2006–3028. [Google Scholar]
- Benotti, M.J.; Trenholm, R.A.; Vanderford, B.J.; Holady, J.C.; Stanford, B.D.; Snyder, S.A. Pharmaceuticals and endocrine disrupting compounds in U.S. drinking water. Environ. Sci. Technol 2009, 43, 597–603. [Google Scholar]
- Bossi, R.; Vejrup, K.V.; Mogensen, B.B.; Asman, W.A. Analysis of polar pesticides in rainwater in Denmark by liquid chromatography-tandem mass spectrometry. J. Chromatogr. A 2002, 957, 27–36. [Google Scholar]
- Brun, G.L.; MacDonald, R.M.; Verge, J.; Aubé, J. Long-term atmospheric deposition of current-use and banned pesticides in Atlantic Canada; 1980–2000. Chemosphere 2008, 71, 314–327. [Google Scholar]
- Jablonowski, N.D.; Schaffer, A.; Burauel, P. Still present after all these years: Persistence plus potential toxicity raise questions about the use of atrazine. Environ. Sci. Pollut. Res. Int 2011, 18, 328–331. [Google Scholar]
- Ross, M.K.; Jones, T.L.; Filipov, N.M. Disposition of the herbicide 2-chloro-4-(ethylamino)-6-(isopropylamino)-s-triazine (Atrazine) and its major metabolites in mice: A liquid chromatography/mass spectrometry analysis of urine, plasma, and tissue levels. Drug Metab. Dispos 2009, 37, 776–786. [Google Scholar]
- US Environmental Protection Agency (EPA), Atrazine, Simazine, and Cyanazine: Notice of Initiation of Special Review; EPA: Washington, DC, USA, 1994.
- Gammon, D.W.; Aldous, C.N.; Carr, W.C., Jr.; Sanborn, J.R.; Pfeifer, K.F. A risk assessment of atrazine use in California: Human health and ecological aspects. Pest Manag. Sci 2005, 61, 331–355. [Google Scholar]
- Curwin, B.D.; Hein, M.J.; Sanderson, W.T.; Striley, C.; Heederik, D.; Kromhout, H.; Reynolds, S.J.; Alavanja, M.C. Urinary pesticide concentrations among children, mothers and fathers living in farm and non-farm households in iowa. Ann. Occup. Hyg 2007, 51, 53–65. [Google Scholar]
- Rayner, J.L.; Enoch, R.R.; Wolf, D.C.; Fenton, S.E. Atrazine-induced reproductive tract alterations after transplacental and/or lactational exposure in male Long-Evans rats. Toxicol. Appl. Pharmacol 2007, 218, 238–248. [Google Scholar]
- US Environmental Protection Agency (EPA), Interim Registration Eligibility Decision for Atrazine; EPA: Washington, DC, USA, 2003.
- Bayer, S.A.; Wills, K.V.; Triarhou, L.C.; Verina, T.; Thomas, J.D.; Ghetti, B. Selective vulnerability of late-generated dopaminergic neurons of the substantia nigra in weaver mutant mice. Proc. Natl. Acad. Sci. USA 1995, 92, 9137–9140. [Google Scholar]
- Van den Munckhof, P.; Luk, K.C.; Ste-Marie, L.; Montgomery, J.; Blanchet, P.J.; Sadikot, A.F.; Drouin, J. Pitx3 is required for motor activity and for survival of a subset of midbrain dopaminergic neurons. Development 2003, 130, 2535–2542. [Google Scholar]
- Rayner, J.L.; Wood, C.; Fenton, S.E. Exposure parameters necessary for delayed puberty and mammary gland development in Long-Evans rats exposed in utero to atrazine. Toxicol. Appl. Pharmacol 2004, 195, 23–34. [Google Scholar]
- Stoker, T.E.; Robinette, C.L.; Cooper, R.L. Maternal exposure to atrazine during lactation suppresses suckling-induced prolactin release and results in prostatitis in the adult offspring. Toxicol. Sci 1999, 52, 68–79. [Google Scholar]
- Cooper, R.L.; Laws, S.C.; Das, P.C.; Narotsky, M.G.; Goldman, J.M.; Lee Tyrey, E.; Stoker, T.E. Atrazine and reproductive function: Mode and mechanism of action studies. Birth Defects Res. B Dev. Reprod. Toxicol 2007, 80, 98–112. [Google Scholar]
- Heindel, J.J. Role of exposure to environmental chemicals in the developmental basis of disease and dysfunction. Reprod. Toxicol 2007, 23, 257–259. [Google Scholar]
- Barker, D.J.; Osmond, C.; Law, C.M. The intrauterine and early postnatal origins of cardiovascular disease and chronic bronchitis. J. Epidemiol. Community Health 1989, 43, 237–240. [Google Scholar]
- Das, P.C.; McElroy, W.K.; Cooper, R.L. Differential modulation of catecholamines by chlorotriazine herbicides in pheochromocytoma (PC12) cells in vitro. Toxicol. Sci. 2000, 56, 324–331. [Google Scholar]
- Das, P.C.; McElroy, W.K.; Cooper, R.L. Potential mechanisms responsible for chlorotriazine-induced alterations in catecholamines in pheochromocytoma (PC12) cells. Life Sci 2003, 73, 3123–3138. [Google Scholar]
- Filipov, N.M.; Stewart, M.A.; Carr, R.L.; Sistrunk, S.C. Dopaminergic toxicity of the herbicide atrazine in rat striatal slices. Toxicology 2007, 232, 68–78. [Google Scholar]
- Cooper, J.R.; Bloom, F.E.; Roth, R.H. The Biochemical Basis of Neuropharmacology; Oxford University Press: New York NY, USA, 2003. [Google Scholar]
- Caudle, W.M.; Richardson, J.R.; Wang, M.; Miller, G.W. Perinatal heptachlor exposure increases expression of presynaptic dopaminergic markers in mouse striatum. Neurotoxicology 2005, 26, 721–728. [Google Scholar]
- Hermanson, E.; Joseph, B.; Castro, D.; Lindqvist, E.; Aarnisalo, P.; Wallen, A.; Benoit, G.; Hengerer, B.; Olson, L.; Perlmann, T. Nurr1 regulates dopamine synthesis and storage in MN9D dopamine cells. Exp. Cell Res 2003, 288, 324–334. [Google Scholar]
- Smits, S.M.; Ponnio, T.; Conneely, O.M.; Burbach, J.P.; Smidt, M.P. Involvement of Nurr1 in specifying the neurotransmitter identity of ventral midbrain dopaminergic neurons. Eur. J. Neurosci 2003, 18, 1731–1738. [Google Scholar]
- LaVoie, M.J.; Hastings, T.G. Dopamine quinone formation and protein modification associated with the striatal neurotoxicity of methamphetamine: Evidence against a role for extracellular dopamine. J. Neurosci 1999, 19, 1484–1491. [Google Scholar]
- Miller, G.W.; Gainetdinov, R.R.; Levey, A.I.; Caron, M.G. Dopamine transporters and neuronal injury. Trends Pharmacol. Sci 1999, 20, 424–429. [Google Scholar]
- Chiueh, C.C.; Andoh, T.; Lai, A.R.; Lai, E.; Krishna, G. Neuroprotective strategies in Parkinson’s disease: Protection against progressive nigral damage induced by free radicals. Neurotox. Res 2000, 2, 293–310. [Google Scholar]
- Berman, S.B.; Hastings, T.G. Dopamine oxidation alters mitochondrial respiration and induces permeability transition in brain mitochondria: Implications for Parkinson’s disease. J. Neurochem 1999, 73, 1127–1137. [Google Scholar]


| Genes | Primers | Length (bp) | Annealing temperature (°C) |
|---|---|---|---|
| Nurr1 | F: 5′-TGATGATCTCCATAGAGCCAGTCAG-3′ R: 5′-CCAATCCGGCAATGACCAG-3′ | 129 | 57.4 |
| TH | F: 5′-AGCTGTGCAGCCCTACCAAGA-3′ R: 5′-GTGTGTACGGGTCAAACTTCACAGA-3′ | 140 | 57.4 |
| DAT | F: 5′-GTACTGGCGGCTATGCTGGAA-3′ R: 5′-GGGTCTGAAGGTCACAATGCTG-3′ | 82 | 57.4 |
| VAMT | F: 5′-CCTTCGAAGTCCACCTGCTAA-3′ R: 5′-CATCACCGATGGGATATGACTG-3′ | 116 | 57.4 |
| β-actin | F: 5′-GGAAATCGTGCGTGACATTAAAG-3′ R: 5′-CGGCAGTGGCCATCTCTT-3′ | 85 | 57.4 |
© 2014 by the authors; licensee MDPI, Basel, Switzerland This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Sun, Y.; Li, Y.-S.; Yang, J.-W.; Yu, J.; Wu, Y.-P.; Li, B.-X. Exposure to Atrazine during Gestation and Lactation Periods: Toxicity Effects on Dopaminergic Neurons in Offspring by Downregulation of Nurr1 and VMAT2. Int. J. Mol. Sci. 2014, 15, 2811-2825. https://doi.org/10.3390/ijms15022811
Sun Y, Li Y-S, Yang J-W, Yu J, Wu Y-P, Li B-X. Exposure to Atrazine during Gestation and Lactation Periods: Toxicity Effects on Dopaminergic Neurons in Offspring by Downregulation of Nurr1 and VMAT2. International Journal of Molecular Sciences. 2014; 15(2):2811-2825. https://doi.org/10.3390/ijms15022811
Chicago/Turabian StyleSun, Yan, Yan-Shu Li, Jun-Wei Yang, Jia Yu, Yan-Ping Wu, and Bai-Xiang Li. 2014. "Exposure to Atrazine during Gestation and Lactation Periods: Toxicity Effects on Dopaminergic Neurons in Offspring by Downregulation of Nurr1 and VMAT2" International Journal of Molecular Sciences 15, no. 2: 2811-2825. https://doi.org/10.3390/ijms15022811
APA StyleSun, Y., Li, Y.-S., Yang, J.-W., Yu, J., Wu, Y.-P., & Li, B.-X. (2014). Exposure to Atrazine during Gestation and Lactation Periods: Toxicity Effects on Dopaminergic Neurons in Offspring by Downregulation of Nurr1 and VMAT2. International Journal of Molecular Sciences, 15(2), 2811-2825. https://doi.org/10.3390/ijms15022811




