β-Conglycinin Reduces the Tight Junction Occludin and ZO-1 Expression in IPEC-J2
Abstract
:1. Introduction
2. Results
2.1. TEER
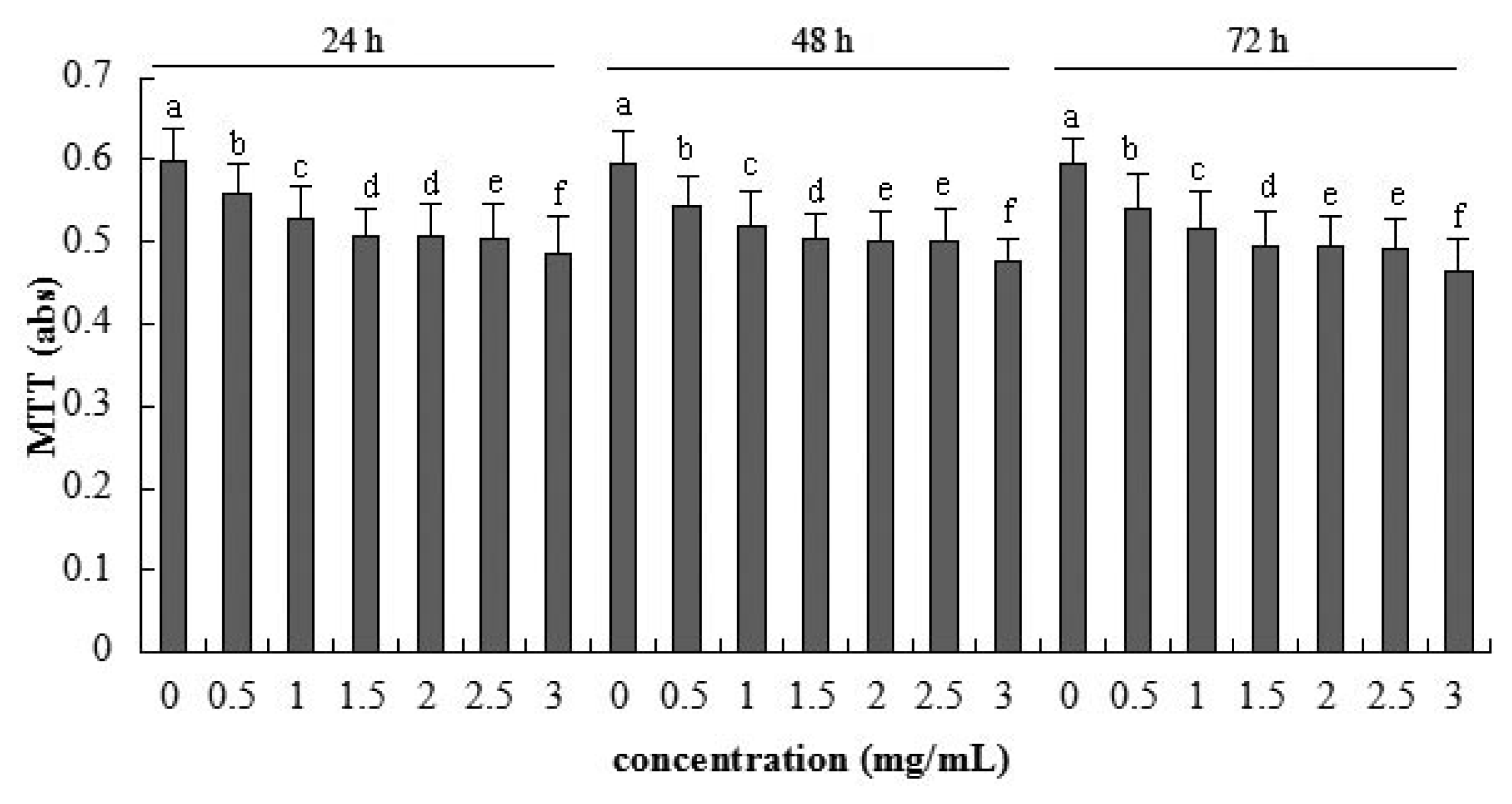
2.2. Cellular Metabolic Activity Detected by MTT Assay
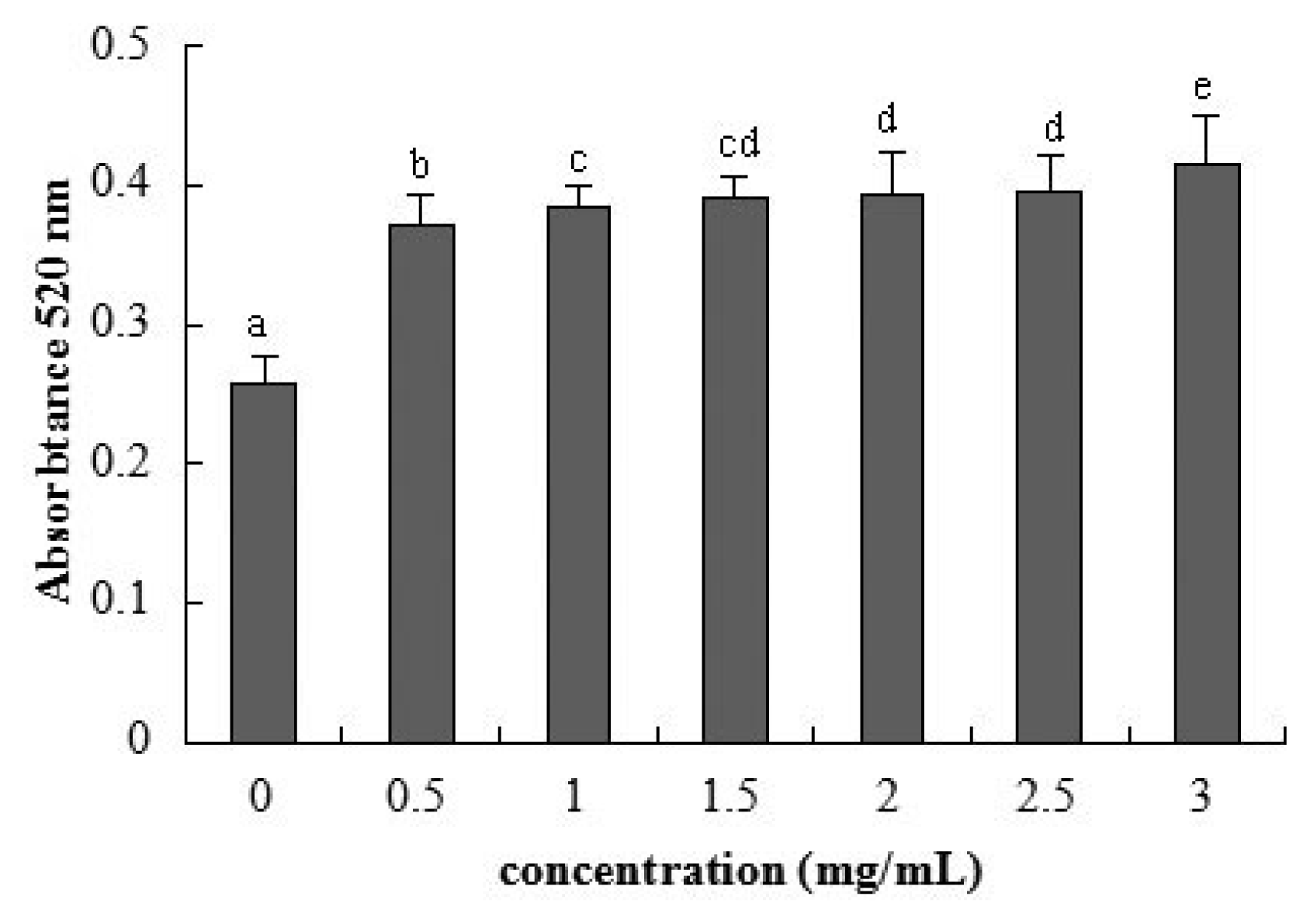
2.3. Cellular Integrity Assessed by AP Activity
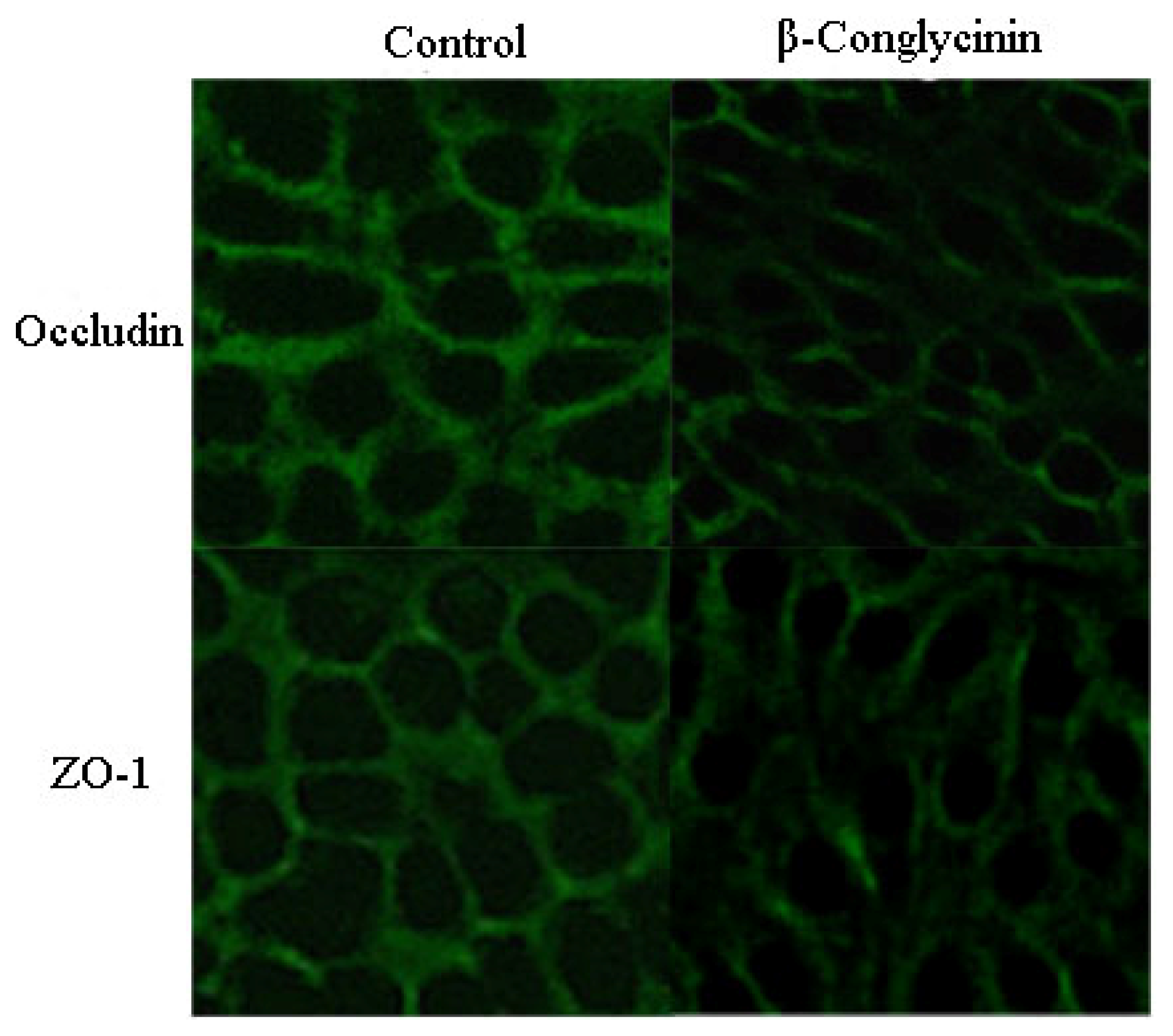
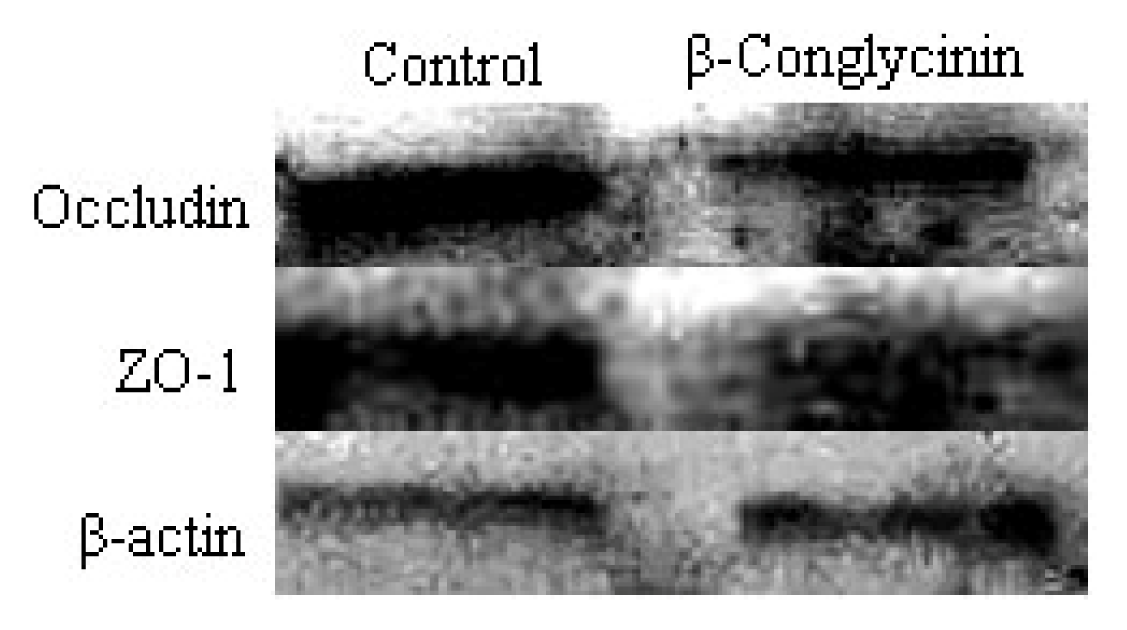
2.4. Tight Junction Distribution and Expression
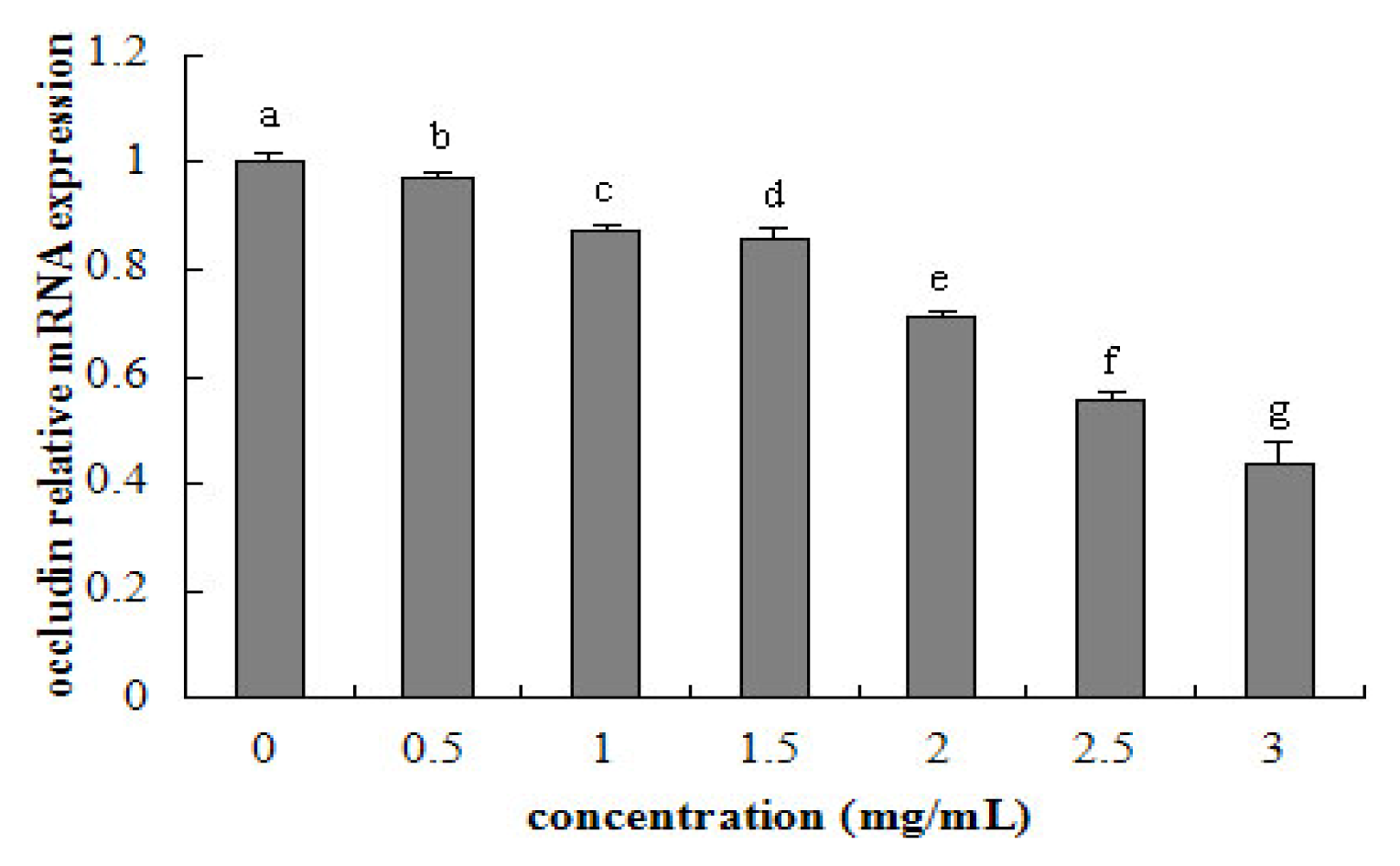
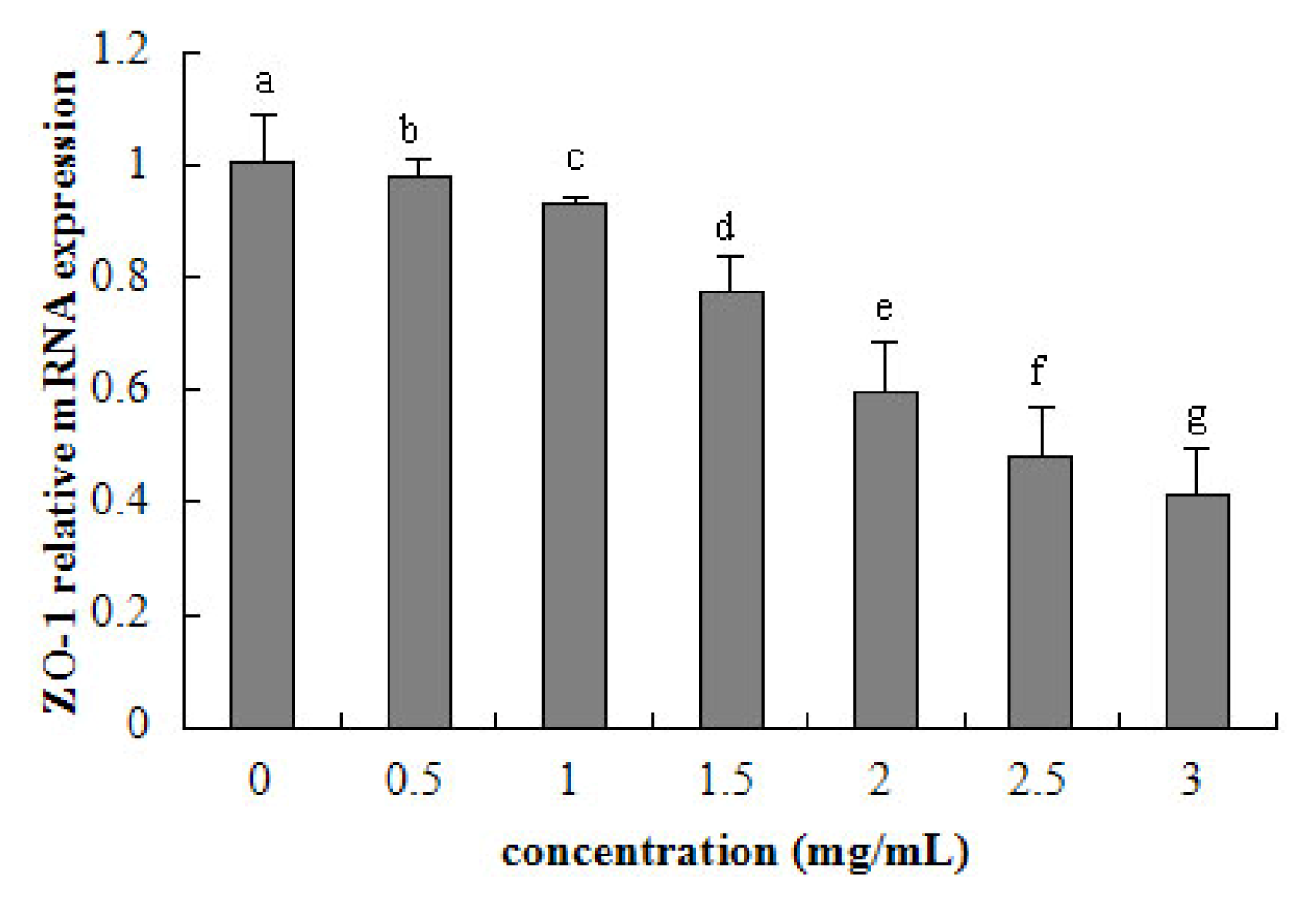
2.5. Tight Junction mRNA Expression
3. Discussion
4. Methods and Materials
4.1. Preparation of β-Conglycinin
4.2. Cells and Culture Conditions
4.3. Trans-Epithelial Electrical Resistance (TEER) Measurement
4.4. MTT Assay
4.5. Alkaline Phosphatase Activity Assay
4.6. Analysis of Tight Junction Proteins Structure by Immunofluorescence
4.7. Western Blot Analysis
4.8. RNA Extraction
4.9. Reverse Transcription
4.10. Real-Time Quantitative PCR Analysis
4.11. Statistical Analysis
5. Conclusion
Acknowledgments
Conflicts of Interest
References
- Friedman, M.; Brandon, D.L. Nutritional and health benefits of soy proteins. J. Agric. Food Chem 2001, 49, 1069–1086. [Google Scholar]
- Ogawa, T.; Bando, N.; Tsuji, H.; Nishikawa, K.; Kitamura, K. Alpha-subunit of β-conglycinin, an allergenic protein recognized by IgE antibodies of soybean-sensitive patients with atopic-dermatitis. Biosci. Biotechnol. Biochem 1995, 59, 831–833. [Google Scholar]
- Krishnan, H.B.; Kim, W.S.; Jang, S.; Kerley, M.S. All three subunits of soybean β-conglycinin are potential food allergens. J. Agric. Food Chem 2009, 57, 938–943. [Google Scholar]
- Hao, Y.; Zhan, Z.F.; Guo, P.F.; Piao, X.S.; Li, D.F. Soybean β-conglycinin-induced gut hypersensitivity reaction in a piglet model. Arch. Anim. Nutr 2009, 63, 188–202. [Google Scholar]
- Mariani, V.; Palermo, S.; Fiorentini, S.; Lanubile, A.; Giuffra, E. Gene expression study of two widely used pig intestinal epithelial cell lines: IPEC-J2 and IPI-2I. Vet. Immunol. Immunopathol 2009, 131, 278–284. [Google Scholar]
- Pitman, R.S.; Blumberg, R.S. First line of defense: The role of the intestinal epithelium as an active component of the mucosal immune system. J. Gastroenterol 2000, 35, 805–814. [Google Scholar]
- Zheng, S.G. The Identification of Potential Allergenic Subunits and Epitope-containing Peptide Fragments in Soybean Protein Glycinin and β-Conglycinin. Ph.D. Thesis, Jilin Agricultural University, Changchun, China, 2009. [Google Scholar]
- Adachi, A.; Horikawa, T.; Shimizu, H.; Sarayama, Y.; Ogawa, T.; Sjolander, S.; Tanaka, A.; Moriyama, T. Soybean β-conglycinin as the main allergen in a patient with food-dependent exercise-induced anaphylaxis by tofu: Food processing alters pepsin resistance. Clin. Exp. Allergy 2009, 39, 167–173. [Google Scholar]
- Lallès, J.P.; Tukur, H.M.; Salgado, P.; Mills, C.E.N.; Morgan, M.R.A.; Quillien, L.; Levieux, D.; Toullec, R. Immunochemical studies on gastric and intestinal digestion of soybean glycinin and β-conglycinin in vivo. J. Agric. Food Chem. 1999, 47, 2797–2806. [Google Scholar]
- Wang, T.; Qin, G.X.; Zhao, Y.; Sun, Z.W. Comparative study on the stability of soybean (Glycine max) β-conglycinin in vivo. Food Agric. Immunol. 2009, 20, 295–304. [Google Scholar]
- Zhao, Y.; Qin, G.X.; Sun, Z.W.; Zhang, B.; Wang, T. Stability and immunoreactivity of glycinin and β-conglycinin to hydrolysis in vitro. Food Agric. Immunol. 2010b, 21, 253–263. [Google Scholar]
- Astwood, J.D.; Leach, J.N.; Fuchs, R.L. Stability of food allergens to digestion in vitro. Nat. Biotechnol 1996, 14, 1269–1273. [Google Scholar]
- Moreno, F.J.; Clemente, A. 2S albumin storage proteins: What makes them food allergens? Open Biochem. J 2008, 2, 16–28. [Google Scholar]
- Dunsford, B.R.; Knabe, D.A.; Hanesly, W.E. Effect of dietary soybean meal on the microscopic anatomy of the small intestine in the early weaned pigs. J. Anim. Sci 1989, 67, 1855–1863. [Google Scholar]
- Li, D.; Nelssen, J.L.; Reddy, P.G.; Belcha, F.; Hancock, J.D.; Allee, G.L.; Goodband, R.D. Transient hypersensitivity to soybean meal in the early-weaned pig. J. Anim. Sci 1990, 68, 1790–1799. [Google Scholar]
- Qin, G.X.; Xu, L.M.; Jiang, H.L.; van der Poel, A.F.B.; Bosch, M.W.; Verstegen, M.W.A. The effect of Chinese and Argentine soybeans on nutrient digestibility and organ morphology in Landrace and Chinese Min pigs. Asian Aust. J. Anim. Sci 2002, 15, 555–564. [Google Scholar]
- Qiao, S.; Li, D.; Jiang, J.; Zhou, H.; Li, J.; Thacker, P. Effects of moist extruded full-fat soybeans on Gut morphology and mucosal cell turnover time of weanling pigs. Asian Aust. J. Anim. Sci 2003, 16, 63–69. [Google Scholar]
- Zhao, Y.; Qin, G.X.; Sun, Z.W.; Zhang, B.; Wang, T. Effects of glycinin and β-conglycinin on enterocyte apoptosis, proliferation and migration of piglets. Food Agric. Immunol 2010, 21, 209–218. [Google Scholar]
- Miller, E.R.; Ullrey, D.E. The pig as a model for human nutrition. Annu. Rev. Nutr 1987, 7, 361–382. [Google Scholar]
- Khatri, M.; Dwivedi, V.; Krakowka, S.; Manickam, C.; Ali, A.; Wang, L.; Qin, Z.M.; Renukaradhya, G.J.; Lee, C.W. Swine influenza H1N1 virus induces acute inflammatory Immune responses in pig lungs: A potential animal model for human H1N1 influenza virus. J. Virol 2010, 84, 11210–11218. [Google Scholar]
- Hinnebusch, B.F.; Siddique, W.; Henderson, W.; Malo, M.S.; Zhang, W.; Athaide, C.P.; Abedrapo, M.A.; Chen, X.; Yang, V.W.; Hodin, R.A. Enterocyte differentiation marker intestinal alkaline phosphatase is a target gene of the gut-enriched Krüppel-like factor. Am. J. Physiol. Gastrointest. Liver Physiol 2004, 286, G23–G30. [Google Scholar]
- Sampson, H.A. Allergy clinical immunology disorders. J. Allergy Clin. Immunol 1999, 103, 717–728. [Google Scholar]
- Venter, C.; Pereira, B.; Voigt, K.; Grundy, J.; Clayton, C.B.; Higgins, B.; Arshad, S.H.; Dean, T. Prevalence and cumulative incidence of food hypersensitivity in the first 3 years of life. Allergy 2008, 6, 354–359. [Google Scholar]
- Tanabe, S. Epitope peptides and immunotherapy. Curr. Protein Pept. Sci 2007, 8, 109–118. [Google Scholar]
- Heyman, M.; Ducroc, R.; Desjeux, J.F.; Morgat, J.L. Horseradish peroxidase transport across adult rabbit jejunum in vitro. Am. J. Physiol. 1982, 242, 558–564. [Google Scholar]
- Fujita, M.; Reinhart, F.; Neutra, M. Convergence of apical and basolateral endocytic pathways at apical late endosomes in absorptive cells of suckling rat ileum in vivo. J. Cell Sci. 1990, 97, 385–394. [Google Scholar]
- Laiping, S.A.; Pelton-Henrion, K.; Small, G.; Becker, K.; Oei, E.; Tyorkin, M.; Sperber, K.; Mayer, L. Antigen uptake and trafficking in human intestinal epithelial cells. Dig. Dis. Sci 2000, 45, 1451–1461. [Google Scholar]
- Yu, L.C.H. The epithelial gatekeeper against food allergy. Pediatr. Neonatol 2009, 50, 247–254. [Google Scholar]
- Berin, M.C.; Kiliaan, A.J.; Yang, P.C.; Groot, J.A.; Kitamura, Y.; Perdue, M.H. The influence of mast cells on pathways of transepithelial antigen transport in rat intestine. J. Immunol 1998, 161, 2561–2566. [Google Scholar]
- Yu, L.C.H.; Yang, P.C.; Berin, M.C.; Di Leo, V.; Conrad, D.H.; McKay, D.M.; Satoskar, A.R.; Perdue, M.H. Enhanced transepithelial antigen transport in intestine of allergic mice is mediated by IgE/CD23 and regulated by interleukin-4. Gastroenterology 2001, 121, 370–381. [Google Scholar]
- André, C.; André, F.; Colin, N.; Cavagna, S. Measurement of intestinal permeability to mannitol and lactulose as a means of diagnosing food allergy and evaluating therapeutic effectiveness of sodium cromoglycate. Ann. Allergy 1987, 59, 127–130. [Google Scholar]
- Ventura, M.T.; Polimeno, L.; Amoruso, A.C. Intestinal permeability in patients with adverse reactions to food. Dig. Liver Dis 2006, 38, 732–736. [Google Scholar]
- Song, D.J.; Cho, J.Y.; Miller, M. Anti-Siglec-F antibody inhibits oral egg allergen induced intestinal eosinophilic inflammation in a mouse model. Clin. Immunol 2009, 131, 157–169. [Google Scholar]
- Perrier, C.; Corthésy, B. Gut permeability and food allergies. Clin. Exp. Allergy 2011, 41, 20–28. [Google Scholar]
- Hidalgo, I.J.; Raub, T.J.; Borchardt, R.T. Characterization of the human colon carcinoma cell line (Caco-2) as a model system for intestinal epithelial permeability. Gastroenterology 1989, 96, 736–749. [Google Scholar]
- Wilson, G.; Hassan, I.F.; Dix, C.J.; Williamson, R.; Shah, R.; Mackey, M.; Artursson, P. Transport and permeability properties of human Caco-2 cells: An in vitro model of the intestinal epithelial cell barrier. J. Control Release 1990, 11, 25–40. [Google Scholar]
- Pellegrina, C.D.; Rizzi, C.; Mosconi, S.; Zoccatelli, G.; Peruffo, A.; Chignola, R. Plant lectin as carriers for oral drugs: Is wheat germ agglutinin a suitable candidate? Toxicol. Appl. Pharm 2005, 207, 170–178. [Google Scholar]
- Xu, J.; Zhou, A.; Wang, Z.; Ai, D. Effects of glycinin and β-conglycinin on integrity and immune responses of mouse intestinal epithelial cells. J. Anim. Plant Sci 2010, 20, 170–174. [Google Scholar]
- Kenworthy, R. Observations on the effects of weaning in the young pigs: Clinical and histopathological studies of intestinal function and morphology. Res. Vet. Sci 1976, 21, 69–75. [Google Scholar]
- Bates, J.M.; Akerlund, J.; Mittge, E.; Guillemin, K. Intestinal alkaline phosphatase detoxifies lipopolysaccharide and prevents inflammation in zebrafish in response to the gut microbiota. Cell Host Microbe 2007, 2, 371–382. [Google Scholar]
- Geddes, K.; Philpott, D.J. A new role for intestinal alkaline phosphatase in gut barrier maintenance. Gastroenterology 2008, 135, 8–12. [Google Scholar]
- Yosbida, Y.; Ban, Y.; Kinosbita, S. Tight junction transmembrane protein claudin subtype expression and distribution in human corneal and conjunctival epithelium. Investig. Ophthalmol. Vis. Sci 2009, 50, 2103–2108. [Google Scholar]
- Yu, A.S.; McCarthy, K.M.; Francis, S.A.; McCormack, J.M.; Lai, J.; Rogers, R.A.; Lynch, R.D.; Schneeberger, E.E. Knockdown of occludin expression leads to diverse phenotypic alterations in epithelial cells. Am. J. Physiol. Cell Physiol 2005, 288, C1231–C1241. [Google Scholar]
- Zhao, J.H.; Wang, J.H.; Dong, L.; Shi, H.; Wang, Z.; Ding, H.; Shi, H.; Lu, X. A protease inhibitor against acute stress-induced visceral hypersensitivity and paracellular permeability in rats. Eur. J. Pharmacol 2011, 654, 289–294. [Google Scholar]
- Groschwitz, K.R.; Hogan, S.P. Intestinal barrier function: Molecular regulation and disease pathogenesis. J. Allergy Clin. Immunol 2009, 124, 3–20. [Google Scholar]
- Setsuko, I.; Fumio, Y. Determination of glycinin and β-conglycinin in soybean protein by immunological methods. Agric. Food Chem 1987, 35, 200–205. [Google Scholar]

© 2014 by the authors; licensee MDPI, Basel, Switzerland This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Zhao, Y.; Qin, G.; Han, R.; Wang, J.; Zhang, X.; Liu, D. β-Conglycinin Reduces the Tight Junction Occludin and ZO-1 Expression in IPEC-J2. Int. J. Mol. Sci. 2014, 15, 1915-1926. https://doi.org/10.3390/ijms15021915
Zhao Y, Qin G, Han R, Wang J, Zhang X, Liu D. β-Conglycinin Reduces the Tight Junction Occludin and ZO-1 Expression in IPEC-J2. International Journal of Molecular Sciences. 2014; 15(2):1915-1926. https://doi.org/10.3390/ijms15021915
Chicago/Turabian StyleZhao, Yuan, Guixin Qin, Rui Han, Jun Wang, Xiaodong Zhang, and Dandan Liu. 2014. "β-Conglycinin Reduces the Tight Junction Occludin and ZO-1 Expression in IPEC-J2" International Journal of Molecular Sciences 15, no. 2: 1915-1926. https://doi.org/10.3390/ijms15021915
APA StyleZhao, Y., Qin, G., Han, R., Wang, J., Zhang, X., & Liu, D. (2014). β-Conglycinin Reduces the Tight Junction Occludin and ZO-1 Expression in IPEC-J2. International Journal of Molecular Sciences, 15(2), 1915-1926. https://doi.org/10.3390/ijms15021915



