Abstract
The microcirculation is a portion of the vascular circulatory system that consists of resistance arteries, arterioles, capillaries and venules. It is the place where gases and nutrients are exchanged between blood and tissues. In addition the microcirculation is the major contributor to blood flow resistance and consequently to regulation of blood pressure. Therefore, structural remodeling of this section of the vascular tree has profound implications on cardiovascular pathophysiology. This review is focused on the role that reactive oxygen species (ROS) play on changing the structural characteristics of vessels within the microcirculation. Particular attention is given to the resistance arteries and the functional pathways that are affected by ROS in these vessels and subsequently induce vascular remodeling. The primary sources of ROS in the microcirculation are identified and the effects of ROS on other microcirculatory remodeling phenomena such as rarefaction and collateralization are briefly reviewed.
1. Introduction
Reactive oxygen species (ROS) such as superoxide anion and hydroxyl radical (OH−) are highly reactive chemical molecules that contain oxygen with unpaired electrons in their outer orbit. Within blood vessels, ROS are produced by all the cellular components of the vascular wall including endothelial cells, smooth muscle cells, fibroblasts and immune cells. Accumulating evidence indicates that ROS serve as signaling molecules that modulate acute vascular functions such as vasodilation, vasoconstriction and vascular permeability, as well as long-term vascular changes including structural remodeling of vessel segments and vascular beds [1,2,3,4]. ROS also play pivotal roles in the initiation and development of vascular dysfunctions associated with aging and diseases such as ischemic and chronic heart disease, stroke, hypertension, atherosclerosis, diabetes mellitus and chronic kidney disease [2,4,5,6,7]. An important function of ROS in the vasculature includes their capacity to modulate cellular signaling pathways that induce both acute and chronic changes in cellular phenotype. This particular function of ROS plays a key role in vascular remodeling processes through the modulation of cellular cytoskeletal properties, proliferation, migration and death, as well as, the alteration of the extracellular matrix (ECM) [1,8,9,10]. Collectively, these events are believed to be linked to changes in the mechanical characteristics of the vascular wall. This review will focus on the roles that ROS play in vascular remodeling with particular attention to the microcirculation.
The microcirculation is the most distal segment of the vascular system consisting of a network of arterioles, capillaries, and venules that are interposed between the arterial and venous systems. Its primary function is to regulate blood flow and capillary pressure that are crucial for optimizing the supply of nutrients and oxygen to tissues. In turn, these exchange processes sustain cellular metabolism and remove metabolic waste products. The flow of blood into organs is regulated by the microcirculation to meet the demands of the tissue. Within the microcirculation, the small arteries and arterioles account for the majority of the resistance to blood flow and these vessels are responsible for controlling approximately 80% of the drop in blood pressure that occurs from the large conduit arteries of the systemic circulation to the capillaries. Therefore, much emphasis has been placed on their functional and structural characteristics [11,12,13]. The resistance vessels, located proximal to capillaries, control the inflow of blood into tissues through changes in vascular diameter. As such, at any given time the diameter of a resistance vessel results from the integration of global systemic signals and local modulatory factors. Ultimately, there is a dynamic balance of vasodilatory and constrictive effects that optimize tissue perfusion, while simultaneously contributing to the regulation of systemic arterial pressure. In the microcirculation, circulating hormones, locally-derived cytokines, as well as neuronal and physical factors contribute to the level of vascular tone expressed by the resistance vessels and hence account for acute regulation of diameter.
Over the long term, resistance vessels also display adaptive properties that influence their diameter and responses to vasoactive factors. Often these longer-term adaptive changes result in the structural reorganization of the vascular wall that involves changes in both the cellular and ECM components of the vessel wall and constitutes vascular remodeling [13]. The remodeling process is achieved through a continuum of changes involving mechanisms such as cytoskeletal remodeling, cell migration, cell growth, cell proliferation, apoptosis, and ECM modifications including protein deposition, crosslinking and degradation. Each of these mechanisms is sensitive to and influenced by the oxidative environment. Changes in the oxidative environment are often associated with impairment of local blood flow and also with pathological conditions such as hypertension and diabetes.
Vascular remodeling encompasses a wide variety of structural changes occurring at all levels of the vascular tree, from large vessels down to the microcirculation. Examples include large vessel stiffening, atherogenesis, aneurisms, resistance vessel wall thickening, rarefaction or loss of small vessels and capillaries, as well as vasculogenesis and angiogenesis. In this review, our primary focus will be to provide an overview of the relationship between ROS production and the different mechanisms of remodeling processes that occur at the level of the microcirculation. First, we will discuss some of the most prominent enzymatic systems involved in the production of ROS in the vasculature. Then we will discuss the mechanisms of remodeling found primarily in resistance arteries and arterioles, and how they are affected by ROS. Where needed, we will draw upon information obtained from large vessel studies and cell-based in vitro experiments that provide insight on the mechanisms that modulate microvascular structure. Vascular remodeling of the resistance arteries will be described using the definitions introduced by Mulvany et al. [14]. Consequently, vascular remodeling will be classified based on the changes observed in the passive luminal diameter of vessels and the cross-sectional area of the vascular wall. The remodeling is inward or outward depending on whether the passive luminal diameter decreases or increases, and hypotrophic, eutrophic or hypertrophic if the amount of wall material decreases, remains constant or increases, respectively. We will also briefly cover the role of ROS in rarefaction of the capillary bed and angiogenesis.
2. The Remodeling Process
Vascular remodeling processes involve intracellular and extracellular modifications that change the functional relationship between cells, and between cell and the ECM components of the vessel wall. This results in alterations in vascular function and performance [13,15]. Vascular remodeling may be induced by an array of factors, such as mechanical and hemodynamic forces, as well as neurohumoral or paracrine agents. In addition, multiple pathological conditions are associated with remodeling of the microcirculation, of which hypertension has received considerable attention due to its high incidence and contribution to cardiovascular mortality [16]. Several studies indicate that inward eutrophic and hypertrophic remodeling are the two major remodeling types encountered in the resistance vessels of humans and animal models of hypertension [17,18,19,20,21,22,23,24,25,26]. Inward eutrophic remodeling is believed to occur as vessel wall components rearrange around a smaller luminal diameter without synthesis of new materials or degradation of existing constituents [13]. Hypertrophic remodeling involves an increase in cross-sectional area of the vascular wall that includes cell proliferation and alterations in the amount, composition and arrangement of ECM components such as elastin, fibronectin and collagen [27]. Inward eutrophic remodeling is the most common structural change encountered in the resistance vessels of patients with essential hypertension, as well as in those of spontaneously hypertensive rats (SHRs) [28,29,30]. In secondary forms of hypertension, resistance artery and arteriolar remodeling is inward hypertrophic [27,31,32]. This hypertrophic remodeling has also been observed in hypertensive patients with type 2 diabetes [33,34], and in patients with acromegalia [25] and Cushing syndrome [24]. This suggests that the mechanisms contributing to remodeling can be differently modulated depending on background factors associated with the pathological conditions.
In contrast to hypertension, where inward remodeling of resistance arteries is most prevalent, outward remodeling occurs in vessels with chronically increased blood flow [35]. Flow-induced outward remodeling is believed to be an important physiological adaptation to increased metabolic demands, such as those encountered during exercise training or pregnancy [36,37,38,39]. The main trigger for vessels to remodel outwardly is an increased level of shear stress associated with the augmented flow of blood. During the prolonged increase in shear stress the endothelium releases factors that induce vasodilation and are believed to initiate a structural increase in vascular diameter accompanied by hypertrophy of the media. This outward hypertrophic remodeling results in normalization of shear stress and wall circumferential stress.
Mechanistically, the explanation for the inward or outward remodeling processes observed in resistance vessels may reside in the balance of stimuli that support vasodilation or vasoconstriction. It has been suggested that the intrinsic ability of resistance vessels to effectively control their diameter through the myogenic response plays a role in directing the overall characteristics of the remodeling process [30,40]. The myogenic response is defined as the ability of resistance vessels to constrict or relax in response to increases or decreases in intravascular pressure, respectively [41]. This local pressure-dependent mechanism is believed to help resistance arteries normalize circumferential stress, and during prolonged vasoconstriction to allow for the rearrangement of the cellular and extracellular components of the vascular wall leading to inward eutrophic remodeling [13,42]. When the myogenic response is not sufficient to normalize circumferential stress, the vessel undergoes hypertrophy as a compensatory mechanism [42]. The ultimate purpose of these adaptive changes in the structural diameter of resistance vessel is thought to play a role in protecting the fragile capillaries from pressure overload and ultimately prevent end-organ damage due to changes in perfusion and pressure [17]. The above postulates are supported by a growing body of evidence suggesting that the structure of resistance vessels is plastic and dynamic, with all components of the vessel wall, cellular and extracellular, continually changing to regulate the structural state of the vessel in order to maintain optimal conditions for vasodilation and vasoconstriction [13,15,43,44]. While all determinants of the remodeling process are not yet fully elucidated, current evidence suggests that alterations in intracellular cytoskeletal structure, cell attachment, cell migration, growth and apoptosis as well as ECM reorganization, synthesis and degradation occur to varying degrees to support the transition from prolonged vasodilation or vasoconstriction to the remodeled vascular state [13].
3. Sources of Reactive Oxygen Species in the Microcirculation
3.1. NADPH Oxidases
The nicotinamide-adenine-dinucleotide phosphate (NADPH) oxidases (Noxs) are membrane bound enzymes that transfer electrons from NADPH to molecular oxygen, thus generating NADP+, superoxide and other downstream ROS [45]. There are seven members of the Nox family of Noxs: Nox1, Nox3, Nox4, Nox5, Duox1, Duox2 and the prototypic NADPH oxidase Nox2 (gp91phox). The distribution of the different Nox family members is quite heterogeneous across different tissues. Nox1 is found in colon, prostate, uterus and vascular cells, and its function(s) is mainly related to cell growth [46]. Nox2 is the phagocytic Nox and is widely distributed across tissues [45]. In contrast, Nox3 is found almost exclusively in the inner ear [47]. Nox4 is a widely distributed enzyme abundant in kidney, pancreas, placenta, ovary, testis, skeletal muscle and vascular cells [48,49]. It is for the most part constitutively expressed, but its precise physiological function is not well known. Nox5 is a calcium-dependent enzyme, not found in mice and rats, that is primarily expressed in lymphoid tissues and testis [49]. Duox1 and Duox2 are both found in the thyroid, where they are involved in thyroid hormone synthesis, and in epithelia of the lung and gastrointestinal glandular tissues where they possibly serve in host defense [50,51]. The Noxs are a primary source of superoxide anion in the vasculature. Nox1, Nox2, Nox4, and Nox5 have been identified in blood vessels and their roles have been extensively reviewed elsewhere [52]. It is also apparent that the expression of these specific Noxs varies in different vascular beds, cell types within blood vessels, and even subcellular compartments [53].
The prototypical phagocytic NADPH is composed of five subunits: p47phox, p40phox, p67phox, p22phox and the catalytic subunit gp91phox [54]. Two of the units reside in the membrane, gp91phox and p22phox, where they form a heterodimeric flavoprotein (cytocrome b558). The other three subunits reside in the cytosol. Upon stimulation, p47phox is phosphorylated and the cytosolic units form a complex that includes the small guanosine triphosphate (GTP)ase Rac [55]. This complex is translocated to the membrane where it interacts with gp91phox and p22phox. This interaction activates the enzyme resulting in superoxide anion production. The regulation of Nox activity is intricate, involving different signaling pathways. In addition, the ability of the different Noxs to produce superoxide varies depending on the characteristic of the supportive proteins. In general, Nox1, and 2 require Rac to initiate or enhance their production of superoxide, whereas Nox5, Duox1 and Duox2 do not require Rac [55,56]. Nox4 does not contain any Rac binding sites but its activity may be modulated by Rac1 [9,56]. The role of Rac on the activity of the Noxs also depends on whether the Nox supportive proteins in the cell are p47phox and p67phox or their homologues Noxo1 and Noxa1, respectively. It also appears that different Noxs have different affinities for Rac1 or Rac2 [55], but the overall effect of either Rac on the activity of the Noxs likely depends on the predominant Rac expressed in each specific cell or tissue.
Additional levels of Nox-activity regulation are provided by different enzymes and signaling pathways within the cell. For example, in vascular smooth muscle cells (VSMCs) Nox activity is regulated by protein disulfide isomerase, a chaperone enzyme involved in regulating the redox status of the cell and the processing of proteins [57]. Also in VSMCs and in neutrophils, Nox1 and 2 have been shown to be regulated by the chloride/proton antiporter CIC-3 [58,59]. The enzyme polymerase delta-interacting protein or Poldip2 has also been shown to modulate the activity of Nox4 in VSMCs [60]. Interestingly, Poldip2 is involved in the strengthening of focal adhesions and the formation of stress fibers via Rho dependent pathways [60,61]. This is important in the context of vascular remodeling, as we have shown that vasoconstriction-induced inward remodeling of resistance vessels requires ROS and actin polymerization [15,62]. Furthermore, the transcription and activation of Nox enzymes are induced by a wide variety of stimuli such as G-protein coupled receptor agonists (angiotensin II, serotonin, thrombin, endothelin-1), mechanical stimuli (shear stress, pressure, stretch), growth factors (transforming growth factor β, epidermal growth factor, platelet derived growth factor), and inflammatory cytokines (interleukins, tumor necrosis factor α), most of which have been associated with vascular remodeling processes [9,63,64,65,66].
In the vascular system, increased Nox activity and expression have been described in a number of physiological and pathological conditions associated with vascular remodeling. For example, hypertension in general has been associated with an augmented production of superoxide by Nox in conduit vessels. Nox activity is increased in SHR rats [67], deoxycorticosterone acetate (DOCA) salt sensitive rats [68] or mice [69], and angiotensin II-infused hypertensive rats [70,71]. At the level of the microcirculation, increased activity of Nox has been reported in the coronary and mesenteric arterioles of SHR rats [72]. In addition, it has been reported that in SHRs treated with testosterone addition of the antioxidant and non-specific Nox inhibitor, apocynin, results in decreased migration of VSMCs [73]. In hypertension, angiotensin II appears to be an important player in the activation of Nox and the vascular remodeling process. Angiotensin II infusion induces an increase in Nox activity and remodeling of the mesenteric [74,75] and renal microvasculature [76]. Conversely, the vascular remodeling and increase in Nox activity induced by angiotensin II is diminished in mice treated with apocynin [77]. In addition, treatment with angiotensin-converting enzyme (ACE) inhibitors or angiotensin II receptor blockers results in reduced expression of p22phox, p47phox, gp91phox and reverses the cardiovascular remodeling observed in the SHR [78]. Furthermore, a recent study reported that treatment with atorvastatin down-regulates angiotensin II-induced Nox1 expression and reduces vascular remodeling in rats through a putative antioxidant effect [79].
The activity and expression of the Noxs have also been reported to play important roles in the regulation of angiogenesis. For example, in the angiogenic response to hypoxia-reoxygenation in the heart, the expression of the proangiogenic factor vascular endothelial growth factor (VEGF) is reduced by the pharmacological blockade of Nox or the transcriptional knockdown of the NADPH oxidase subunit p47phox [80]. Similarly, angiopoetin-1-mediated angiogenesis is dependent on the activity of Nox and the presence of H2O2 [81]. A detailed description of the role of ROS and Nox on angiogenesis is beyond the scope of this review, and the reader is referred to several other publications [82,83,84]. Overall, the accumulated evidence supports the concept that alterations in expression and activity of the Nox enzymes are associated with changes in microvascular structure and function.
3.2. Nitric Oxide Synthase
Endothelial nitric oxide synthase (eNOS), one of the three isoforms of NO synthases, uses l-arginine and molecular oxygen to produce l-citrulline and NO. Functionally eNOS is a homo-dimer enzyme that binds calmodulin in the presence of Ca2+ to facilitate the production of NO. The enzyme requires the presence of cofactors such as flavin adenine dinucleotide (FAD), flavin mononucleotide (FMN), and (6R-)5,6,7,8-tetrahydrobiopterin (BH4). The synthesis of NO by eNOS requires the hydroxylation of l-arginine to Nω-hydroxy-l-arginine that is then oxidized to NO and l-citrulline. Nitric oxide causes vascular dilation and participates in blood pressure control. It also has anti-atherosclerotic and vasoprotective effects by interfering with coagulation [85], leukocyte adhesion [86,87,88] and smooth muscle cell proliferation [89]. However, in certain circumstances such as those in which BH4 or l-arginine are deficient, eNOS transfers electrons form NADPH directly to molecular oxygen, resulting in the production of superoxide anion instead of NO, a process known as eNOS uncoupling (Figure 1). The uncoupling of eNOS, in a rodent model of metabolic syndrome, has been associated with increased production of superoxide and hypertrophic remodeling of resistance arteries [90].
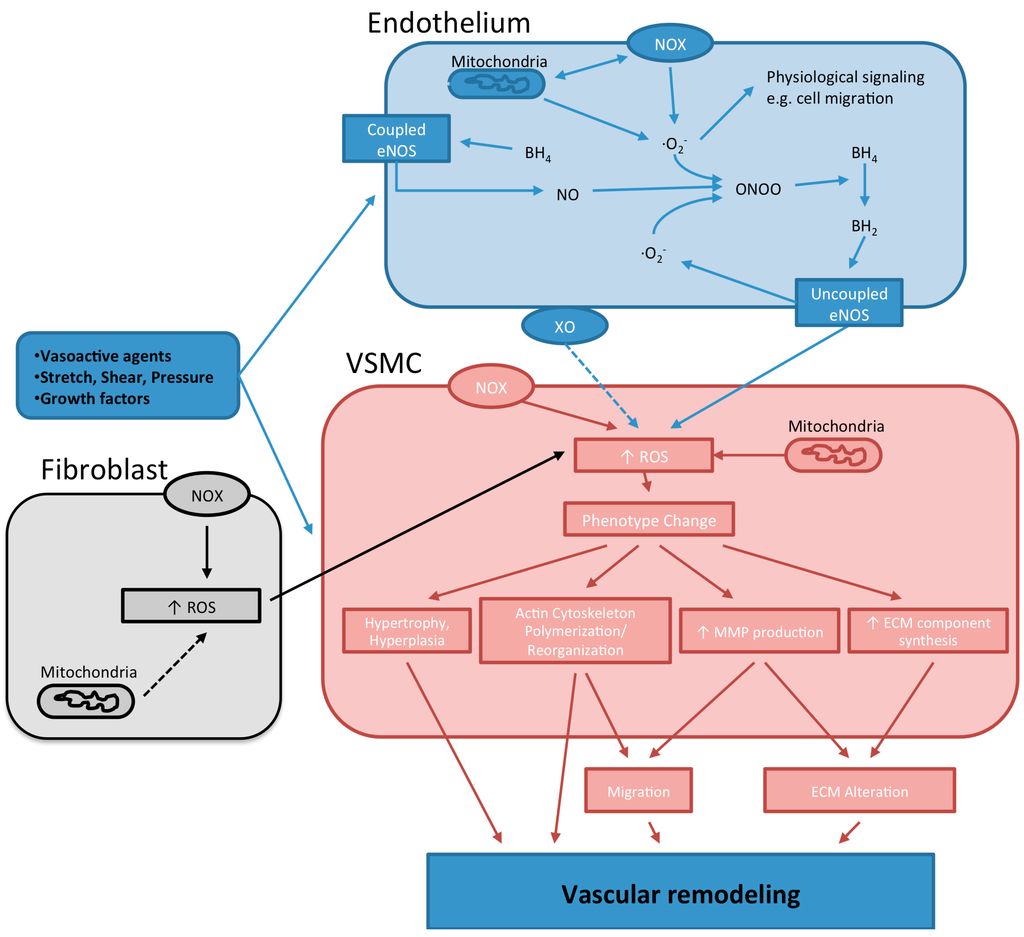
Figure 1.
Reactive oxygen species (ROS)-dependent mechanisms underlying vascular remodeling. Various stimuli activate ROS-generating enzymes located either in the endothelium or the vascular smooth muscle cells (VSMCs). In the endothelium, activation of Nox results in the production of superoxide and may also induce an increased release of superoxide from the mitochondria. The superoxide anion produced can activate physiological signaling pathways and/or interact with nitric oxide (NO) to produce peroxynitrite (ONOO). The latter scenario is particularly important when superoxide is produced in excess or when ROS scavenging molecules are insufficient. Peroxynitrite interacts with tetrahydrobiopterin (BH4) decreasing its availability as a precursor for the synthesis of NO. As a result, endothelial nitric oxide synthase (eNOS) becomes uncoupled and starts to produce superoxide. The superoxide, in turn, interacts with NO to produce ONOO, further reducing the availability of BH4, thus promoting eNOS uncoupling. In VSMCs, exogenous or endogenous ROS induce phenotype changes. These alterations in phenotype are associated with changes in cellular growth and apoptosis that result in the hypertrophy and hyperplasia of VSMCs. Furthermore, VSMCs also reorganize their actin cytoskeleton, increase the production of extracellular matrix (ECM) components and the activity of matrix metalloproteinases (MMPs). The results of these phenotypical changes are increased VSMC proliferation, migration/repositioning and the reorganization of the ECM. These processes lead to changes in the micro architecture of the vascular wall and changes in vessel diameter that underlie vascular remodeling. Dashed arrows represent pathways that are not confirmed.
A number of conditions can result in a reduced availability of l-arginine that uncouples eNOS. Endothelial cells express arginase, an enzyme that uses l-arginine as a substrate to produce l-ornithine and urea. There are two arginase isoforms; arginase I is located in the cytoplasm and mainly found in the liver, while arginase II is located in the mitochondria and has a wide tissue distribution with the highest levels of expression in the kidney and prostate. In certain pathological conditions, arginase I and II become highly expressed and decrease the availability of l-arginine leading to eNOS uncoupling and an increased production of ROS. Results obtained in bovine aortic endothelial cells indicate that peroxynitrite and H2O2 increase arginase activity and lead to increased production of superoxide anion [91]. The activity and expression of arginase appears to be increased through a protein kinase C (PKC) mediated activation of the Rho/Rho kinase (ROCK) pathway. Furthermore, a recent study found that in the aorta of aged mice there is an increase in arginase II activity that causes eNOS uncoupling and impairs endothelial mediated vasorelaxation [92]. In those mice, siRNA treatment targeting arginase II markedly improved acetylcholine dependent vasodilation indicating that arginase downregulation increased the availability of l-arginine for eNOS. Thus, current data suggest that a major factor causing eNOS uncoupling is decreased l-arginine availability as a consequence of increased arginase enzyme activity.
Deficiency of BH4 is another factor that promotes eNOS uncoupling (Figure 1). l-citrulline and NO production by eNOS, in endothelial cells, is closely related to the intracellular levels of BH4 [93], which in turn are dependent upon the fine balance between its synthesis and degradation. Decreased levels of BH4 have been found in DOCA-salt hypertensive rats [94], in the aorta of insulin resistant rats [95], in the plasma of SHRs [96], as well as in patients with diabetes and essential hypertension [97,98]. In all those examples supplementation of BH4 enhanced vasodilation and had an overall beneficial effect on endothelial function. BH4 is one of the most potent naturally occurring reducing agents and is very susceptible to oxidation. It has been proposed that in the initial stages of hypertension, Nox activation leads to the production of superoxide anion that, in turn, reacts with NO producing peroxynitrite and uncoupling eNOS [94]. Peroxynitrite, even at small concentrations, oxidizes BH4 and therefore plays a major role in the uncoupling of eNOS [99,100]. Through a feed-forward mechanism, the production of peroxynitrite continues to amplify eNOS uncoupling and the consumption of BH4. In addition, a diminished biosynthesis of BH4 may further exacerbate eNOS uncoupling in a number of pathological conditions where BH4 is being oxidized. For example, the overexpression of GTP cyclohydrolase I (GTPCH I), a rate limiting enzyme in the synthesis of BH4, has been shown to abrogate superoxide production in mesenteric arteries of DOCA-salt treated mice [101]. In that study, GTPCH I overexpression also reduced microvascular hypertrophic remodeling and attenuated hypertension. Another mechanism that contributes to BH4 depletion and eNOS uncoupling involves the attenuated recycling of 7,8-dihydrobiopterin (BH2), the oxidized form of BH4 [102] (Figure 1). For example, it has been reported that in endothelial cells treated with angiotensin II, BH4 deficiency is the result of diminished dihydrofolate reductase (DHFR) activity, the enzyme responsible for BH2 to BH4 recycling. In those cells, downregulation of DHFR was caused by H2O2 produced as a result of angiotensin II-mediated activation of Nox [103]. Similarly, in DOCA salt-treated mice the increased production of ROS by uncoupled eNOS is markedly reduced in p47phox-knockout DOCA mice, suggesting that Nox contributes to the uncoupling of eNOS in that model of hypertension [94]. In support for a role of the Noxs in diminishing the recycling of BH2 to BH4 by DHFR, a recent study found that Nox1 is involved in the uncoupling of eNOS and the downregulation of DHFR in diabetic mice [104]. In that study the overexpression of DHFR or the administration of folic acid resulted in improved endothelial function and recoupling of eNOS. All this evidence suggests that the depletion of BH4 represents an important pathological mechanism leading to eNOS uncoupling and increased production of ROS in vascular diseases associated with vessel remodeling.
3.3. Xanthine Oxidase
Xanthine oxidase (XO) is another vascular source of ROS that is expressed mainly in the endothelium. XO is one of the two isoforms of xanthine oxidoreductase, the other isoform being xanthine dehydrogenase. Xanthine dehydrogenase produces NADH and uric acid from hypoxanthine or xanthine, while XO produces superoxide and H2O2 by transferring an electron from xanthine or hypoxanthine to molecular oxygen [105,106]. Xanthine dehydrogenase can be converted to XO by the reversible oxidation of cysteine residues, or it can be irreversibly transformed into XO through proteolysis [107,108,109]. XO produces a mix of H2O2 and superoxide (with H2O2 being the main ROS) depending on the concentration of oxygen in tissues, cellular localization of the enzyme or availability of substrates [110]. XO expression and activity are increased by angiotensin II in patients with coronary disease. Its activation is ROS dependent and is inhibited by allopurinol and losartan (an angiotensin II receptor blocker) [111]. Furthermore, oscillatory shear stress triggers Nox dependent XO activation and release of superoxide [112], an important contributor to vascular injury and remodeling. In vitro, XO activation promotes endothelial cell survival and stimulates angiogenesis through the release of VEGF [113]. In contrast, XO-derived superoxide, by promoting endothelial dysfunction, contributes to hypoxia-induced pulmonary hypertension and remodeling [114]. In some models of hypertension such as the SHR and Dahl-salt sensitive rat, inhibition of XO reduces the production of ROS and lowers blood pressure [115,116]. In contrast, XO appears not to be an important contributor to the elevated blood pressure observed in glucocorticoid-induced hypertension [117]. Based on these conflicting data, the role of XO in hypertension, vascular injury and microvascular remodeling remains to be clearly defined.
3.4. Mitochondrial Electron Transport System
The mitochondrial electron transport chain is one of the major sources of ROS in mammalian cells. Superoxide is generated as a byproduct of ATP production in the process of oxidative phosphorylation. During the transfer of electrons from NADH to molecular oxygen in the mitochondria some superoxide is produced, which can escape into the cytosol via anion channels [118,119,120]. In addition, the electron transport chain may become uncoupled during episodes of tissue ischemia or cellular hypoxia resulting in increased production of superoxide. Elevated mitochondrial production of ROS has been associated with an increased production of VEGF and angiogenesis [121]. However, the specific action of mitochondrial ROS on the vasculature may vary with vascular bed and the amount of ROS produced. In pulmonary arteries, hypoxia induces Nox activation, increases ROS production and the contractility of smooth muscle cells. These hypoxic effects can be reduced by rotenone, an inhibitor of mitochondrial complex I, as well as inhibitors of PKCε [122], suggesting that mitochondrial uncoupling participates in the process. In DOCA-salt sensitive rats it has been reported that the use of inhibitors of mitochondrial electron transport complexes II and IV prevent the increase in ROS associated with hypertension [123]. Further evidence on the contribution of mitochondrial ROS to hypertension comes from experiments showing that aldehyde dehydrogenase 2, a mitochondrial enzyme, diminishes the contractile effects of angiotensin II in hypertensive mice by preventing ROS generation [124]. Recently, a positive feedback mechanism has been proposed for the increase in mitochondrial ROS production induced by angiotensin II at the level of the endothelium [125]. In the proposed mechanism, angiotensin II triggers Nox activation, followed by the activation of mitochondrial KATP channels resulting in the depolarization of the mitochondria and ROS production. The increase in cytosolic ROS results in PKC activation that further increases Nox activity. In this paradigm, the mitochondria behave as an amplifier for ROS in a positive feedback loop, which would eventually result in uncoupling of eNOS, endothelial dysfunction and impaired vascular relaxation (Figure 1). In regard to vascular remodeling, a role for mitochondria in the process has been suggested by experiments showing that in a model of vascular injury a reduction in superoxide dismutase-2 (SOD2, MnSOD) activity results in an increased proliferation and migration of neointimal cells [126]. Overall, substantial evidence implies that mitochondrial ROS contributes to vessel remodeling, angiogenesis and blood pressure regulation, but additional work is needed to elucidate the exact mechanisms promoting and regulating mitochondrial ROS production and its effects on vascular remodeling.
4. The Role of ROS in Vascular Remodeling
4.1. ROS in Remodeling of the Microcirculation
ROS serve a physiological role in the vasculature and contribute as secondary messengers in adventitial fibroblasts, VSMCs, and endothelial cells. Overall, increases in the bioavailability of vascular ROS can stimulate collagen deposition, alter the activity of matrix metalloproteinases (MMPs), and promote the rearrangement of the cytoskeleton, leading to cell migration, growth or apoptosis [127] (Figure 1). All these processes have clear repercussions on the structure of the vascular wall, suggesting that ROS play important roles in microvascular remodeling. However, only a small number of studies have directly investigated the involvement of ROS on the structural modification of microvessels (Table 1). In vivo, studies have shown that the inward and hypertrophic microvascular remodeling processes that are observed in animal models of hypertension are decreased by blockade of the angiotensin II type 1 receptor [128], up-regulation of SOD [76] or treatment with the SOD mimetic, tempol [62,76,129,130]. Ex vivo, we have shown that incubation of isolated rat cremaster arterioles with tempol or apocynin prevents the inward remodeling induced by prolonged agonist-induced vasoconstriction [62]. Touyz and Schiffrin [131] also showed that VSMCs isolated from arterioles of hypertensive patients produce greater amounts of ROS than normotensive controls in response to angiotensin II stimulation. These results suggest that superoxide is an important ROS associated with the pathological microvascular remodeling observed in hypertension. They also suggest that ROS-dependent signaling by angiotensin II is an important mechanism associated with the inward and hypertrophic types of resistance vessel remodeling in hypertension.

Table 1.
Representative studies showing ROS contribution to remodeling of resistance arteries.
| Vascular Bed | Experimental System | Stimulus | ROS Species | Type of Remodeling | ROS Inhibitor | Ref. |
|---|---|---|---|---|---|---|
| Mouse mesenteric arteriole | PPARγ KO mice | angiotensin II | superoxide, reduced SOD3 expression | Eutrophic remodeling, Hypertrophic remodeling | - | [75] |
| Human subcutaneous arteriole | Human | Cushing syndrome | superoxide | Hypertrophic remodeling | - | [24] |
| Mouse mesenteric arteriole | (NZO) mice | - | superoxide, peroxynitrite | Hypertrophic remodeling | Tempol | [90] |
| Rat mesenteric arteriole | Wistar rats (female) ovareiectomized | high flow | superoxide | Hypertrophic remodeling | - | [39] |
| Rat mesenteric arteriole | Zucker rats | high flow, hyperglycemia | superoxide | Hypertrophic remodeling | Tempol | [132] |
| Mouse mesenteric arteriole | BALB/c male mice | angiotensin II | superoxide | Hypertrophic remodeling | Apocynin | [77] |
| Rat mesenteric arteriole | Wistar rats | angiotensin II | superoxide | Inward eutrophic remodeling | Atorvastatin ** | [79] |
| Mouse basilary artery | PPAR-gamma KO mice | - | superoxide | Inward hypertrophic remodeling | Tempol | [32] |
| Rat cremasteric arteriole | Sprague-Dawley rat | norepinephrine, angiotensin II | superoxide, hydrogen peroxide | Inward remodeling | Tempol, Apocynin | [62] |
| Rat middle cerebral artery | SPSHR rats | serotonin | superoxide | Inward remodeling | Tempol | [130] |
| Rat mesenteric arteriole | Wistar rats | low flow | superoxide | Inward remodeling | Tempol, Apocynin | [133] |
| Rat mesenteric arteriole | Sprague-Dawley rat | angiotensin II | superoxide | Inward remodeling | - | [71] |
| Mouse aferent arteriole | SOD1 tg, SOD1 KO mice | angiotensin II | superoxide | Inward remodeling | Tempol | [76] |
| Rat middle cerebral artery, basilary artery | SHR | - | superoxide | Inward remodeling, Hypertrophic remodeling | Telmisartan # (ARB) | [128] |
| Rat mesenteric arteriole | Wistar rats | low flow, high flow | superoxide | Inward remodeling, Outward remodeling | Tempol | [134] |
| Rat/Mouse mesenteric arteriole | Wistar rats, eNOS KO mice | low flow, high flow | superoxide, hydrogen peroxide | Inward remodeling, Outward remodeling | Apocynin, Catalase | [135] |
| Rat mesenteric arteriole | Wistar rats | high flow | superoxide | Outward hypertrophic remodeling | Tempol, Perindopril *, Candesartan # | [136] |
| Rat mesenteric arteriole | Zucker rats | high flow | superoxide | Outward hypertrophic remodeling | Tempol, Catalase, SOD | [137] |
| Rat mesenteric arteriole | Wistar rats | high flow | superoxide | Outward remodeling | Tempol, Apocynin | [138] |
PPARγ, KO-Peroxisome proliferator-activator receptor; NZO-New, Zealand obese; SHR, Spontaneously hypertensive rats; SPSHR, Stroke prone spontaneously hypertensive rats; SOD1 tg, Superoxide dismutase 1 transgenic; SOD1 KO, Superoxide dismutase 1 knock out; # Angiotensin II receptor blocker; * Angiotensin-converting-enzyme inhibitor; ** Statin.
Angiotensin II increases ROS production and signaling by stimulating the activity of Noxs and by activating redox-sensitive genes [139,140,141]. Although angiotensin II is usually associated with vasoconstriction and hypertension, ROS and angiotensin II-dependent signaling are also associated with the outward remodeling induced by high-flow conditions in mesenteric resistance arteries [136,138]. Multiple studies have shown the effects of high or low flow conditions in resistance arteries [39,133,134,135,142,143]. High flow induces outward hypertrophic remodeling, while low flow induces inward hypotrophic remodeling. Interestingly, both remodeling processes are associated with angiotensin II-dependent signaling and an increased production of ROS by the vessel wall. The proportion of the increase in ROS that is dependent on angiotensin II remains to be fully determined, but reports indicate that the specific effects that ROS and angiotensin II have on the vascular wall during high or low flow conditions are different. It appears that superoxide mediates the luminal enlargement effect of high flow, while angiotensin II mediates the hypertrophic effect through extracellular-signal-regulated kinases (ERK)1/2 activation [136]. In contrast, angiotensin II-dependent constriction appears to mediate low flow-induced inward remodeling with only a marginal effect of superoxide on the cross-sectional area of the wall [133]. It is believed that hemodynamics and the local signaling environment, in particular the local production of vasodilators and vasoconstrictors, affect the contractile and synthetic state of cells within the vascular wall and thus guide the type of remodeling [142]. This is consistent with the fact that multiple types of vascular remodeling can coexist in a vascular bed or in the same individual in different vascular beds. The effects of high or low flow conditions on resistance artery remodeling highlight the role that the contractile state of the vasculature has on the type of remodeling. High flow is associated with vasodilation and low flow with vasoconstriction in concert with increased or decreased production of NO, respectively [35]. In both the inward and outward remodeling processes, it appears that ROS participate in part via their capacity to activate MMPs [35,62,144,145]. The specific vascular cells, signaling cascades, and ROS molecules involved in each type of remodeling, however, are not yet fully determined. Furthermore, pathological states such as those encountered in obesity and diabetes modulate the effects that ROS have on the remodeling process. For example, mesenteric arteries from obese Zucker rats develop outward remodeling when exposed to high flow. The presence of diabetes in those rats reduces the high-flow dependent outward remodeling despite the presence of increased ROS production [132,137,146,147].
4.2. Reactive Oxygen Species and the Phenotype of Vascular Smooth Muscle Cells
An important aspect of the vascular remodeling processes relies on the capacity of VSMCs to actively contract the vessel, produce ECM proteins, degrade those proteins, change position within the vascular wall and/or proliferate. VSMCs can perform both contractile and synthetic functions. These functions are associated with specific cellular morphologies and the cellular expression of different marker proteins, as well as proliferative and migratory capabilities. The particular phenotype VSMCs adopt depends on a wide array of environmental cues, such as physical factors (stretch and shear stress), biochemical factors, and biophysical signals contained within neighboring ECM components. Within the adult vessel wall, VSMCs are generally believed to exist in a low proliferative state with reduced synthetic activity known as the contractile phenotype. Some of the most relevant markers present in VSMCs with the contractile phenotype are smooth muscle myosin heavy chain, smoothelin, and desmin. However, during development or in pathological conditions such as those found in vascular injury or atherosclerosis, VSMCs lose those contractile marker proteins [148]. This results in a change to a more synthetic phenotype, characterized by increased VSMC migration and proliferation, hypertrophy, and ECM protein deposition [148]. In hypertension, the role of VSMC phenotype switching is evidenced by the enhanced proliferation of cultured VSMCs from SHRs [149,150] and the expression of genes associated with the synthetic phenotype, such as cellular retinol binding protein (CREB-1). Also, in stroke-prone SHRs, the presence of brain lesions is associated with the expression of markers specific for the synthetic phonotype in VSMCs, such as non-muscle myosin heavy chain [151]. Furthermore, in patients with essential hypertension that develop preeclampsia a reduction in VSMC contractile markers has been shown to predict the persistence of hypertension after delivery [152]. Similarly, reduced smooth muscle contractile markers (α-smooth muscle actin, desmin, smooth muscle myosin heavy chain) were found in patients with pulmonary hypertension [153]. Although the mechanisms involved in the development of a particular VSMC phenotype are not yet fully determined, a series of physical and biochemical factors have been proposed as contributors to this phenotypic modulation. Growth factors, such as platelet-derived growth factor-BB (PDGF-BB) and transforming growth factor-β1 (TGF-β1), or modulators of tone such as NO, angiotensin II and endothelin-1, in addition to mechanical stimulation of vessels, have been shown to induce phenotypical changes in VSMCs [148,154]. Many of these factors also promote the production of ROS, suggesting that ROS may be involved in the phenotypic modulation of VSMCs. Whether such phenotypical changes occur at the level of the resistance arteries in the microcirculation remains to be fully elucidated. Our previous finding—that a number of VSMCs reposition during the remodeling induced by prolonged exposure to vasoconstrictor agonists in isolated arterioles—suggests that VSMCs may change phenotype rapidly during the inward remodeling process [155]. However, whether this represents a full phenotypic change from a contractile to a synthetic phenotype is controversial. It could be a normal property of VSMCs, especially when one considers that the remodeling observed in hypertension at the level of the resistance arteries is for the most part eutrophic; that is, there is no hypertrophy or hyperplasia of VSMCs.
The overall role of ROS in VSMC phenotypic modulation is not fully understood. However, a number of studies clearly indicate that ROS are involved in the process. Studies in atherosclerosis have shown that superoxide induces the synthetic phenotype as well as cytoskeletal changes in VSMCs [156,157]. Furthermore, superoxide anion (in conjunction with H2O2) increases PDGF [158,159] and collagen production in VSMCs [160], which, as mentioned above, are characteristic of the synthetic phenotype. In animal models of vascular injury, it has been shown that superoxide increases VSMC migration leading to vascular remodeling of conduit arteries [126,161]. A similar role has been found for H2O2, which enhances hypertrophy, proliferation, and migration of VSMCs in culture [162]. However, the role of H2O2 on inducing the synthetic phenotype in VSMCs is controversial, and it has been shown that in cultured human VSMCs the production of H2O2 is necessary to maintain the contractile phenotype [163]. The specific location of ROS production within the cell may also be important, as it is believed that in VSMCs, Nox1 and Nox4 may have opposite effects due in part to their subcellular localization [164,165]. In addition, Nox4 is constitutively active and appears to produce mostly H2O2, while Nox1 produces mostly superoxide. It also appears that the local environment plays a role on the effect that specific ROS have on modulating the VSMC phenotype. For example in pulmonary arterial smooth muscle cells, ROS produced by Nox4 promote proliferation [166], whereas Nox4 appears to maintain the contractile phenotype of VSMCs from the systemic circulation [167]. Although little is known about the role that ROS play on the phenotypic modulation of VSMCs in resistance vessels, it is likely that ROS have functions similar to those observed in conduit arteries. In addition, it has been shown that endothelial dysfunction in resistance arteries is associated with low production of NO and increased production of ROS. NO is known to maintain VSMCs in a low proliferative state [168]; thus a dysfunction in the production of NO might result in phenotypic changes in VSMCs. However, it remains to be determined if it is only the reduction of NO, the increase in ROS, or both that change the proliferative capacity and the phenotype of VSMCs. As the origin and characteristics of VSMCs vary along the vascular tree [169] and possibly within the same vessel, further investigation is needed on the exact role of ROS-induced VSMC phenotypic modulation at the level of the microcirculation in different vascular beds.
4.3. ROS-Induced Vascular Smooth Muscle Cell Migration
The vascular remodeling process, especially that associated with vascular injury, involves the migration of VSMCs. Induction of VSMC migration is mediated by a wide range of signaling molecules, such as angiotensin II, PDGF, VEGF, thrombin, arachidonic acid, and norepinephrine [141,170,171,172,173,174,175]. Most of these molecules also generate ROS, and the involvement of ROS in VSMC migration is well documented in neointimal formation and vessel injury repair [176]. A few studies have also looked at the migration of VSMCs in animal models of hypertension and diabetes [73,177,178,179,180,181,182,183]. In vitro, a seminal study detailing the importance of ROS in VSMC migration, determined that H2O2 is required for PDGF to stimulate cell motility, tyrosine phosphorylation and activation of mitogen-activated protein kinase [184]. In that study, the scavenging of H2O2 with catalase or the use of the antioxidant N-acetylcysteine inhibited all the effects of PDGF. As neointimal formation is associated with PDGF signaling, these results support a role for ROS in VSMC migration during vascular injury. An additional in vitro study showed that hyperinsulinemia-induced VSMC migration is accompanied by an increase in Nox activity and enhanced mitochondrial production of ROS [185]. In that study, treatment with diphenylene iodonium (DPI) to inhibit ROS production diminished VSMC motility, which suggests that ROS are involved in diabetes-related VSMC migration. Further support for a role of ROS in VSMC migration associated with vascular remodeling comes from a recent study in which antioxidant treatment decreased the motility of VSMCs isolated from mice treated with angiotensin II [141]. Inhibition of c-Src signaling has also been shown to cause ROS suppression and a subsequent reduction in the motility of VSMCs isolated from a testosterone-infused SHR model of hypertension that has increased Nox1 and Nox4 activity [73]. Other evidence for a role of Nox1 in the modulation of VSMC motility comes from a study in Nox1 knockout mice that showed Nox1 is necessary for the phosphorylation of ERK1/2, transactivation of epidermal growth factor receptors (EGFR) and activation of MMP-9. In turn, MMP-9 is responsible for the shedding of N-cadherins and the increase VSMC motility observed upon thrombin stimulation [186].
In the microcirculation little is known about ROS-induced VSMC migration, although a number of vascular remodeling processes, such as arterialization and inward remodeling, are likely to involve VSMC movement. Arterialization occurs in processes such as collateral formation, when a capillary, arteriole or small artery is enlarged to increase blood flow to a tissue that has become ischemic due to an arterial blockade. During collateralization it has been shown that VSMCs both migrate and proliferate to increase the thickness of the medial layer and allow for enlargement of vascular diameter and normalization of circumferential stress [187,188,189]. In addition to arterialization, evidence suggests that VSMC migration also occurs during the process of inward remodeling in resistance arteries [155]. During prolonged vasoconstriction we have shown that VSMCs move and change their position within the wall of arterioles that remodeled inwardly. ROS are likely involved in this process as vasoconstriction-induced inward remodeling has been shown to be blocked by either scavengers of ROS, or inhibitors of ROS production [62]. Inward remodeling is also blocked by inhibition of the pathways associated with activation of the small GTPases Rho and Rac [15]. Interestingly, these GTPases are regulated by ROS and associated with its production. The precise role of ROS in mediating VSMC movements in arterialization or inward remodeling requires further investigation to determine whether the mechanisms reported for ROS-mediated VSMC migration elsewhere apply to the microcirculation [10,190].
4.4. Reactive Oxygen Species and the Actin Cytoskeleton
Resistance vessels are able to modulate their diameters via the constriction/relaxation of smooth muscle cells within the vessel wall. Contractile stimuli leads to cross bridge cycling between actin and myosin filaments, which shortens smooth muscle cells, thus generating tension that contracts the vessel. In contrast, vasodilatory stimuli inhibit the myosin filaments from interacting with the actin cytoskeleton, which in turn causes smooth muscle cells to lengthen and increase the vessel’s luminal diameter. Historically, the role of the actin cytoskeleton during the contractile process was thought to be static; the filamentous actin that comprises the actin cytoskeleton was believed to be a stable structure that remained relatively unchanged and provided a fixed scaffolding for the myosin filaments to move against and generate tension. A number of studies have challenged this notion, and it is now emerging that the actin cytoskeleton is a dynamic structure that does indeed undergo rapid reorganization, in part via filamentous (F)-actin polymerization, in response to contractile stimuli [191,192,193,194,195,196].
We have observed that reorganization of the actin cytoskeleton also occurs during inward eutrophic remodeling in resistance vessels [15,195]. Following a 4 h exposure of isolated arterioles to constriction agonists, actin polymerization promoted inward remodeling through processes that require ROS generation. Inhibition of either actin polymerization via the addition of cytochalasin D, or ROS generation with apocynin, was sufficient to block the remodeling process [15,62]. Together these observations have led us to hypothesize that one of the roles of ROS during the inward remodeling process is to facilitate actin polymerization/reorganization.
It has been demonstrated that ROS can increase the ratio of F-actin to total actin. In post-hypoxic endothelial cells, the reintroduction of oxygen generates ROS and increases the pool of F-actin. This increase is attenuated by the overexpression of SOD, suggesting that superoxide promotes actin polymerization [197,198]. In addition, Moldovan et al. [199], have shown in a wound healing model of endothelial cells that the rate of actin monomer incorporation into F-actin was greater in areas with high levels of measured superoxide, and that this incorporation of monomers was blocked in the presence of either the superoxide dismutase mimetic, manganese (III) tetrakis(1-methyl-4-pyridyl)porphyrin (MnTMPyP), or the inhibitor of ROS generation, DPI. Together, these studies indicate that in specific cell types, ROS increases the rate of actin polymerization thereby increasing the proportion of F- to total-actin. It remains to be determined whether VSMCs in vivo undergo a similar response to ROS vis a vis the actin cytoskeleton. However, our observation that inward remodeling in response to vasoconstrictor stimuli requires both the production of ROS and the polymerization of actin supports a model in which ROS facilitate reorganization of the underlying cytoskeletal architecture of VSMCs.
Actin polymerization involves the assembly of G-actin monomers into filaments (F-actin) that are characterized by a fast growing barbed end and a slower growing pointed end. The rate-limiting step in this process is the nucleation of monomers into stable oligomers. Kinetically, these reactions are not favored due to the inherent instability of actin dimers. Three classes of actin nucleating proteins have been identified that function to counteract the kinetically unfavorable conditions associated with nucleation: Actin related protein 2/actin related protein 3 (Arp2/3) complex, formins, and tandem monomer-binding nucleators (for review see [200]). In smooth muscle cells, the most studied nucleator is the Arp2/3 complex. It is a seven-subunit complex that exerts its activity predominantly by binding to existing filaments and forming new actin branches. Its polymerizing activity is greatly enhanced via association with a number of nucleation promotion factors (NPF). These function by binding to and activating Arp2/3 as well as recruiting G-actin monomers to the nucleating complex. Thus nucleation is promoted by NPF activation of Arp2/3 and by increasing the local G-actin concentration. The NPF, neuronal Wiskott-Aldrich Syndrome protein (nWASP), has been shown to play an important role in smooth muscle actin polymerization. Inhibition of nWASP, by expressing a dominant negative mutant, blocks actin polymerization in response to contractile stimulation in tracheal smooth muscle cells [201]. In addition, the adaptor protein, CrkII, also plays a role in Arp2/3 mediated actin polymerization by forming a multi-protein complex with Arp2/3 and nWASP that in turn facilitates polymerization. Mutants of CrkII, that failed to associate with nWASP, inhibit force generation and actin polymerization in response to contractile agonists in tracheal smooth muscle cells [202]. Though it has been well established in tracheal smooth muscle cells that Arp2/3 activation increases actin polymerization, there are a limited number of studies in vessels and VSMCs corroborating these results and their relationship with ROS. In mesenteric arterioles, inhibition of CrkII interactions with nWASP and Arp2/3 attenuates the increase in actin polymerization induced by contractile stimulation [203]. In isolated VSMCs, exposure to H2O2 up-regulates ARP2C (a component of Arp2/3) and down-regulates the actin depolymerizing proteins cofilin and destrin [204]. This presumably favors actin polymerization, but actin polymerization was not assessed in that study. Also in VSMCs, exposure to phorbol esters induces the localization of Arp2/3 complexes to microdomains associated with actin polymerization and newly formed podosomes [205]. This evidence suggests that ROS are involved in actin polymerization and cell migration events in vascular tissues and support our hypothesis that ROS-mediated cytoskeletal modifications are involved in the inward remodeling process associated with hypertension and other cardiovascular morbidities.
The evidence for ROS directly modulating actin nucleation and assembly in vivo is not well characterized in the literature. Standard cell free protocols investigating actin polymerization include reducing agents/antioxidants, such as dithiothreitol (DTT) or beta-mercaptoethanol, to stabilize nucleating actin oligomers, thereby facilitating the polymerizing process. Additional cell free studies indicate that ROS can oxidize actin and decrease the rate of polymerization [206,207]. However, in cell cultures it would appear that the effects of ROS on polymerization are cell-type specific as well as concentration dependent. It was demonstrated that H2O2 induces F-actin fragmentation and depolymerization in fibroblasts, while the same concentration of H2O2 (100–250 μm) increases actin stress fibers in vascular endothelial cells [208]. A much lower concentration of hydrogen peroxide (1 μM) exposure increased actin stress fiber formation in serum-starved fibroblasts [209]. Moreover, as previously indicated, Moldovan and Crawford demonstrated a positive effect of ROS on actin polymerization in vascular endothelial cells. Thus, in the context of vascular remodeling, the in vivo effects of ROS on actin polymerization in VSMCs need further study. Though the direct effect of ROS on actin polymerization in vivo is unclear, there is evidence that ROS can facilitate filament assembly via activation of signaling cascade(s) that modulate actin nucleating factors and complexes.
The members of the Rho family of GTPases are central regulators of a number of cellular processes associated with vascular remodeling including cell-cell interactions, cell motility, polarity, morphogenesis, and cellular interactions with the ECM. They modulate these processes, in part, through reorganization of the actin cytoskeleton. Within the Rho family, Rho, Rac and Cdc42 are closely associated with remodeling of the actin cytoskeleton. Typical of GTPases, they are activated upon exchange of guanosine diphosphate (GDP) for GTP. In their GTP bound conformation, they interact with downstream effector proteins that transmit activating stimuli, both internal and external, into a cellular response that effects both actin polymerization as well as the bundling of actin filaments into stress fibers. For a general review of Rho GTPases see [210].
Both Rac and Cdc42 have been implicated in facilitating actin polymerization through Arp2/3 activation [211,212,213], whereas Rho appears to initiate elongation of existing filaments via the activation of formins [214,215]. A number of studies have corroborated the role of GTPases in facilitating actin polymerization and/or actin remodeling in smooth muscle cells. Inhibition of Cdc42 blocked tension development and actin polymerization in airway smooth muscle cells exposed to acetylcholine [216]. In an investigation examining VSMC hypertrophy, it was demonstrated that exposure to Leptin increased the F to G-actin ratio in isolated rat portal vein strips, and this coincided with an increase in the activation of RhoA. We have demonstrated that inward remodeling of resistance vessels requires actin polymerization, and that blocking RhoA or Rac1 pathways attenuates remodeling [15].
A small number of studies have demonstrated that ROS can positively modulate the activity of GTPases and their downstream targets in VSMCs. ROS generated by a xanthine-xanthine oxidase mixture contracted rat aortic rings and translocated Rho to the membrane, indicative of Rho activation. This contraction was attenuated in the presence of the Rho kinase inhibitor, Y-27632 [217]. In a rat model of hypertension, pulmonary arteries had elevated basal levels of ROS and activated RhoA compared to control animals. Treatment with the antioxidant, tiron, decreased the level of activated RhoA to that of control animals [218]. In human omental arteries, RhoA activity was increased 3-fold in response to ROS treatment [219].
Cdc42 is negatively regulated by Cdc42GAP (GTPase activating protein), which enhances the GTP hydrolysis of Cdc42, thereby inactivating it. In smooth muscle cells, Cdc42GAP activity is inhibited by exogenously added H2O2 as well as endogenously produced ROS generated in response to exposure to contractile agonists. Inhibitors of ROS restore Cdc42GAP activity, thereby down-regulating Cdc42 activity in smooth muscle cells exposed to vasoconstrictor agonists [220,221].
These results support a model in which ROS generated by vasoconstrictor stimuli facilitates tension development and arterial remodeling by inducing an increase in actin polymerization in VSMCs. The available data suggests that the rate of actin monomer incorporation is enhanced in response to ROS, though it would appear that this is a consequence of ROS upregulating the activity of positive regulators and/or down regulating inhibitors of actin polymerization, which offsets the negative direct effect ROS has on actin polymerization, via the oxidation of actin.
4.5. ROS-Induced Cellular Growth and Apoptosis
In addition to the ROS-induced effects on the phenotypical changes and migration of VSMCs, oxidative stress is also associated with cellular growth and apoptosis [222]. The effects of superoxide anion appear to be mostly mitogenic, whereas those of H2O2 seem to induce apoptosis [222]. However, there are contradictory results that show exposure of VSMCs to H2O2 results in increased DNA synthesis, a necessary prerequisite for cell growth [223]. In general, exogenously generated ROS have varying effects on VSMC growth and apoptosis that appear to depend on the origin of the cells and the ROS generating system. In addition, these differential effects of ROS are related to the type of ROS, the concentration of the oxidant, the amount of time the cell is exposed to the oxidant, and the cellular localization targeted by the oxidant. For example, in VSMCs, H2O2 at high concentrations induces apoptosis [224,225], while at lower concentrations it stimulates growth and differentiation [226].
In hypertension-induced remodeling it has been shown that ROS contribute as mediators of VSMC growth [139,227,228]. VSMC hypertrophy and hyperplasia have been observed in the vessels of animals treated with angiotensin II. As mentioned above, angiotensin II is known to activate Nox and increase the production of superoxide anion. Therefore an association appears to exist between angiotensin II stimulation and VSMC hypertrophy/hyperplasia. However, a study in which mice overexpressing human SOD were exposed to angiotensin II suggest the effects of superoxide influence only the pressor response to angiotensin II and not its effects on vascular hypertrophy [229]. Furthermore, evidence from mice overexpressing human catalase suggests that in angiotensin II treated mice, H2O2 and not superoxide, plays an important role in the hypertrophy of the arterial wall, while its effect on the increase in blood pressure is negligible [230]. Contrary to this, in rat VSMCs superoxide produced by XO appears to have mitogenic effects [222]. In humans, XO derived superoxide does not affect proliferation but increases protein synthesis and hypertrophy of VSMCs [231]. Regardless of the type of ROS involved in the induction of VSMC growth, ROS function as a secondary messenger in the pathways normally associated with cell growth and hypertrophy, such as those including mitogen-activated protein kinase (MAPK), Akt and c-Jun [228,232]. For example, in VSMCs stimulated with angiotensin II, p-38 MAPK is an essential component of ROS mediated hypertrophy [233]. In addition, the ROS-induced hypertrophy/proliferation of murine VSMCs was inhibited by the overexpression of Cu/Zn-SOD or catalase, which diminish the epidermal growth factor (EGF)-induced phosphorylation of ERK1/2 or p-38 MAPK [234]. Overall, data suggest that ROS play an important role in hypertension-induced VSMC hypertrophy and hyperplasia, but the precise mechanisms by which different ROS participate in vascular hypertrophy remain to be fully elucidated.
Apoptosis contributes to the structural changes taking place in the vascular wall under physiological and pathological conditions. In hypertensive mRen2 rats, angiotensin II induced production of ROS is associated with increased apoptosis and vascular remodeling [235]. Inhibition of ROS generation with apocynin blocks the formation of abdominal aortic aneurysm in a murine model by reducing apoptosis of medial cells [236]. Similarly, enhanced VSMC apoptosis has been observed in mesenteric resistance arteries of SHRs [237], as well as in Angiotensin II infused rats [238]. Some of the mechanisms believed to be involved in the apoptosis of VSMCs in hypertension are covered in more detail in [239] and include: activation of angiotensin II receptor type 2 [240] as well as L-type calcium channels [241]. The evidence presented above suggests that ROS play an important role on the dynamic reduction and increase in VSMC number and size in the vascular wall.
4.6. ROS-Induced ECM Reorganization
Remodeling of the vasculature also progresses through the degradation, synthesis and reorganization of the ECM in the vascular wall. The most important enzymes associated with ECM reorganization are the MMPs. The MMPs are a group of zinc-dependent endopeptidases that collectively are able to degrade a wide array of ECM proteins such as collagen, elastin, gelatin, and fibronectin. Within the ECM, MMPs are involved in the remodeling of the vessel wall, cleavage of cell surface receptors [242], shedding of precursor signaling molecules [243] and the modulation of chemokine-cytokine signaling [244]. An increased activity of MMPs and subsequent ECM reorganization has been postulated to contribute to vascular remodeling in senescent rats [245,246]. Also, increased circulating levels of MMPs and their endogenous inhibitors (TIMPs) have been found in association with vascular remodeling in human hypertension [247]. Thus it has been suggested that plasma concentrations of MMP-2, MMP-9 and TIMP-1 could serve as markers of cardiovascular remodeling in hypertension or in type 2 diabetic patients where MMP-9 in particular is elevated [248,249]. Similarly, in the SHR, circulating levels of MMP-9 are increased compared with normotensive rats [250], which suggests that in hypertension the activity MMPs is up-regulated and contributing to vascular remodeling.
Although evidence indicates MMPs are involved in the activation of enzymes that produce ROS, numerous studies have shown that ROS, in particular superoxide and H2O2, are involved in the increased expression and activation of MMPs in VSMCs [176,186,251,252,253]. An example of the former is that MT1-MMP (a membrane bound MMP) has been shown to be necessary for the activation of Nox in the advanced glycation end products (AGE)-induced ROS increase observed in diabetic VSMCs [254]. In that study, direct AGE receptor (RAGE) interaction with MT1-MMP was shown to be required for the activation of Nox through a pathway involving Rac1/p47phox. Conversely, many examples exist that indicate multiple ROS producing enzymes and oxidant molecules increase the expression and activity of MMPs. XO, for example has been reported to be involved in the activation of MMP-2 [255]. Other studies investigating the role of angiotensin-II-induced MMP activation in VSMCs have shown that MMP activation, specifically that of MMP-1 and MMP-2, occurs downstream of Nox-dependent ROS production [256,257,258]. In animal models of hypertension, results suggest that vascular remodeling is associated with both MMP-dependent activation of ROS producing enzymes and ROS-dependent upregulation and activation of MMPs. This is particularly evident in the murine two-kidney one-clip model (2K-1C) of hypertension, where inhibition of MMP activity with doxycycline reduces blood pressure, ameliorates the endothelial dysfunction associated with reduced levels of NO, and reduces the levels of ROS in addition to reducing MMP activity in conduit arteries [259]. In the same model of hypertension, the inhibition of NF κB resulted in the reduction of the transcription rate of MMP-2, MMP-9 and ROS production [260]. Furthermore, antioxidant treatment with tempol, in 2K-1C mice, reduced MMP-2 activity and attenuated vascular remodeling suggesting a role for superoxide in this process [261]. These studies reveal that at the level of conduit arteries MMPs play an important role in the remodeling of the vessel wall, and that ROS, in most cases, are responsible for inducing their increased activity.
Interestingly, only a small number of studies have investigated the role of MMPs in microvascular remodeling. In one study performed in rat mesenteric arteries, the maintenance of constriction, by angiotensin-II or phenylephrine, appears to require MMP-2 and MMP-7 to transactivate EGFR and maintain tone through PI3K dependent signaling [253]. The transactivation of EGFR in turn also increases the production of ROS. Also the remodeling process induced by altered flow in mesentery resistance arteries has been associated with inflammation and increased MMP-1 and MMP-9 activity [142]. The inflammatory process facilitates remodeling while the type of remodeling, inward or outward, appears to be determined by the hemodynamic conditions present in the vessel (low flow or high flow) [142]. Recently, we have shown that ROS are involved in mediating MMP activation during the process of vasoconstriction-induced resistance vessel remodeling [62]. Our study demonstrated that MMP activity was increased and necessary to induce inward eutrophic remodeling in vessels constricted with norepinephrine and angiotensin-II. ROS inhibition prevented MMP activation and inward remodeling, suggesting that in vasoconstriction-induced inward remodeling, ROS signaling is needed for MMP activation. The source of ROS and MMPs in our study appeared to be VSMCs and fibroblasts present within the vascular wall. In comparison, the source of MMPs involved in high-flow induced outward remodeling is likely macrophages residing in the vascular wall or adjacent to the blood vessel.
4.7. Reactive Oxygen Species Contribution to Rarefaction
Rarefaction is defined as the disappearance of capillaries and pre-capillary arterioles in a vascular bed that results in a reduced spatial density of microvascular networks [262]. Rarefaction is one of the most common findings in clinical and experimental hypertension. The density of capillaries can decrease through vessel destruction and/or deficient angiogenesis. The current paradigm indicates that during rarefaction the endothelium suffers a series of functional changes that are associated with decreased vascular relaxation and result in microvessel constriction, loss of blood perfusion and eventual disappearance of capillaries. Recent data suggest that abnormal endothelial apoptosis causes rarefaction [263,264]. The exact mechanism of enhanced endothelial apoptosis is still under investigation, but in vitro experiments with vascular endothelial cells suggest that during rarefaction, ROS may induce apoptotic cell death through Nox activation and mitochondrial dysfunction [125,265,266]. In support of the above statement, oxidative stress has been shown to be involved in the promotion of rarefaction through endothelial apoptosis in hypertensive rats, while treatment with antioxidants has resulted in a reduction of microvessel loss [267,268]. Similarly, in the SHR the addition of tempol or apocynin reversed the impaired collateral growth associated with hypertension, suggesting that ROS dampen collateral formation in hypertension [269]. Although activation of the Noxs contributes significantly to the endothelial dysfunction leading to rarefaction, it appears that not all Nox enzymes have deleterious effects. Recently, in a model of kidney injury, rarefaction and oxidative stress were shown to increase following Nox4 silencing, but not after Nox2 silencing [270]. This suggests that Nox4, which is the major Nox isoform in renal tissues, has a protective effect in the kidney.
The angiogenesis responsible for the formation of new microvessels that counterbalances rarefaction appears to be impaired in the normotensive offspring of individuals with essential hypertension [271,272]. Impaired angiogenesis is also present in animal models of aging [273], where this phenomenon is associated with reduced expression of pro-angiogenic factors and receptors [274,275,276,277,278]. Furthermore, in diabetes, which is associated with severe peripheral impairment of angiogenesis, there is increased production of ROS through mechanisms involving the Noxs and XO [279,280]. Also in diabetes, the inhibition of eNOS uncoupling or the addition of BH4 results in decreased superoxide production, suggesting that angiogenesis impairment in diabetes is associated with a reduction in antioxidant defenses combined with eNOS uncoupling and an increase in ROS production [281]. A few studies have also shown that increased superoxide production, formation of peroxynitrite, as well as reduced NO bioavailability diminish the proangiogenic responses of patients with type 1 or type 2 diabetes [282,283]. This further suggests that ROS production in diabetes affects peripheral angiogenesis through different mechanisms. In contrast, in the retina of diabetic patients, ROS production leads to PKC-dependent or AGE-dependent increases in VEGF expression [284,285]. In the retina of diabetic patients the migration of endothelial cells and the proliferation of smooth muscle cells can also be stimulated by H2O2 [285]. While increased peripheral angiogenesis would be welcomed, in the retina the increase in angiogenesis is abnormal and promotes diabetic retinopathy. It therefore appears that the role of ROS signaling in the modulation of angiogenesis is beneficial under physiological conditions. However, under certain circumstances, the induction of pathways that promote angiogenesis are overly enhanced by the presence of oxidative stress and their effects are deleterious [286]. An important issue to consider is that ROS can regulate angiogenesis in several distinct ways, including the modulation of cytokine receptors, the modulation of the expression of genes involved in cell survival and the modulation of multiple signaling pathways involved in vessel formation. A more in-depth review covering the multifaceted roles of ROS in angiogenesis can be found in reference [83].
5. ROS and the Myogenic Response
As mentioned above, it has been postulated that presence and modulation of the myogenic response in resistance vessels is intrinsically associated with the type and level of remodeling these arteries develop in association with hypertension and other pathophysiological conditions [42,287]. Myogenic vasoconstriction is a vascular response in which increased intravascular pressure results in VSMC activation and contraction. The increase in intravascular pressure is usually associated with VSMC stretch or an increase in tension at the anchoring points of the cell with the ECM or neighboring cells. Therefore evidence for an involvement of ROS in myogenic phenomena comes from studies that show VSMC stretching is associated with ROS production [288,289,290]. Those studies further suggest that the primary sources of ROS associated with VSMC stretching are the Noxs [291]. In whole blood vessels, increased intravascular pressure has also been associated with an increased production of ROS [292,293,294,295,296,297]. This appears to take place both in conduit arteries as well as in myogenically active resistance vessels. The addition of exogenous scavengers of ROS to resistance vessels has been shown to inhibit the myogenic vasoconstriction induced by intravascular pressure increases [298]. This suggests that in myogenically active vessels, ROS produced in response to intravascular pressure elevation is associated with signaling pathways that induce VSMC contraction, whereas in conduit vessels (which are not myogenically active) these pathways may not be present. A recent study suggests that in the myogenically active middle cerebral artery, ROS-dependent pathways associated with myogenic vasoconstriction include oxidation of the phosphatase PTEN and activation of the protein kinase Akt [298]. That study also indicates that mitochondria are the main sources that provide ROS during pressure-induced myogenic vasoconstriction. Because other studies indicate that the Noxs are the main sources of ROS in stretched VSMCs or vessels exposed to increased intraluminal pressure, it remains to be determined whether the sources of ROS associated with myogenic vasoconstriction vary depending on the vascular bed studied and the myogenic properties of the vessel.
Recently, it was discovered that G-protein couple receptors are mechanosensitive and that the angiotensin II type 1a receptor (AT1aR) is required for the mesenteric and renal arteries of the mouse to be myogenically active [299]. Because AT1R activation is associated with Nox activation and ROS production, it is likely that myogenic vasoconstriction in these vessels is associated with superoxide production by the Noxs. We recently discovered that ROS activate integrins in VSMCs stimulated with lysophosphatidic acid (LPA) [300]. Furthermore, we showed that ROS produced by isolated resistance arteries stimulated with LPA increase the level of myogenic vasoconstriction of these vessels in response to sequential increments in intravascular pressure. Because we have previously determined that blockade of αvβ3 and α5β1 integrins in these vessels inhibits myogenic vasoconstriction [301], it is tempting to speculate that ROS produced subsequent to stretch activation of AT1aR activates integrins that then participate in promoting a number of the VSMC intracellular changes associated with both myogenic vasoconstriction as well as vascular remodeling phenomena.
6. Conclusions
All cells present within the wall of microvessels produce ROS. The role of these ROS on vascular pathophysiological processes appear to vary depending on the source of ROS within the cell, the specific cell that produces ROS and the overall amount of bioavailable ROS. The scientific literature consistently indicates that while ROS play important roles in physiological cell signaling pathways, excessive production of ROS or a reduced capacity to scavenge these radicals can damage microvessels and induce pathophysiological remodeling of the microcirculation. The remodeling of microvessels induced by ROS modify the structural characteristics of the vascular wall and may include modifications in cytoskeletal structures, cellular attachments, cell phenotype, apotosis and composition of the ECM. A better understanding of the pathways associated with ROS-induced remodeling of microvessels should provide targets for the development of strategies to manipulate vascular remodeling and ameliorate the detrimental consequences of vascular disease.
Acknowledgments
This work was supported by the National Heart, Lung and Blood Institute (R01 HL088105 to Luis A. Martinez-Lemus, and P01 HL095486 to Gerald A. Meininger).
Conflicts of Interest
The authors declare no conflict of interest.
References
- Gu, Y.; Dee, C.M.; Shen, J. Interaction of free radicals, matrix metalloproteinases and caveolin-1 impacts blood-brain barrier permeability. Front. Biosci. Schol. Ed. 2011, 3, 1216–1231. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Horke, S.; Forstermann, U. Vascular oxidative stress, nitric oxide and atherosclerosis. Atherosclerosis 2014, 237, 208–219. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Horke, S.; Forstermann, U. Oxidative stress in vascular disease and its pharmacological prevention. Trends Pharmacol. Sci. 2013, 34, 313–319. [Google Scholar] [CrossRef] [PubMed]
- Montezano, A.C.; Touyz, R.M. Reactive oxygen species, vascular Noxs, and hypertension: Focus on translational and clinical research. Antioxid. Redox Signal. 2014, 20, 164–182. [Google Scholar] [CrossRef] [PubMed]
- Rubattu, S.; Mennuni, S.; Testa, M.; Mennuni, M.; Pierelli, G.; Pagliaro, B.; Gabriele, E.; Coluccia, R.; Autore, C.; Volpe, M. Pathogenesis of chronic cardiorenal syndrome: Is there a role for oxidative stress? Int. J. Mol. Sci. 2013, 14, 23011–23032. [Google Scholar] [CrossRef] [PubMed]
- Rochette, L.; Zeller, M.; Cottin, Y.; Vergely, C. Diabetes, oxidative stress and therapeutic strategies. Biochim. Biophys. Acta 2014, 1840, 2709–2729. [Google Scholar] [CrossRef] [PubMed]
- Hafstad, A.D.; Nabeebaccus, A.A.; Shah, A.M. Novel aspects of ROS signalling in heart failure. Basic Res. Cardiol. 2013, 108, 359. [Google Scholar] [CrossRef]
- Satoh, K.; Nigro, P.; Berk, B.C. Oxidative stress and vascular smooth muscle cell growth: A mechanistic linkage by cyclophilin A. Antioxid. Redox Signal. 2010, 12, 675–682. [Google Scholar] [CrossRef] [PubMed]
- Brandes, R.P.; Weissmann, N.; Schroder, K. Nox family NADPH oxidases in mechano-transduction: Mechanisms and consequences. Antioxid. Redox Signal. 2014, 20, 887–898. [Google Scholar] [CrossRef] [PubMed]
- San Martin, A.; Griendling, K.K. Redox control of vascular smooth muscle migration. Antioxid. Redox Signal. 2010, 12, 625–640. [Google Scholar] [CrossRef] [PubMed]
- Mulvany, M.J.; Aalkjaer, C. Structure and function of small arteries. Physiol. Rev. 1990, 70, 921–961. [Google Scholar] [PubMed]
- Martinez-Lemus, L.A. The dynamic structure of arterioles. Basic. Clin. Pharmacol. Toxicol. 2012, 110, 5–11. [Google Scholar] [CrossRef] [PubMed]
- Martinez-Lemus, L.A.; Hill, M.A.; Meininger, G.A. The plastic nature of the vascular wall: A continuum of remodeling events contributing to control of arteriolar diameter and structure. Physiology (Bethesda) 2009, 24, 45–57. [Google Scholar] [CrossRef]
- Mulvany, M.J.; Baumbach, G.L.; Aalkjaer, C.; Heagerty, A.M.; Korsgaard, N.; Schiffrin, E.L.; Heistad, D.D. Vascular remodeling. Hypertension 1996, 28, 505–506. [Google Scholar] [PubMed]
- Staiculescu, M.C.; Galinanes, E.L.; Zhao, G.; Ulloa, U.; Jin, M.; Beig, M.I.; Meininger, G.A.; Martinez-Lemus, L.A. Prolonged vasoconstriction of resistance arteries involves vascular smooth muscle actin polymerization leading to inward remodelling. Cardiovasc. Res. 2013, 98, 428–436. [Google Scholar] [CrossRef] [PubMed]
- Centers for Disease Control and Prevention (CDC). Vital signs: Prevalence, treatment, and control of hypertension—United States, 1999–2002 and 2005–2008. MMWR Morb. Mortal. Wkly Rep. 2011, 60, 103–108. [Google Scholar]
- Feihl, F.; Liaudet, L.; Levy, B.I.; Waeber, B. Hypertension and microvascular remodelling. Cardiovasc. Res. 2008, 78, 274–285. [Google Scholar] [CrossRef] [PubMed]
- Agabiti-Rosei, E.; Heagerty, A.M.; Rizzoni, D. Effects of antihypertensive treatment on small artery remodelling. J. Hypertens. 2009, 27, 1107–1114. [Google Scholar] [CrossRef] [PubMed]
- Dupuis, F.; Vincent, J.M.; Limiñana, P.; Chillon, J.M.; Capdeville-Atkinson, C.; Atkinson, J. Effects of suboptimal doses of the AT1 receptor blocker, telmisartan, with the angiotensin-converting enzyme inhibitor, ramipril, on cerebral arterioles in spontaneously hypertensive rat. J. Hypertens. 2010, 28, 1566–1573. [Google Scholar] [CrossRef] [PubMed]
- Hassona, M.D. H.; Abouelnaga, Z.A.; Elnakish, M.T.; Awad, M.M.; Alhaj, M.; Goldschmidt-Clermont, P.J.; Hassanain, H.H. Vascular hypertrophy-associated hypertension of profilin1 transgenic mouse model leads to functional remodeling of peripheral arteries. Am. J. Physiol. Heart Circ. Physiol. 2010, 298, H2112–H2120. [Google Scholar] [CrossRef] [PubMed]
- Izzard, A.S.; Horton, S.; Heerkens, E.H.; Shaw, L.; Heagerty, A.M. Middle cerebral artery structure and distensibility during developing and established phases of hypertension in the spontaneously hypertensive rat. J. Hypertens. 2006, 24, 875–880. [Google Scholar] [CrossRef] [PubMed]
- Louis, H.; Kakou, A.; Regnault, V.; Labat, C.; Bressenot, A.; Gao-Li, J.; Gardner, H.; Thornton, S.N.; Challande, P.; Li, Z.; Lacolley, P. Role of α 1β 1-integrin in arterial stiffness and angiotensin-induced arterial wall hypertrophy in mice. Am. J. Physiol. Heart Circ. Physiol. 2007, 293, H2597–H2604. [Google Scholar] [CrossRef] [PubMed]
- Ong, S.S.; Baker, P.N.; Mayhew, T.M.; Dunn, W.R. Remodeling of myometrial radial arteries in preeclampsia. Am. J. Obstet. Gynecol. 2005, 192, 572–579. [Google Scholar] [CrossRef] [PubMed]
- Rizzoni, D.; Porteri, E.; de Ciuceis, C.; Rodella, L.F.; Paiardi, S.; Rizzardi, N.; Platto, C.; Boari, G.E.M.; Pilu, A.; Tiberio, G.A.M.; et al. Hypertrophic remodeling of subcutaneous small resistance arteries in patients with Cushing's syndrome. J. Clin. Endocrinol. Metab. 2009, 94, 5010–5018. [Google Scholar] [CrossRef] [PubMed]
- Rizzoni, D.; Porteri, E.; Giustina, A.; de Ciuceis, C.; Sleiman, I.; Boari, G.E.; Castellano, M.; Muiesan, M.L.; Bonadonna, S.; Burattin, A.; et al. Acromegalic patients show the presence of hypertrophic remodeling of subcutaneous small resistance arteries. Hypertension 2004, 43, 561–565. [Google Scholar] [CrossRef] [PubMed]
- Rizzoni, D.; Rossi, G.P.; Porteri, E.; Sticchi, D.; Rodella, L.; Rezzani, R.; Sleiman, I.; de Ciuceis, C.; Paiardi, S.; Bianchi, R.; et al. Bradykinin and matrix metalloproteinases are involved the structural alterations of rat small resistance arteries with inhibition of ACE and NEP. J. Hypertens. 2004, 22, 759–766. [Google Scholar] [CrossRef] [PubMed]
- Rizzoni, D.; Porteri, E.; Guefi, D.; Piccoli, A.; Castellano, M.; Pasini, G.; Muiesan, M.L.; Mulvany, M.J.; Rosei, E.A. Cellular hypertrophy in subcutaneous small arteries of patients with renovascular hypertension. Hypertension 2000, 35, 931–935. [Google Scholar] [CrossRef] [PubMed]
- Mulvany, M.J. Small artery remodelling in hypertension. Basic Clin. Pharmacol. Toxicol. 2012, 110, 49–55. [Google Scholar] [CrossRef] [PubMed]
- Heagerty, A.M.; Aalkjaer, C.; Bund, S.J.; Korsgaard, N.; Mulvany, M.J. Small artery structure in hypertension: Dual processes of remodeling and growth. Hypertension 1993, 21, 391–397. [Google Scholar] [CrossRef] [PubMed]
- Heagerty, A.M.; Heerkens, E.H.; Izzard, A.S. Small artery structure and function in hypertension. J. Cell. Mol. Med. 2010, 14, 1037–1043. [Google Scholar] [PubMed]
- Rizzoni, D.; Porteri, E.; Castellano, M.; Bettoni, G.; Muiesan, M.L.; Muiesan, P.; Giulini, S.M.; Agabiti-Rosei, E. Vascular hypertrophy and remodeling in secondary hypertension. Hypertension 1996, 28, 785–690. [Google Scholar] [CrossRef] [PubMed]
- Beyer, A.M.; Baumbach, G.L.; Halabi, C.M.; Modrick, M.L.; Lynch, C.M.; Gerhold, T.D.; Ghoneim, S.M.; de Lange, W.J.; Keen, H.L.; Tsai, Y.S.; et al. Interference with PPARγ signaling causes cerebral vascular dysfunction, hypertrophy, and remodeling. Hypertension 2008, 51, 867–871. [Google Scholar] [CrossRef] [PubMed]
- Rizzoni, D.; Porteri, E.; Guelfi, D.; Muiesan, M.L.; Valentini, U.; Cimino, A.; Girelli, A.; Rodella, L.; Bianchi, R.; Sleiman, I.; et al. Structural alterations in subcutaneous small arteries of normotensive and hypertensive patients with non-insulin-dependent diabetes mellitus. Circulation 2001, 103, 1238–1244. [Google Scholar] [CrossRef] [PubMed]
- Rizzoni, D.; Agabiti Rosei, E. Small artery remodeling in hypertension and diabetes. Curr. Hypertens. Rep. 2006, 8, 90–5. [Google Scholar] [CrossRef] [PubMed]
- Vessières, E.; Freidja, M.L.; Loufrani, L.; Fassot, C.; Henrion, D. Flow (shear stress)-mediated remodeling of resistance arteries in diabetes. Vascul. Pharmacol. 2012, 57, 173–178. [Google Scholar] [CrossRef] [PubMed]
- Amaral, S.L.; Michelini, L.C. Effect of gender on training-induced vascular remodeling in SHR. Braz. J. Med. Biol. Res. 2011, 44, 814–826. [Google Scholar] [CrossRef] [PubMed]
- Cipolla, M.J.; Sweet, J.G.; Chan, S.L. Cerebral vascular adaptation to pregnancy and its role in the neurological complications of eclampsia. J. Appl. Physiol. 2011, 110, 329–339. [Google Scholar] [CrossRef] [PubMed]
- Hale, S.A.; Weger, L.; Mandala, M.; Osol, G. Reduced NO signaling during pregnancy attenuates outward uterine artery remodeling by altering MMP expression and collagen and elastin deposition. Am. J. Physiol. Heart Circ. Physiol. 2011, 301, H1266–H1275. [Google Scholar] [CrossRef] [PubMed]
- Tarhouni, K.; Guihot, A.L.; Freidja, M.L.; Toutain, B.; Henrion, B.; Baufreton, C.; Pinaud, F.; Procaccio, V.; Grimaud, L.; Ayer, A.; et al. Key role of estrogens and endothelial estrogen receptor α in blood flow-mediated remodeling of resistance arteries. Arterioscler. Thromb. Vasc. Biol. 2013, 33, 605–611. [Google Scholar] [CrossRef] [PubMed]
- Sonoyama, K.; Greenstein, A.; Price, A.; Khavandi, K.; Heagerty, T. Vascular remodeling: Implications for small artery function and target organ damage. Ther. Adv. Cardiovasc. Dis. 2007, 1, 129–137. [Google Scholar] [CrossRef] [PubMed]
- Hill, M.A.; Meininger, G.A.; Davis, M.J.; Laher, I. Therapeutic potential of pharmacologically targeting arteriolar myogenic tone. Trends Pharmacol. Sci. 2009, 30, 363–374. [Google Scholar] [CrossRef] [PubMed]
- Heerkens, E.H. J.; Izzard, A.S.; Heagerty, A.M. Integrins, vascular remodeling, and hypertension. Hypertension 2007, 49, 1–4. [Google Scholar] [CrossRef] [PubMed]
- Tuna, B.G.; Schoorl, M.J.; Bakker, E.N.; de Vos, J.; VanBavel, E. Smooth muscle contractile plasticity in rat mesenteric small arteries: Sensitivity to specific vasoconstrictors, distension and inflammatory cytokines. J. Vasc. Res. 2013, 50, 249–262. [Google Scholar] [CrossRef] [PubMed]
- Martinez-Lemus, L.A.; Galinanes, E.L. Matrix metalloproteinases and small artery remodeling. Drug Discov. Today Dis. Models 2011, 8, 21–28. [Google Scholar] [CrossRef] [PubMed]
- Nauseef, W.M. Biological roles for the NOX family NADPH oxidases. J. Biol. Chem. 2008, 283, 16961–16965. [Google Scholar] [CrossRef] [PubMed]
- Suh, Y.A.; Arnold, R.S.; Lassegue, B.; Shi, J.; Xu, X.; Sorescu, D.; Chung, A.B.; Griendling, K.K.; Lambeth, J.D. Cell transformation by the superoxide-generating oxidase Mox1. Nature 1999, 401, 79–82. [Google Scholar] [CrossRef] [PubMed]
- Banfi, B.; Malgrange, B.; Knisz, J.; Steger, K.; Dubois-Dauphin, M.; Krause, K.H. NOX3, a superoxide-generating NADPH oxidase of the inner ear. J. Biol. Chem. 2004, 279, 46065–46072. [Google Scholar] [PubMed]
- Cave, A.C.; Brewer, A.C.; Narayanapanicker, A.; Ray, R.; Grieve, D.J.; Walker, S.; Shah, A.M. NADPH oxidases in cardiovascular health and disease. Antioxid. Redox Signal. 2006, 8, 691–728. [Google Scholar] [CrossRef] [PubMed]
- Cheng, G.; Cao, Z.; Xu, X.; van Meir, E.G.; Lambeth, J.D. Homologs of gp91phox: Cloning and tissue expression of Nox3, Nox4, and Nox5. Gene 2001, 269, 131–140. [Google Scholar] [CrossRef] [PubMed]
- Fischer, H. Mechanisms and function of DUOX in epithelia of the lung. Antioxid. Redox Signal. 2009, 11, 2453–2465. [Google Scholar] [CrossRef] [PubMed]
- Krause, K.H. Tissue distribution and putative physiological function of NOX family NADPH oxidases. Jpn. J. Infect. Dis. 2004, 57, S28–S29. [Google Scholar] [PubMed]
- Lassègue, B.; Griendling, K.K. NADPH Oxidases: Functions and Pathologies in the Vasculature. Arterioscler. Thromb. Vasc. Biol. 2010, 30, 653–661. [Google Scholar] [CrossRef] [PubMed]
- Hilenski, L.L.; Clempus, R.E.; Quinn, M.T.; Lambeth, J.D.; Griendling, K.K. Distinct subcellular localizations of nox1 and nox4 in vascular smooth muscle cells. Arterioscler. Thromb. Vasc. Biol. 2004, 24, 677–683. [Google Scholar] [CrossRef] [PubMed]
- Babior, B.M. NADPH oxidase. Curr. Opin. Immunol. 2004, 16, 42–47. [Google Scholar] [CrossRef] [PubMed]
- Miyano, K.; Sumimoto, H. Role of the small GTPase Rac in p22phox-dependent NADPH oxidases. Biochimie 2007, 89, 1133–44. [Google Scholar] [CrossRef] [PubMed]
- Hordijk, P.L. Regulation of NADPH oxidases: The role of Rac proteins. Circ. Res. 2006, 98, 453–462. [Google Scholar] [CrossRef] [PubMed]
- Janiszewski, M.; Lopes, L.R.; Carmo, A.O.; Pedro, M.A.; Brandes, R.P.; Santos, C.X.; Laurindo, F.R. Regulation of NAD(P)H oxidase by associated protein disulfide isomerase in vascular smooth muscle cells. J. Biol. Chem. 2005, 280, 40813–40819. [Google Scholar] [PubMed]
- Miller, F.J., Jr.; Filali, M.; Huss, G.J.; Stanic, B.; Chamseddine, A.; Barna, T.J.; Lamb, F.S. Cytokine activation of nuclear factor kappa B in vascular smooth muscle cells requires signaling endosomes containing Nox1 and ClC-3. Circ. Res. 2007, 101, 663–671. [Google Scholar] [CrossRef] [PubMed]
- Moreland, J.G.; Davis, A.P.; Matsuda, J.J.; Hook, J.S.; Bailey, G.; Nauseef, W.M.; Lamb, F.S. Endotoxin priming of neutrophils requires NADPH oxidase-generated oxidants and is regulated by the anion transporter ClC-3. J. Biol. Chem. 2007, 282, 33958–33967. [Google Scholar] [CrossRef] [PubMed]
- Lyle, A.N.; Deshpande, N.N.; Taniyama, Y.; Seidel-Rogol, B.; Pounkova, L.; Du, P.; Papaharalambus, C.; Lassegue, B.; Griendling, K.K. Poldip2, a novel regulator of Nox4 and cytoskeletal integrity in vascular smooth muscle cells. Circ. Res. 2009, 105, 249–259. [Google Scholar] [CrossRef] [PubMed]
- Manickam, N.; Patel, M.; Griendling, K.K.; Gorin, Y.; Barnes, J.L. RhoA/Rho kinase mediates TGF-β-induced kidney myofibroblast activation through Poldip2/Nox4-derived reactive oxygen species. Am. J. Physiol. Renal. Physiol. 2014, 307. [Google Scholar] [CrossRef]
- Martinez-Lemus, L.A.; Zhao, G.; Galiñanes, E.L.; Boone, M. Inward remodeling of resistance arteries requires reactive oxygen species-dependent activation of matrix metalloproteinases. Am. J. Physiol. Heart Circ. Physiol. 2011, 300, H2005–H2015. [Google Scholar] [CrossRef] [PubMed]
- Brandes, R.P.; Kreuzer, J. Vascular NADPH oxidases: Molecular mechanisms of activation. Cardiovasc. Res. 2005, 65, 16–27. [Google Scholar] [CrossRef] [PubMed]
- Brandes, R.P.; Schroder, K. Differential vascular functions of Nox family NADPH oxidases. Curr. Opin. Lipidol. 2008, 19, 513–518. [Google Scholar] [CrossRef] [PubMed]
- Brandes, R.P.; Weissmann, N.; Schroder, K. Nox family NADPH oxidases: Molecular mechanisms of activation. Free Radic. Biol. Med. 2014, 76, 208–226. [Google Scholar] [CrossRef]
- Martinez-Lemus, L.A. Persistent agonist-induced vasoconstriction is not required for angiotensin II to mediate inward remodeling of isolated arterioles with myogenic tone. J. Vasc. Res. 2008, 45, 211–221. [Google Scholar] [CrossRef] [PubMed]
- Briones, A.M.; Tabet, F.; Callera, G.E.; Montezano, A.C.; Yogi, A.; He, Y.; Quinn, M.T.; Salaices, M.; Touyz, R.M. Differential regulation of Nox1, Nox2 and Nox4 in vascular smooth muscle cells from WKY and SHR. J. Am. Soc. Hypertens. 2011, 5, 137–153. [Google Scholar] [CrossRef] [PubMed]
- Beswick, R.A.; Dorrance, A.M.; Leite, R.; Webb, R.C. NADH/NADPH oxidase and enhanced superoxide production in the mineralocorticoid hypertensive rat. Hypertension 2001, 38, 1107–1111. [Google Scholar] [CrossRef] [PubMed]
- Fujii, A.; Nakano, D.; Katsuragi, M.; Ohkita, M.; Takaoka, M.; Ohno, Y.; Matsumura, Y. Role of gp91phox-containing NADPH oxidase in the deoxycorticosterone acetate-salt-induced hypertension. Eur. J. Pharmcol. 2006, 552, 131–134. [Google Scholar] [CrossRef]
- Polichnowski, A.J.; Jin, C.; Yang, C.; Cowley, A.W. Role of renal perfusion pressure versus angiotensin ii on renal oxidative stress in angiotensin II-induced hypertensive rats. Hypertension 2010, 55, 1425–1430. [Google Scholar] [CrossRef] [PubMed]
- Zhou, M.S.; Jaimes, E.A.; Raij, L. Vascular but not cardiac remodeling is associated with superoxide production in angiotensin II hypertension. J. Hypertens. 2005, 23, 1737–1743. [Google Scholar] [CrossRef] [PubMed]
- Bonacasa, B.; Sanchez, M.L.; Rodriguez, F.; Lopez, B.; Quesada, T.; Fenoy, F.J.; Hernandez, I. 2-Methoxyestradiol attenuates hypertension and coronary vascular remodeling in spontaneously hypertensive rats. Maturitas 2008, 61, 310–316. [Google Scholar] [CrossRef] [PubMed]
- Chignalia, A.Z.; Schuldt, E.Z.; Camargo, L.L.; Montezano, A.C.; Callera, G.E.; Laurindo, F.R.; Lopes, L.R.; Avellar, M.C.W.; Carvalho, M.H.C.; Fortes, Z.B.; et al. Testosterone induces vascular smooth muscle cell migration by NADPH oxidase and c-Src-dependent pathways. Hypertension 2012, 59, 1263–1271. [Google Scholar] [CrossRef] [PubMed]
- Diep, Q.N.; Amiri, F.; Touyz, R.M.; Cohn, J.S.; Endemann, D.; Neves, M.F.; Schiffrin, E.L. PPARα activator effects on Ang II-induced vascular oxidative stress and inflammation. Hypertension 2002, 40, 866–871. [Google Scholar] [CrossRef] [PubMed]
- Marchesi, C.; Rehman, A.; Rautureau, Y.; Kasal, D.A.; Briet, M.; Leibowitz, A.; Simeone, S.M.C.; Ebrahimian, T.; Neves, M.F.; Offermanns, S.; et al. Protective role of vascular smooth muscle cell PPARγ in angiotensin II-induced vascular disease. Cardiovasc. Res. 2013, 97, 562–570. [Google Scholar] [CrossRef] [PubMed]
- Carlström, M.; Lai, E.Y.; Ma, Z.; Steege, A.; Patzak, A.; Eriksson, U.J.; Lundberg, J.O.; Wilcox, C.S.; Persson, A.E.G. Superoxide dismutase 1 limits renal microvascular remodeling and attenuates arteriole and blood pressure responses to angiotensin II via modulation of nitric oxide bioavailability. Hypertension 2010, 56, 907–913. [Google Scholar] [CrossRef] [PubMed]
- Virdis, A.; Neves, M.F.; Amiri, F.; Touyz, R.M.; Schiffrin, E.L. Role of NAD(P)H oxidase on vascular alterations in angiotensin II-infused mice. J. Hypertens. 2004, 22, 535–542. [Google Scholar] [CrossRef] [PubMed]
- Ito, N.; Ohishi, M.; Yamamoto, K.; Tatara, Y.; Shiota, A.; Hayashi, N.; Komai, N.; Yanagitani, Y.; Rakugi, H.; Ogihara, T. Renin-angiotensin inhibition reverses advanced cardiac remodeling in aging spontaneously hypertensive rats. Am. J. Hypertens. 2007, 20, 792–799. [Google Scholar] [CrossRef] [PubMed]
- Briones, A.M.; Rodriguez-Criado, N.; Hernanz, R.; Garcia-Redondo, A.B.; Rodrigues-Diez, R.R.; Alonso, M.J.; Egido, J.; Ruiz-Ortega, M.; Salaices, M. Atorvastatin prevents angiotensin II-induced vascular remodeling and oxidative stress. Hypertension 2009, 54, 142–149. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.-X.; Zeng, H.; Tuo, Q.-H.; Yu, H.; Meyrick, B.; Aschner, J.L. NADPH oxidase modulates myocardial Akt, ERK1/2 activation, and angiogenesis after hypoxia-reoxygenation. Am. J. Physiol. Heart Circ. Physiol. 2007, 292, H1664–H1674. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.M.; Kim, K.E.; Koh, G.Y.; Ho, Y.-S.; Lee, K.-J. Hydrogen peroxide produced by angiopoietin-1 mediates angiogenesis. Cancer Res. 2006, 66, 6167–6174. [Google Scholar] [CrossRef] [PubMed]
- Ago, T.; Kuroda, J.; Kamouchi, M.; Sadoshima, J.; Kitazono, T. Pathophysiological roles of NADPH oxidase/Nox family proteins in the vascular system review and perspective. Circ. J. 2011, 75, 1791–1800. [Google Scholar] [CrossRef] [PubMed]
- Bir, S.C.; Kolluru, G.K.; Fang, K.; Kevil, C.G. Redox balance dynamically regulates vascular growth and remodeling. Semin. Cell Dev. Biol. 2012, 23, 745–757. [Google Scholar] [CrossRef] [PubMed]
- Wilkinson-Berka, J.L.; Rana, I.; Armani, R.; Agrotis, A. Reactive oxygen species, Nox and angiotensin II in angiogenesis: Implications for retinopathy. Clin. Sci. 2013, 124, 597–615. [Google Scholar] [CrossRef] [PubMed]
- Catani, M.V.; Bernassola, F.; Rossi, A.; Melino, G. Inhibition of clotting factor XIII activity by nitric oxide. Biochem. Biophys. Res. Commun. 1998, 249, 275–278. [Google Scholar] [CrossRef] [PubMed]
- Kubes, P.; Kanwar, S.; Niu, X.F.; Gaboury, J.P. Nitric oxide synthesis inhibition induces leukocyte adhesion via superoxide and mast cells. FASEB J. 1993, 7, 1293–1299. [Google Scholar] [PubMed]
- Kurose, I.; Wolf, R.; Grisham, M.B.; Tak Yee, A.; Specian, R.D.; Granger, D.N. Microvascular responses to inhibition of nitric oxide production: Role of active oxidants. Circ. Res. 1995, 76, 30–39. [Google Scholar] [CrossRef] [PubMed]
- Zou, M.H.; Cohen, R.A.; Ullrich, V. Peroxynitrite and vascular endothelial dysfunction in diabetes mellitus. Endothelium 2004, 11, 89–97. [Google Scholar] [CrossRef] [PubMed]
- Tsihlis, N.D.; Oustwani, C.S.; Vavra, A.K.; Jiang, Q.; Keefer, L.K.; Kibbe, M.R. Nitric oxide inhibits vascular smooth muscle cell proliferation and neointimal hyperplasia by increasing the ubiquitination and degradation of ubcH10. Cell. Biochem. Biophys. 2011, 60, 89–97. [Google Scholar] [CrossRef] [PubMed]
- Marchesi, C.; Ebrahimian, T.; Angulo, O.; Paradis, P.; Schiffrin, E.L. Endothelial nitric oxide synthase uncoupling and perivascular adipose oxidative stress and inflammation contribute to vascular dysfunction in a rodent model of metabolic syndrome. Hypertension 2009, 54, 1384–1392. [Google Scholar] [CrossRef] [PubMed]
- Chandra, S.; Romero, M.J.; Shatanawi, A.; Alkilany, A.M.; Caldwell, R.B.; Caldwell, R.W. Oxidative species increase arginase activity in endothelial cells through the RhoA/Rho kinase pathway. Br. J. Pharmacol. 2012, 165, 506–519. [Google Scholar] [CrossRef] [PubMed]
- Shin, W.; Berkowitz, D.E.; Ryoo, S. Increased arginase II activity contributes to endothelial dysfunction through endothelial nitric oxide synthase uncoupling in aged mice. Exp. Mol. Med. 2012, 44, 594–602. [Google Scholar] [CrossRef] [PubMed]
- Werner-Felmayer, G.; Werner, E.R.; Fuchs, D.; Hausen, A.; Reibnegger, G.; Schmidt, K.; Weiss, G.; Wachter, H. Pteridine biosynthesis in human endothelial cells. Impact on nitric oxide-mediated formation of cyclic GMP. J. Biol. Chem. 1993, 268, 1842–1846. [Google Scholar] [PubMed]
- Landmesser, U.; Dikalov, S.; Price, S.R.; McCann, L.; Fukai, T.; Holland, S.M.; Mitch, W.E.; Harrison, D.G. Oxidation of tetrahydrobiopterin leads to uncoupling of endothelial cell nitric oxide synthase in hypertension. J. Clin. Investig. 2003, 111, 1201–1209. [Google Scholar] [CrossRef] [PubMed]
- Shinozaki, K.; Kashiwagi, A.; Nishio, Y.; Okamura, T.; Yoshida, Y.; Masada, M.; Toda, N.; Kikkawa, R. Abnormal biopterin metabolism is a major cause of impaired endothelium-dependent relaxation through nitric oxide/O2− imbalance in insulin-resistant rat aorta. Diabetes 1999, 48, 2437–2445. [Google Scholar] [CrossRef] [PubMed]
- Hong, H.-J.; Hsiao, G.; Cheng, T.-H.; Yen, M.-H. Supplemention with tetrahydrobiopterin suppresses the development of hypertension in spontaneously hypertensive rats. Hypertension 2001, 38, 1044–1048. [Google Scholar] [CrossRef] [PubMed]
- Heitzer, T.; Krohn, K.; Albers, S.; Meinertz, T. Tetrahydrobiopterin improves endothelium-dependent vasodilation by increasing nitric oxide activity in patients with Type II diabetes mellitus. Diabetologia 2000, 43, 1435–1438. [Google Scholar] [CrossRef] [PubMed]
- Higashi, Y.; Sasaki, S.; Nakagawa, K.; Fukuda, Y.; Matsuura, H.; Oshima, T.; Chayama, K. Tetrahydrobiopterin enhances forearm vascular response to acetylcholine in both normotensive and hypertensive individuals. Am. J. Hypertens. 2002, 15, 326–332. [Google Scholar] [CrossRef] [PubMed]
- Milstien, S.; Katusic, Z. Oxidation of tetrahydrobiopterin by peroxynitrite: Implications for vascular endothelial function. Biochem. Biophys. Res. Commun. 1999, 263, 681–684. [Google Scholar] [CrossRef] [PubMed]
- Laursen, J.B.; Somers, M.; Kurz, S.; McCann, L.; Warnholtz, A.; Freeman, B.A.; Tarpey, M.; Fukai, T.; Harrison, D.G. Endothelial regulation of vasomotion in apoE-deficient mice: Implications for interactions between peroxynitrite and tetrahydrobiopterin. Circulation 2001, 103, 1282–1288. [Google Scholar] [CrossRef] [PubMed]
- Du, Y.H.; Guan, Y.Y.; Alp, N.J.; Channon, K.M.; Chen, A.F. Endothelium-specific GTP cyclohydrolase i overexpression attenuates blood pressure progression in salt-sensitive low-renin hypertension. Circulation 2008, 117, 1045–1054. [Google Scholar] [CrossRef] [PubMed]
- Crabtree, M.J.; Tatham, A.L.; Hale, A.B.; Alp, N.J.; Channon, K.M. Critical role for tetrahydrobiopterin recycling by dihydrofolate reductase in regulation of endothelial nitric-oxide synthase coupling: Relative importance of the de novo biopterin synthesis versus salvage pathways. J. Biol. Chem. 2009, 284, 28128–28136. [Google Scholar] [CrossRef] [PubMed]
- Chalupsky, K.; Cai, H. Endothelial dihydrofolate reductase: Critical for nitric oxide bioavailability and role in angiotensin II uncoupling of endothelial nitric oxide synthase. Proc. Natl. Acad. Sci. USA 2005, 102, 9056–9061. [Google Scholar] [CrossRef] [PubMed]
- Youn, J.Y.; Gao, L.; Cai, H. The p47 phox- and NADPH oxidase organiser 1 (NOXO1)-dependent activation of NADPH oxidase 1 (NOX1) mediates endothelial nitric oxide synthase (eNOS) uncoupling and endothelial dysfunction in a streptozotocin-induced murine model of diabetes. Diabetologia 2012, 55, 2069–2079. [Google Scholar] [CrossRef] [PubMed]
- Stipek, S.; Novak, L.; Crkovska, J.; Zima, T.; Platenik, J. Xanthine oxidoreductase. Biochemical, biological and pathogenic functions. Sb. Lek. 1994, 95, 289–295. [Google Scholar] [PubMed]
- Garattini, E.; Mendel, R.; Romão, M.J.; Wright, R.; Terao, M. Mammalian molybdo-flavoenzymes, an expanding family of proteins: Structure, genetics, regulation, function and pathophysiology. Biochem. J. 2003, 372, 15–32. [Google Scholar] [CrossRef] [PubMed]
- Berry, C.E.; Hare, J.M. Xanthine oxidoreductase and cardiovascular disease: Molecular mechanisms and pathophysiological implications. J. Physiol. 2004, 555, 589–606. [Google Scholar] [CrossRef] [PubMed]
- Sakuma, S.; Fujimoto, Y.; Sakamoto, Y.; Uchiyama, T.; Yoshioka, K.; Nishida, H.; Fujita, T. Peroxynitrite induces the conversion of xanthine dehydrogenase to oxidase in rabbit liver. Biochem. Biophys. Res. Commun. 1997, 230, 476–479. [Google Scholar] [CrossRef] [PubMed]
- Kuwabara, Y.; Nishino, T.; Okamoto, K.; Matsumura, T.; Eger, B.T.; Pai, E.F. Unique amino acids cluster for switching from the dehydrogenase to oxidase form of xanthine oxidoreductase. Proc. Natl. Acad. Sci. USA 2003, 100, 8170–8175. [Google Scholar] [CrossRef] [PubMed]
- Kelley, E.E.; Khoo, N.K. H.; Hundley, N.J.; Malik, U.Z.; Freeman, B.A.; Tarpey, M.M. Hydrogen peroxide is the major oxidant product of xanthine oxidase. Free Radic. Biol. Med. 2010, 48, 493–498. [Google Scholar] [CrossRef] [PubMed]
- Landmesser, U.; Spiekermann, S.; Preuss, C.; Sorrentino, S.; Fischer, D.; Manes, C.; Mueller, M.; Drexler, H. Angiotensin II induces endothelial xanthine oxidase activation: Role for endothelial dysfunction in patients with coronary disease. Arterioscler. Thromb. Vasc. Biol. 2007, 27, 943–948. [Google Scholar] [CrossRef] [PubMed]
- McNally, J.S.; Davis, M.E.; Giddens, D.P.; Saha, A.; Hwang, J.; Dikalov, S.; Jo, H.; Harrison, D.G. Role of xanthine oxidoreductase and NAD(P)H oxidase in endothelial superoxide production in response to oscillatory shear stress. Am. J. Physiol. Heart Circ. Physiol. 2003, 285, H2290–H2297. [Google Scholar] [PubMed]
- Kou, B.; Ni, J.; Vatish, M.; Singer, D.R. Xanthine oxidase interaction with vascular endothelial growth factor in human endothelial cell angiogenesis. Microcirculation 2008, 15, 251–267. [Google Scholar] [CrossRef] [PubMed]
- Jankov, R.P.; Kantores, C.; Pan, J.; Belik, J. Contribution of xanthine oxidase-derived superoxide to chronic hypoxic pulmonary hypertension in neonatal rats. Am. J. Physiol. Lung Cell. Mol. Physiol. 2008, 294, L233–L245. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, H.; Delano, F.A.; Parks, D.A.; Jamshidi, N.; Granger, D.N.; Ishii, H.; Suematsu, M.; Zweifach, B.W.; Schmid-Schönbein, G.W. Xanthine oxidase activity associated with arterial blood pressure in spontaneously hypertensive rats. Proc. Natl. Acad. Sci. USA 1998, 95, 4754–4759. [Google Scholar] [CrossRef] [PubMed]
- Swei, A.; Lacy, F.; Delano, F.A.; Parks, D.A.; Schmid-Schönbein, G.W. A mechanism of oxygen free radical production in the Dahl hypertensive rat. Microcirculation 1999, 6, 179–187. [Google Scholar] [CrossRef] [PubMed]
- Ong, S.L. H.; Vickers, J.J.; Zhang, Y.; McKenzie, K.U. S.; Walsh, C.E.; Whitworth, J.A. Role of xanthine oxidase in dexamethasone-induced hypertension in rats. Clin. Exp. Pharmacol. Physiol. 2007, 34, 517–519. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Wu, Y.; Wu, C.; Gunst, S.J. Integrin-linked kinase regulates N-WASp-mediated actin polymerization and tension development in tracheal smooth muscle. J. Biol. Chem. 2007, 282, 34568–34580. [Google Scholar] [CrossRef] [PubMed]
- Lustgarten, M.S.; Bhattacharya, A.; Muller, F.L.; Jang, Y.C.; Shimizu, T.; Shirasawa, T.; Richardson, A.; van Remmen, H. Complex I generated, mitochondrial matrix-directed superoxide is released from the mitochondria through voltage dependent anion channels. Biochem. Biophys. Res. Commun. 2012, 422, 515–521. [Google Scholar] [CrossRef] [PubMed]
- Aon, M.A.; Cortassa, S.; O’Rourke, B. Percolation and criticality in a mitochondrial network. Proc. Natl. Acad. Sci. USA 2004, 101, 4447–4452. [Google Scholar] [CrossRef] [PubMed]
- Xia, C.; Meng, Q.; Liu, L.Z.; Rojanasakul, Y.; Wang, X.R.; Jiang, B.H. Reactive oxygen species regulate angiogenesis and tumor growth through vascular endothelial growth factor. Cancer Res. 2007, 67, 10823–10830. [Google Scholar] [CrossRef] [PubMed]
- Rathore, R.; Zheng, Y.-M.; Niu, C.-F.; Liu, Q.-H.; Korde, A.; Ho, Y.-S.; Wang, Y.-X. Hypoxia activates NADPH oxidase to increase [ROS]i and [Ca2+]i through the mitochondrial ROS-PKCɛ signaling axis in pulmonary artery smooth muscle cells. Free Radic. Biol. Med. 2008, 45, 1223–1231. [Google Scholar] [CrossRef] [PubMed]
- Viel, E.C.; Benkirane, K.; Javeshghani, D.; Touyz, R.M.; Schiffrin, E.L. Xanthine oxidase and mitochondria contribute to vascular superoxide anion generation in DOCA-salt hypertensive rats. Am. J. Physiol. Heart Circ. Physiol. 2008, 295, H281–H288. [Google Scholar] [CrossRef] [PubMed]
- Choi, H.; Tostes, R.C.; Webb, R.C. Mitochondrial aldehyde dehydrogenase prevents ROS-induced vascular contraction in angiotensin-II hypertensive mice. J. Am. Soc. Hypertens. 2011, 5, 154–160. [Google Scholar] [CrossRef] [PubMed]
- Doughan, A.K.; Harrison, D.G.; Dikalov, S.I. Molecular mechanisms of angiotensin II-mediated mitochondrial dysfunction: Linking mitochondrial oxidative damage and vascular endothelial dysfunction. Circ. Res. 2008, 102, 488–496. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.N.; Shi, N.; Chen, S.Y. Manganese superoxide dismutase inhibits neointima formation through attenuation of migration and proliferation of vascular smooth muscle cells. Free Radic. Biol. Med. 2012, 52, 173–181. [Google Scholar] [CrossRef] [PubMed]
- Intengan, H.D.; Schiffrin, E.L. Vascular remodeling in hypertension: Roles of apoptosis, inflammation, and fibrosis. Hypertension 2001, 38, 581–587. [Google Scholar] [CrossRef] [PubMed]
- Kumai, Y.; Ooboshi, H.; Ago, T.; Ishikawa, E.; Takada, J.; Kamouchi, M.; Kitazono, T.; Ibayashi, S.; Iida, M. Protective effects of angiotensin II Type 1 receptor blocker on cerebral circulation independent of blood pressure. Exp. Neurol. 2008, 210, 441–448. [Google Scholar] [CrossRef] [PubMed]
- Bonacasa, B.; Hernández, I.; Fenoy, F.J.; Quesada, T.; López, B. Effect of tempol on myocardial vascular remodeling in female spontaneously hypertensive rats. Histol. Histopathol. 2012, 27, 1047–1054. [Google Scholar] [PubMed]
- Pires, P.W.; Deutsch, C.; McClain, J.L.; Rogers, C.T.; Dorrance, A.M. Tempol, a superoxide dismutase mimetic, prevents cerebral vessel remodeling in hypertensive rats. Microvasc. Res. 2010, 80, 445–452. [Google Scholar] [CrossRef] [PubMed]
- Touyz, R.M.; Schiffrin, E.L. Increased generation of superoxide by angiotensin II in smooth muscle cells from resistance arteries of hypertensive patients: Role of phospholipase d-dependent NAD(P)H oxidase-sensitive pathways. J. Hypertens. 2001, 19, 1245–1254. [Google Scholar] [CrossRef] [PubMed]
- Belin De Chantemèle, E.J.; Vessières, E.; Guihot, A.L.; Toutain, B.; Maquignau, M.; Loufrani, L.; Henrion, D. Type 2 diabetes severely impairs structural and functional adaptation of rat resistance arteries to chronic changes in blood flow. Cardiovasc. Res. 2009, 81, 788–796. [Google Scholar] [CrossRef] [PubMed]
- Baron-Menguy, C.; Toutain, B.; Cousin, M.; Dumont, O.; Guihot, A.L.; Vessières, E.; Subra, J.F.; Custaud, M.A.; Loufrani, L.; Henrion, D. Involvement of angiotensin II in the remodeling induced by a chronic decrease in blood flow in rat mesenteric resistance arteries. Hypertens. Res. 2010, 33, 857–866. [Google Scholar] [CrossRef] [PubMed]
- Freidja, M.L.; Vessieres, E.; Clere, N.; Desquiret, V.; Guihot, A.L.; Toutain, B.; Loufrani, L.; Jardel, A.; Procaccio, V.; Faure, S.; et al. Heme oxygenase-1 induction restores high-blood-flow-dependent remodeling and endothelial function in mesenteric arteries of old rats. J. Hypertens. 2011, 29, 102–112. [Google Scholar] [CrossRef] [PubMed]
- Freidja, M.L.; Toutain, B.; Caillon, A.; Desquiret, V.; Lambert, D.; Loufrani, L.; Procaccio, V.; Henrion, D. Heme oxygenase 1 is differentially involved in blood flow-dependent arterial remodeling: Role of inflammation, oxidative stress, and nitric oxide. Hypertension 2011, 58, 225–231. [Google Scholar] [CrossRef] [PubMed]
- Cousin, M.; Custaud, M.A.; Baron-Menguy, C.; Toutain, B.; Dumont, O.; Guihot, A.L.; Vessieres, E.; Subra, J.F.; Henrion, D.; Loufrani, L. Role of angiotensin II in the remodeling induced by a chronic increase in flow in rat mesenteric resistance arteries. Hypertension 2010, 55, 109–115. [Google Scholar] [CrossRef] [PubMed]
- Bouvet, C.; de Chantemèle, E.B.; Guihot, A.L.; Vessières, E.; Bocquet, A.; Dumont, O.; Jardel, A.; Loufrani, L.; Moreau, P.; Henrion, D. Flow-induced remodeling in resistance arteries from obese Zucker rats is associated with endothelial dysfunction. Hypertension 2007, 50, 248–254. [Google Scholar] [CrossRef] [PubMed]
- Belin De Chanteméle, E.; Vessiéres, E.; Dumont, O.; Guihot, A.L.; Toutain, B.; Loufrani, L.; Henrion, D. Reactive oxygen species are necessary for high flow (shear Stress)-induced diameter enlargement of rat resistance arteries. Microcirculation 2009, 16, 391–402. [Google Scholar] [CrossRef] [PubMed]
- Lv, P.; Miao, S.B.; Shu, Y.N.; Dong, L.H.; Liu, G.; Xie, X.L.; Gao, M.; Wang, Y.C.; Yin, Y.J.; Wang, X.J.; et al. Phosphorylation of smooth muscle 22α facilitates angiotensin II-induced ROS production via activation of the PKCδ-P47phox axis through release of PKCδ and actin dynamics and is associated with hypertrophy and hyperplasia of vascular smooth muscle cells in vitro and in vivo. Circ. Res. 2012, 111, 697–707. [Google Scholar] [CrossRef] [PubMed]
- Murdoch, C.E.; Alom-Ruiz, S.P.; Wang, M.; Zhang, M.; Walker, S.; Yu, B.; Brewer, A.; Shah, A.M. Role of endothelial Nox2 NADPH oxidase in angiotensin II-induced hypertension and vasomotor dysfunction. Basic Res. Cardiol. 2011, 106, 527–538. [Google Scholar] [CrossRef] [PubMed]
- Sakurada, T.; Ishizawa, K.; Imanishi, M.; Izawa-Ishizawa, Y.; Fujii, S.; Tominaga, E.; Tsuneishi, T.; Horinouchi, Y.; Kihira, Y.; Ikeda, Y.; et al. Nitrosonifedipine ameliorates angiotensin II-induced vascular remodeling via antioxidative effects. Naunyn. Schmiedebergs Arch. Pharmacol. 2013, 386, 29–39. [Google Scholar] [CrossRef] [PubMed]
- Bakker, E.N.T.P.; Matlung, H.L.; Bonta, P.; de Vries, C.J.; van Rooijen, N.; Vanbavel, E. Blood flow-dependent arterial remodelling is facilitated by inflammation but directed by vascular tone. Cardiovasc. Res. 2008, 78, 341–348. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Pakeerappa, P.; Lee, H.J.; Fisher, S.A. Induction of PDE5 and de-sensitization to endogenous NO signaling in a systemic resistance artery under altered blood flow. J. Mol. Cell. Cardiol. 2009, 47, 57–65. [Google Scholar] [CrossRef] [PubMed]
- Haas, T.L.; Doyle, J.L.; Distasi, M.R.; Norton, L.E.; Sheridan, K.M.; Unthank, J.L. Involvement of MMPs in the outward remodeling of collateral mesenteric arteries. Am. J. Physiol. Heart Circ. Physiol. 2007, 293, H2429–H2437. [Google Scholar] [CrossRef] [PubMed]
- Castier, Y.; Brandes, R.P.; Leseche, G.; Tedgui, A.; Lehoux, S. p47phox-dependent NADPH oxidase regulates flow-induced vascular remodeling. Circ. Res. 2005, 97, 533–540. [Google Scholar] [CrossRef] [PubMed]
- Freidja, M.L.; Tarhouni, K.; Toutain, B.; Fassot, C.; Loufrani, L.; Henrion, D. The AGE-breaker ALT-711 restores high blood flow-dependent remodeling in mesenteric resistance arteries in a rat model of type 2 diabetes. Diabetes 2012, 61, 1562–1572. [Google Scholar] [CrossRef] [PubMed]
- Gao, L.; Mann, G.E. Vascular NAD(P)H oxidase activation in diabetes: A double-edged sword in redox signalling. Cardiovasc. Res. 2009, 82, 9–20. [Google Scholar] [CrossRef] [PubMed]
- Rensen, S.S.M.; Doevendans, P.A.F.M.; van Eys, G.J.J.M. Regulation and characteristics of vascular smooth muscle cell phenotypic diversity. Neth. Heart J. 2007, 15, 100–108. [Google Scholar] [CrossRef] [PubMed]
- Hu, W.Y.; Fukuda, N.; Kanmatsuse, K. Growth characteristics, angiotensin II generation, and microarray-determined gene expression in vascular smooth muscle cells from young spontaneously hypertensive rats. J. Hypertens. 2002, 20, 1323–1333. [Google Scholar] [CrossRef] [PubMed]
- Lin, Z.H.; Fukuda, N.; Jin, X.Q.; Yao, E.H.; Ueno, T.; Endo, M.; Saito, S.; Matsumoto, K.; Mugishima, H. Complement 3 is involved in the synthetic phenotype and exaggerated growth of vascular smooth muscle cells from spontaneously hypertensive rats. Hypertension 2004, 44, 42–47. [Google Scholar] [CrossRef] [PubMed]
- Fujita, Y.; Lin, J.X.; Takahashi, R.; Tomimoto, H. Cilostazol alleviates cerebral small-vessel pathology and white-matter lesions in stroke-prone spontaneously hypertensive rats. Brain Res. 2008, 1203, 170–176. [Google Scholar] [CrossRef] [PubMed]
- Nagai, Y.; Saito, Y.; Hamada, K.; Hara, N.; Nakanishi, K.; Masaki, K.; Tanaka, M.; Ger, Y.C.; Nakamura, K. Renal vascular walls in patients with preeclampsia superimposed on essential hypertension. Am. J. Kidney Dis. 2001, 37, 728–735. [Google Scholar] [CrossRef] [PubMed]
- Quarck, R.; Wynants, M.; Ronisz, A.; Sepulveda, M.R.; Wuytack, F.; van Raemdonck, D.; Meyns, B.; Delcroix, M. Characterization of proximal pulmonary arterial cells from chronic thromboembolic pulmonary hypertension patients. Respir. Res. 2012, 13. [Google Scholar] [CrossRef]
- Owens, G.K.; Kumar, M.S.; Wamhoff, B.R. Molecular regulation of vascular smooth muscle cell differentiation in development and disease. Physiol. Rev. 2004, 84, 767–801. [Google Scholar] [CrossRef] [PubMed]
- Martinez-Lemus, L.A.; Hill, M.A.; Bolz, S.S.; Pohl, U.; Meininger, G.A. Acute mechanoadaptation of vascular smooth muscle cells in response to continuous arteriolar vasoconstriction: Implications for functional remodeling. FASEB J. 2004, 18, 708–710. [Google Scholar] [PubMed]
- De la Cuesta, F.; Zubiri, I.; Maroto, A.S.; Posada, M.; Padial, L.R.; Vivanco, F.; Alvarez-Llamas, G.; Barderas, M.G. Deregulation of smooth muscle cell cytoskeleton within the human atherosclerotic coronary media layer. J. Proteomics 2013, 82, 155–165. [Google Scholar] [CrossRef] [PubMed]
- Itoh, S.; Umemoto, S.; Hiromoto, M.; Toma, Y.; Tomochika, Y.; Aoyagi, S.; Tanaka, M.; Fujii, T.; Matsuzaki, M. Importance of NAD(P)H oxidase-mediated oxidative stress and contractile type smooth muscle myosin heavy chain SM2 at the early stage of atherosclerosis. Circulation 2002, 105, 2288–2295. [Google Scholar] [CrossRef] [PubMed]
- Absood, A.; Furutani, A.; Kawamura, T.; Graham, L.M. Differential PDGF secretion by graft and aortic SMC in response to oxidized LDL. Am. J. Physiol. Heart Circ. Physiol. 2002, 283, H725–H732. [Google Scholar] [PubMed]
- Ashino, T.; Yamamoto, M.; Yoshida, T.; Numazawa, S. Redox-sensitive transcription factor Nrf2 regulates vascular smooth muscle cell migration and neointimal hyperplasia. Arterioscler. Thromb. Vasc. Biol. 2013, 33, 760–768. [Google Scholar] [CrossRef] [PubMed]
- Patel, R.; Cardneau, J.D.; Colles, S.M.; Graham, L.M. Synthetic smooth muscle cell phenotype is associated with increased nicotinamide adenine dinucleotide phosphate oxidase activity: Effect on collagen secretion. J. Vasc. Surg. 2006, 43, 364–371. [Google Scholar] [CrossRef] [PubMed]
- Touré, F.; Fritz, G.; Li, Q.; Rai, V.; Daffu, G.; Zou, Y.S.; Rosario, R.; Ramasamy, R.; Alberts, A.S.; Yan, S.F.; et al. Formin mDia1 mediates vascular remodeling via integration of oxidative and signal transduction pathways. Circ. Res. 2012, 110, 1279–1293. [Google Scholar] [CrossRef] [PubMed]
- Sung, H.J.; Eskin, S.G.; Sakurai, Y.; Yee, A.; Kataoka, N.; McIntire, L.V. Oxidative stress produced with cell migration increases synthetic phenotype of vascular smooth muscle cells. Ann. Biomed. Eng. 2005, 33, 1546–1554. [Google Scholar] [CrossRef] [PubMed]
- Su, B.; Mitra, S.; Gregg, H.; Flavahan, S.; Chotani, M.A.; Clark, K.R.; Goldschmidt-Clermont, P.J.; Flavahan, N.A. Redox regulation of vascular smooth muscle cell differentiation. Circ. Res. 2001, 89, 39–46. [Google Scholar] [CrossRef] [PubMed]
- Lee, M.Y.; Griendling, K.K. Redox signaling, vascular function, and hypertension. Antioxid. Redox Signal. 2008, 10, 1045–1059. [Google Scholar] [CrossRef] [PubMed]
- Dikalov, S.I.; Dikalova, A.E.; Bikineyeva, A.T.; Schmidt, H.H.H.W.; Harrison, D.G.; Griendling, K.K. Distinct roles of Nox1 and Nox4 in basal and angiotensin II-stimulated superoxide and hydrogen peroxide production. Free Radic. Biol. Med. 2008, 45, 1340–1351. [Google Scholar] [CrossRef] [PubMed]
- Mittal, M.; Roth, M.; König, P.; Hofmann, S.; Dony, E.; Goyal, P.; Selbitz, A.C.; Schermuly, R.T.; Ghofrani, H.A.; Kwapiszewska, G.; et al. Hypoxia-dependent regulation of nonphagocytic NADPH oxidase subunit NOX4 in the pulmonary vasculature. Circ. Res. 2007, 101, 258–267. [Google Scholar] [CrossRef] [PubMed]
- Clempus, R.E.; Sorescu, D.; Dikalova, A.E.; Pounkova, L.; Jo, P.; Sorescu, G.P.; Lassègue, B.; Griendling, K.K. Nox4 is required for maintenance of the differentiated vascular smooth muscle cell phenotype. Arterioscler. Thromb. Vasc. Biol. 2007, 27, 42–48. [Google Scholar] [CrossRef] [PubMed]
- Zuckerbraun, B.S.; Stoyanovsky, D.A.; Sengupta, R.; Shapiro, R.A.; Ozanich, B.A.; Rao, J.; Barbato, J.E.; Tzeng, E. Nitric oxide-induced inhibition of smooth muscle cell proliferation involves S-nitrosation and inactivation of RhoA. Am. J. Physiol.Cell. Physiol. 2007, 292, C824–C831. [Google Scholar] [CrossRef] [PubMed]
- Majesky, M.W. Developmental basis of vascular smooth muscle diversity. Arterioscler. Thromb. Vasc. Biol. 2007, 27, 1248–1258. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Facemire, C.S.; Banes, A.J.; Faber, J.E. Different alpha-adrenoceptors mediate migration of vascular smooth muscle cells and adventitial fibroblasts in vitro. Am. J. Physiol. Heart Circ. Physiol. 2002, 282, H2364–H2370. [Google Scholar] [PubMed]
- Nishio, E.; Watanabe, Y. The involvement of reactive oxygen species and arachidonic acid in alpha 1-adrenoceptor-induced smooth muscle cell proliferation and migration. Br. J. Pharmacol 1997, 121, 665–670. [Google Scholar] [CrossRef] [PubMed]
- Xi, X.P.; Graf, K.; Goetze, S.; Fleck, E.; Hsueh, W.A.; Law, R.E. Central role of the MAPK pathway in ang II-mediated DNA synthesis and migration in rat vascular smooth muscle cells. Arterioscler. Thromb. Vasc. Biol. 1999, 19, 73–82. [Google Scholar] [CrossRef] [PubMed]
- Kim, N.; Hwangbo, C.; Lee, S.; Lee, J.H. Eupatolide inhibits PDGF-induced proliferation and migration of aortic smooth muscle cells through ROS-dependent heme oxygenase-1 induction. Phytother. Res. 2013, 27, 1700–1707. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Castresana, M.R.; Newman, W.H. Reactive oxygen and NF-κB in VEGF-induced migration of human vascular smooth muscle cells. Biochem. Biophys. Res. Commun. 2001, 285, 669–674. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Castresana, M.R.; Newman, W.H. Reactive oxygen species-sensitive p38 MAPK controls thrombin-induced migration of vascular smooth muscle cells. J. Mol. Cell. Cardiol. 2004, 36, 49–56. [Google Scholar] [CrossRef] [PubMed]
- Xu, S.; Shriver, A.S.; Jagadeesha, D.K.; Chamseddine, A.H.; Szócs, K.; Weintraub, N.L.; Griendling, K.K.; Bhalla, R.C.; Miller, F.J., Jr. Increased expression of Nox1 in neointimal smooth muscle cells promotes activation of matrix metalloproteinase-9. J. Vasc. Res. 2012, 49, 242–248. [Google Scholar] [CrossRef] [PubMed]
- Hsieh, C.C.; Lau, Y.T. Migration of vascular smooth muscle cells is enhanced in cultures derived from spontaneously hypertensive rat. Pflugers Arch. 1998, 435, 286–292. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.M.; Kim, H.J.; Won, K.J.; Choi, W.S.; Lee, K.Y.; Bae, Y.M.; Park, P.J.; Park, T.K.; Lee, Y.L.; Lee, C.K.; et al. Contribution of soluble intercellular adhesion molecule-1 to the migration of vascular smooth muscle cells. Eur. J. Pharmcol. 2008, 579, 260–268. [Google Scholar] [CrossRef]
- Zhang, L.; Xie, P.; Wang, J.; Yang, Q.; Fang, C.; Zhou, S.; Li, J. Impaired peroxisome proliferator-activated receptor-γ contributes to phenotypic modulation of vascular smooth muscle cells during hypertension. J. Biol. Chem. 2010, 285, 13666–13677. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J.; Kyotani, Y.; Ito, S.; Nagayama, K.; Ozawa, K.; Yoshizumi, M. Comparison of the effects of Src inhibition and mitogen-activated protein kinase inhibition on the migration of vascular smooth muscle cells stimulated by angiotensin II. J. Nara Med. Assoc. 2012, 63, 25–35. [Google Scholar]
- Panchatcharam, M.; Miriyala, S.; Yang, F.; Leitges, M.; Chrzanowska-Wodnicka, M.; Quilliam, L.A.; Anaya, P.; Morris, A.J.; Smyth, S.S. Enhanced proliferation and migration of vascular smooth muscle cells in response to vascular injury under hyperglycemic conditions is controlled by β3 integrin signaling. Int. J. Biochem. Cell. Biol. 2010, 42, 965–974. [Google Scholar] [CrossRef] [PubMed]
- Watson, P.A.; Nesterova, A.; Burant, C.F.; Klemm, D.J.; Reusch, J.E.B. Diabetes-related changes in cAMP response element-binding protein content enhance smooth muscle cell proliferation and migration. J. Biol. Chem. 2001, 276, 46142–46150. [Google Scholar] [CrossRef] [PubMed]
- Yamaguchi, H.; Igarashi, M.; Hirata, A.; Sugae, N.; Tsuchiya, H.; Jimbu, Y.; Tominaga, M.; Kato, T. Altered PDGF-BB-induced p38 MAP kinase activation in diabetic vascular smooth muscle cells: Roles of protein kinase C-δ. Arterioscler. Thromb. Vasc. Biol. 2004, 24, 2095–2101. [Google Scholar] [CrossRef] [PubMed]
- Sundaresan, M.; Yu, Z.X.; Ferrans, V.J.; Irani, K.; Finkel, T. Requirement for generation of H2O2 for platelet-derived growth factor signal transduction. Science 1995, 270, 296–299. [Google Scholar] [CrossRef] [PubMed]
- Abhijit, S.; Bhaskaran, R.; Narayanasamy, A.; Chakroborty, A.; Manickam, N.; Dixit, M.; Mohan, V.; Balasubramanyam, M. Hyperinsulinemia-induced vascular smooth muscle cell (VSMC) migration and proliferation is mediated by converging mechanisms of mitochondrial dysfunction and oxidative stress. Mol. Cell. Biochem. 2013, 373, 95–105. [Google Scholar] [CrossRef] [PubMed]
- Jagadeesha, D.K.; Takapoo, M.; Banfi, B.; Bhalla, R.C.; Miller, F.J., Jr. Nox1 transactivation of epidermal growth factor receptor promotes N-cadherin shedding and smooth muscle cell migration. Cardiovasc. Res. 2012, 93, 406–413. [Google Scholar] [CrossRef] [PubMed]
- Mac Gabhann, F.; Peirce, S.M. Collateral capillary arterialization following arteriolar ligation in murine skeletal muscle. Microcirculation 2010, 17, 333–347. [Google Scholar] [PubMed]
- Unthank, J.L.; Fath, S.W.; Burkhart, H.M.; Miller, S.C.; Dalsing, M.C. Wall remodeling during luminal expansion of mesenteric arterial collaterals in the rat. Circ. Res. 1996, 79, 1015–1023. [Google Scholar] [CrossRef] [PubMed]
- Wolf, C.; Cai, W.J.; Vosschulte, R.; Koltai, S.; Mousavipour, D.; Scholz, D.; Afsah-Hedjri, A.; Schaper, W.; Schaper, J. Vascular remodeling and altered protein expression during growth of coronary collateral arteries. J. Mol. Cell. Cardiol. 1998, 30, 2291–305. [Google Scholar] [CrossRef] [PubMed]
- Gerthoffer, W.T. Mechanisms of vascular smooth muscle cell migration. Circ. Res. 2007, 100, 607–621. [Google Scholar] [CrossRef] [PubMed]
- Hong, Z.; Sun, Z.; Li, M.; Li, Z.; Bunyak, F.; Ersoy, I.; Trzeciakowski, J.P.; Staiculescu, M.C.; Jin, M.; Martinez-Lemus, L.; et al. Vasoactive agonists exert dynamic and coordinated effects on vascular smooth muscle cell elasticity, cytoskeletal remodelling and adhesion. J. Physiol. 2014, 592, 1249–1266. [Google Scholar] [CrossRef] [PubMed]
- Hong, Z.; Sun, Z.; Li, Z.; Mesquitta, W.T.; Trzeciakowski, J.P.; Meininger, G.A. Coordination of fibronectin adhesion with contraction and relaxation in microvascular smooth muscle. Cardiovasc. Res. 2012, 96, 73–80. [Google Scholar] [CrossRef] [PubMed]
- Yamin, R.; Morgan, K.G. Deciphering actin cytoskeletal function in the contractile vascular smooth muscle cell. J. Physiol. 2012, 590, 4145–4154. [Google Scholar] [CrossRef] [PubMed]
- Lehman, W.; Morgan, K.G. Structure and dynamics of the actin-based smooth muscle contractile and cytoskeletal apparatus. J. Muscle Res. Cell. Motil. 2012, 33, 461–469. [Google Scholar] [CrossRef] [PubMed]
- Castorena-Gonzalez, J.A.; Staiculescu, M.C.; Foote, C.A.; Polo-Parada, L.; Martinez-Lemus, L.A. The obligatory role of the actin cytoskeleton on inward remodeling induced by dithiothreitol activation of endogenous transglutaminase in isolated arterioles. Am. J. Physiol. Heart Circ. Physiol. 2014, 306, H485–H495. [Google Scholar] [CrossRef] [PubMed]
- Gunst, S.J.; Zhang, W. Actin cytoskeletal dynamics in smooth muscle: A new paradigm for the regulation of smooth muscle contraction. Am. J. Physiol. Cell. Physiol. 2008, 295, C576–C587. [Google Scholar] [CrossRef] [PubMed]
- Crawford, L.E.; Milliken, E.E.; Irani, K.; Zweier, J.L.; Becker, L.C.; Johnson, T.M.; Eissa, N.T.; Crystal, R.G.; Finkel, T.; Goldschmidt-Clermont, P.J. Superoxide-mediated actin response in post-hypoxic endothelial cells. J. Biol. Chem. 1996, 271, 26863–26867. [Google Scholar] [CrossRef] [PubMed]
- Zweier, J.L.; Broderick, R.; Kuppusamy, P.; Thompson-Gorman, S.; Lutty, G.A. Determination of the mechanism of free radical generation in human aortic endothelial cells exposed to anoxia and reoxygenation. J. Biol. Chem. 1994, 269, 24156–24162. [Google Scholar] [PubMed]
- Moldovan, L.; Moldovan, N.I.; Sohn, R.H.; Parikh, S.A.; Goldschmidt-Clermont, P.J. Redox changes of cultured endothelial cells and actin dynamics. Circ. Res. 2000, 86, 549–557. [Google Scholar] [CrossRef] [PubMed]
- Firat-Karalar, E.N.; Welch, M.D. New mechanisms and functions of actin nucleation. Curr. Opin. Cell. Biol. 2011, 23, 4–13. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Wu, Y.; Du, L.; Tang, D.D.; Gunst, S.J. Activation of the Arp2/3 complex by N-WASp is required for actin polymerization and contraction in smooth muscle. Am. J. Physiol. Cell. Physiol. 2005, 288, C1145–C1460. [Google Scholar] [CrossRef] [PubMed]
- Tang, D.D.; Zhang, W.; Gunst, S.J. The adapter protein CrkII regulates neuronal Wiskott-Aldrich syndrome protein, actin polymerization, and tension development during contractile stimulation of smooth muscle. J. Biol. Chem. 2005, 280, 23380–23389. [Google Scholar] [CrossRef] [PubMed]
- Anfinogenova, Y.; Wang, R.; Li, Q.F.; Spinelli, A.M.; Tang, D.D. Abl silencing inhibits CAS-mediated process and constriction in resistance arteries. Circ. Res. 2007, 101, 420–428. [Google Scholar] [CrossRef] [PubMed]
- Al Ghouleh, I.; Rodriguez, A.; Pagano, P.J.; Csanyi, G. Proteomic analysis identifies an NADPH oxidase 1 (Nox1)-mediated role for actin-related protein 2/3 complex subunit 2 (ARPC2) in promoting smooth muscle cell migration. Int. J. Mol. Sci. 2013, 14, 20220–20235. [Google Scholar] [CrossRef] [PubMed]
- Kaverina, I.; Stradal, T.E.; Gimona, M. Podosome formation in cultured A7r5 vascular smooth muscle cells requires Arp2/3-dependent de-novo actin polymerization at discrete microdomains. J. Cell Sci. 2003, 116, 4915–4924. [Google Scholar] [CrossRef] [PubMed]
- DalleDonne, I.; Milzani, A.; Colombo, R. H2O2-treated actin: Assembly and polymer interactions with cross-linking proteins. Biophys. J. 1995, 69, 2710–2719. [Google Scholar] [CrossRef] [PubMed]
- Milzani, A.; DalleDonne, I.; Colombo, R. Prolonged oxidative stress on actin. Arch. Biochem. Biophys. 1997, 339, 267–274. [Google Scholar] [CrossRef] [PubMed]
- Huot, J.; Houle, F.; Marceau, F.; Landry, J. Oxidative stress-induced actin reorganization mediated by the p38 mitogen-activated protein kinase/heat shock protein 27 pathway in vascular endothelial cells. Circ. Res. 1997, 80, 383–392. [Google Scholar] [CrossRef] [PubMed]
- Aghajanian, A.; Wittchen, E.S.; Campbell, S.L.; Burridge, K. Direct activation of RhoA by reactive oxygen species requires a redox-sensitive motif. PLoS One 2009, 4, e8045. [Google Scholar] [CrossRef] [PubMed]
- Jaffe, A.B.; Hall, A. Rho GTPases: Biochemistry and biology. Annu. Rev. Cell. Dev. Biol. 2005, 21, 247–69. [Google Scholar] [CrossRef] [PubMed]
- Ten Klooster, J.P.; Evers, E.E.; Janssen, L.; Machesky, L.M.; Michiels, F.; Hordijk, P.; Collard, J.G. Interaction between Tiam1 and the Arp2/3 complex links activation of Rac to actin polymerization. Biochem. J. 2006, 397, 39–45. [Google Scholar] [CrossRef] [PubMed]
- Millius, A.; Watanabe, N.; Weiner, O.D. Diffusion, capture and recycling of SCAR/WAVE and Arp2/3 complexes observed in cells by single-molecule imaging. J. Cell. Sci 2012, 125, 1165–1176. [Google Scholar] [CrossRef] [PubMed]
- Kraemer, A.; Goodwin, M.; Verma, S.; Yap, A.S.; Ali, R.G. Rac is a dominant regulator of cadherin-directed actin assembly that is activated by adhesive ligation independently of Tiam1. Am. J. Physiol. Cell. Physiol. 2007, 292, C1061–C1069. [Google Scholar] [CrossRef] [PubMed]
- Sun, H.; Schlondorff, J.S.; Brown, E.J.; Higgs, H.N.; Pollak, M.R. Rho activation of mDia formins is modulated by an interaction with inverted formin 2 (INF2). Proc. Natl. Acad. Sci. USA 2011, 108, 2933–2938. [Google Scholar] [CrossRef] [PubMed]
- Otomo, T.; Otomo, C.; Tomchick, D.R.; Machius, M.; Rosen, M.K. Structural basis of Rho GTPase-mediated activation of the formin mDia1. Mol. Cell. 2005, 18, 273–2781. [Google Scholar] [CrossRef] [PubMed]
- Tang, D.D.; Gunst, S.J. The small GTPase Cdc42 regulates actin polymerization and tension development during contractile stimulation of smooth muscle. J. Biol. Chem. 2004, 279, 51722–51728. [Google Scholar] [CrossRef] [PubMed]
- Jin, L.; Ying, Z.; Webb, R.C. Activation of Rho/Rho kinase signaling pathway by reactive oxygen species in rat aorta. Am. J. Physiol Heart Circ. Physiol. 2004, 287, H1495–H1500. [Google Scholar] [CrossRef] [PubMed]
- Jernigan, N.L.; Walker, B.R.; Resta, T.C. Reactive oxygen species mediate RhoA/Rho kinase-induced Ca2+ sensitization in pulmonary vascular smooth muscle following chronic hypoxia. Am. J. Physiol. Lung Cell. Mol. Physiol. 2008, 295, L515–L529. [Google Scholar] [CrossRef] [PubMed]
- Mishra, N.; Nugent, W.H.; Mahavadi, S.; Walsh, S.W. Mechanisms of enhanced vascular reactivity in preeclampsia. Hypertension 2011, 58, 867–873. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.F.; Tang, D.D. Role of p47(phox) in regulating Cdc42GAP, vimentin, and contraction in smooth muscle cells. Am. J. Physiol. Cell. Physiol. 2009, 297, C1424–C1433. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.F.; Spinelli, A.M.; Tang, D.D. Cdc42GAP, reactive oxygen species, and the vimentin network. Am. J. Physiol. Cell. Physiol. 2009, 297, C299–C309. [Google Scholar] [CrossRef] [PubMed]
- Li, P.F.; Dietz, R.; von Harsdorf, R. Differential effect of hydrogen peroxide and superoxide anion on apoptosis and proliferation of vascular smooth muscle cells. Circulation 1997, 96, 3602–3609. [Google Scholar] [CrossRef] [PubMed]
- Rao, G.N.; Berk, B.C. Active oxygen species stimulate vascular smooth muscle cell growth and proto-oncogene expression. Circ. Res. 1992, 70, 593–599. [Google Scholar] [CrossRef] [PubMed]
- Deshpande, N.N.; Sorescu, D.; Seshiah, P.; Ushio-Fukai, M.; Akers, M.; Yin, Q.; Griendling, K.K. Mechanism of hydrogen peroxide-induced cell cycle arrest in vascular smooth muscle. Antioxid. Redox Signal. 2002, 4, 845–854. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Li, W.; Su, J.; Liu, W.; Altura, B.T.; Altura, B.M. Hydrogen peroxide induces apoptosis in cerebral vascular smooth muscle cells: Possible relation to neurodegenerative diseases and strokes. Brain Res. Bull. 2003, 62, 101–106. [Google Scholar] [CrossRef] [PubMed]
- Zafari, A.M.; Ushio-Fukai, M.; Akers, M.; Yin, Q.; Shah, A.; Harrison, D.G.; Taylor, W.R.; Griendling, K.K. Role of NADH/NADPH oxidase-derived H2O2 in angiotensin II-induced vascular hypertrophy. Hypertension 1998, 32, 488–495. [Google Scholar] [CrossRef] [PubMed]
- Al Ghouleh, I.; Frazziano, G.; Rodriguez, A.I.; Csányi, G.; Maniar, S.; St Croix, C.M.; Kelley, E.E.; Egaña, L.A.; Song, G.J.; Bisello, A.; et al. Aquaporin 1, Nox1, and Ask1 mediate oxidant-induced smooth muscle cell hypertrophy. Cardiovasc. Res. 2013, 97, 134–142. [Google Scholar] [CrossRef] [PubMed]
- Usui, T.; Okada, M.; Hara, Y.; Yamawaki, H. Death-associated protein kinase 3 mediates vascular inflammation and development of hypertension in spontaneously hypertensive rats. Hypertension 2012, 60, 1031–1039. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.D.; Johns, D.G.; Xu, S.; Cohen, R.A. Role of superoxide anion in regulating pressor and vascular hypertrophic response to angiotensin II. Am. J. Physiol. Heart Circ. Physiol. 2002, 282, H1697–H1702. [Google Scholar] [PubMed]
- Zhang, Y.; Griendling, K.K.; Dikalova, A.; Owens, G.K.; Taylor, W.R. Vascular hypertrophy in angiotensin II-induced hypertension is mediated by vascular smooth muscle cell-derived H2O2. Hypertension 2005, 46, 732–737. [Google Scholar] [CrossRef] [PubMed]
- Matesanz, N.; Lafuente, N.; Azcutia, V.; Martín, D.; Cuadrado, A.; Nevado, J.; Rodríguez-Mañas, L.; Sánchez-Ferrer, C.F.; Peiró, C. Xanthine oxidase-derived extracellular superoxide anions stimulate activator protein 1 activity and hypertrophy in human vascular smooth muscle via c-Jun N-terminal kinase and p38 mitogen-activated protein kinases. J. Hypertens. 2007, 25, 609–618. [Google Scholar] [CrossRef] [PubMed]
- Taniyama, Y.; Ushio-Fukai, M.; Hitomi, H.; Rocic, P.; Kingsley, M.J.; Pfahnl, C.; Weber, D.S.; Alexander, R.W.; Griendling, K.K. Role of p38 MAPK and MAPKAPK-2 in angiotensin II-induced Akt activation in vascular smooth muscle cells. Am. J. Physiol. Cell Physiol. 2004, 287, C494–C499. [Google Scholar] [CrossRef] [PubMed]
- Ushio-Fukai, M.; Alexander, R.W.; Akers, M.; Griendling, K.K. p38 mitogen-activated protein kinase is a critical component of the redox-sensitive signaling pathways activated by angiotensin II. Role in vascular smooth muscle cell hypertrophy. J. Biol. Chem. 1998, 273, 15022–15029. [Google Scholar] [CrossRef] [PubMed]
- Shi, M.; Yang, H.; Motley, E.D.; Guo, Z. Overexpression of Cu/Zn-superoxide dismutase and/or catalase in mice inhibits aorta smooth muscle cell proliferation. Am. J. Hypertens. 2004, 17, 450–456. [Google Scholar] [CrossRef] [PubMed]
- Wei, Y.; Whaley-Connell, A.T.; Chen, K.; Habibi, J.; Uptergrove, G.M.E.; Clark, S.E.; Stump, C.S.; Ferrario, C.M.; Sowers, J.R. NADPH oxidase contributes to vascular inflammation, insulin resistance, and remodeling in the transgenic (mRen2) rat. Hypertension 2007, 50, 384–391. [Google Scholar] [CrossRef] [PubMed]
- Xiong, W.; Mactaggart, J.; Knispel, R.; Worth, J.; Zhu, Z.; Li, Y.; Sun, Y.; Baxter, B.T.; Johanning, J. Inhibition of reactive oxygen species attenuates aneurysm formation in a murine model. Atherosclerosis 2009, 202, 128–34. [Google Scholar] [CrossRef] [PubMed]
- Rizzoni, D.; Rodella, L.; Porteri, E.; Rezzani, R.; Guelfi, D.; Piccoli, A.; Castellano, M.; Muiesan, M.L.; Bianchi, R.; Rosei, E.A. Time course of apoptosis in small resistance arteries of spontaneously hypertensive rats. J. Hypertens. 2000, 18, 885–891. [Google Scholar] [CrossRef] [PubMed]
- Diep, Q.N.; Li, J.S.; Schiffrin, E.L. In vivo study of AT1 and AT2 angiotensin receptors in apoptosis in rat blood vessels. Hypertension 1999, 34, 617–624. [Google Scholar] [CrossRef] [PubMed]
- Irani, K. Oxidant signaling in vascular cell growth, death, and survival: A review of the roles of reactive oxygen species in smooth muscle and endothelial cell mitogenic and apoptotic signaling. Circ. Res. 2000, 87, 179–183. [Google Scholar] [CrossRef] [PubMed]
- Matsubara, H. Pathophysiological role of angiotensin II type 2 receptor in cardiovascular and renal diseases. Circ. Res. 1998, 83, 1182–1191. [Google Scholar] [CrossRef] [PubMed]
- Tao, J.; Zhang, Y.; Tuck, W.S.; Li, S. Urocortin II inhibits the apoptosis of mesenteric arterial smooth muscle cells via L-type calcium channels in spontaneously hypertensive rats. Cell. Physiol. Biochem. 2006, 17, 111–120. [Google Scholar] [CrossRef] [PubMed]
- Rodrigues, S.F.; Tran, E.D.; Fortes, Z.B.; Schmid-Schönbein, G.W. Matrix metalloproteinases cleave the β2-adrenergic receptor in spontaneously hypertensive rats. Am. J. Physiol. Heart Circ. Physiol. 2010, 299, H25–H35. [Google Scholar] [CrossRef] [PubMed]
- Abdalvand, A.; Morton, J.S.; Bourque, S.L.; Quon, A.L.; Davidge, S.T. Matrix metalloproteinase enhances big-endothelin-1 constriction in mesenteric vessels of pregnant rats with reduced uterine blood flow. Hypertension 2013, 61, 488–493. [Google Scholar] [CrossRef] [PubMed]
- Van Lint, P.; Libert, C. Chemokine and cytokine processing by matrix metalloproteinases and its effect on leukocyte migration and inflammation. J. Leukoc. Biol. 2007, 82, 1375–1381. [Google Scholar] [CrossRef] [PubMed]
- Márquez-Martín, A.; Jiménez-Altayó, F.; Dantas, A.P.; Caracuel, L.; Planas, A.M.; Vila, E. Middle cerebral artery alterations in a rat chronic hypoperfusion model. J. Appl. Physiol. 2012, 112, 511–518. [Google Scholar] [CrossRef] [PubMed]
- Spiers, J.P.; Kelso, E.J.; Siah, W.F.; Edge, G.; Song, G.; McDermott, B.J.; Hennessy, M. Alterations in vascular matrix metalloproteinase due to ageing and chronic hypertension: Effects of endothelin receptor blockade. J. Hypertens. 2005, 23, 1717–1724. [Google Scholar] [CrossRef] [PubMed]
- Marchesi, C.; Dentali, F.; Nicolini, E.; Maresca, A.M.; Tayebjee, M.H.; Franz, M.; Guasti, L.; Venco, A.; Schiffrin, E.L.; Lip, G.Y.H.; et al. Plasma levels of matrix metalloproteinases and their inhibitors in hypertension: A systematic review and meta-analysis. J. Hypertens. 2012, 30, 3–16. [Google Scholar] [CrossRef] [PubMed]
- Kadoglou, N.P.E.; Vrabas, I.S.; Sailer, N.; Kapelouzou, A.; Fotiadis, G.; Noussios, G.; Karayannacos, P.E.; Angelopoulou, N. Exercise ameliorates serum MMP-9 and TIMP-2 levels in patients with type 2 diabetes. Diabetes Metab. 2010, 36, 144–151. [Google Scholar] [CrossRef] [PubMed]
- Tayebjee, M.H.; Karalis, I.; Nadar, S.K.; Beevers, D.G.; MacFadyen, R.J.; Lip, G.Y.H. Circulating matrix metalloproteinase-9 and tissue inhibitors of metalloproteinases-1 and -2 levels in gestational hypertension. Am. J. Hypertens. 2005, 18, 325–329. [Google Scholar] [CrossRef] [PubMed]
- Fontana, V.; Silva, P.S.; Belo, V.A.; Antonio, R.C.; Ceron, C.S.; Biagi, C.; Gerlach, R.F.; Tanus-Santos, J.E. Consistent alterations of circulating matrix metalloproteinases levels in untreated hypertensives and in spontaneously hypertensive rats: A relevant pharmacological target. Basic Clin. Pharmacol. Toxicol. 2011, 109, 130–137. [Google Scholar] [CrossRef] [PubMed]
- Estrada-Gutierrez, G.; Cappello, R.E.; Mishra, N.; Romero, R.; Strauss, J.F., III; Walsh, S.W. Increased expression of matrix metalloproteinase-1 in systemic vessels of preeclamptic women: A critical mediator of vascular dysfunction. Am. J. Pathol. 2011, 178, 451–460. [Google Scholar] [CrossRef] [PubMed]
- Hu, T.; Luan, R.; Zhang, H.; Lau, W.B.; Wang, Q.; Zhang, Y.; Wang, H.C.; Tao, L. Hydrogen peroxide enhances osteopontin expression and matrix metalloproteinase activity in aortic vascular smooth muscle cells. Clin. Exp. Pharmacol. Physiol. 2009, 36, 626–630. [Google Scholar] [CrossRef] [PubMed]
- Nagareddy, P.R.; Chow, F.L.; Hao, L.; Wang, X.; Nishimura, T.; MacLeod, K.M.; McNeill, J.H.; Fernandez-Patron, C. Maintenance of adrenergic vascular tone by MMP transactivation of the EGFR requires PI3K and mitochondrial ATP synthesis. Cardiovasc. Res. 2009, 84, 368–377. [Google Scholar] [CrossRef] [PubMed]
- Kamioka, M.; Ishibashi, T.; Ohkawara, H.; Nagai, R.; Sugimoto, K.; Uekita, H.; Matsui, T.; Yamagishi, S.I.; Ando, K.; Sakamoto, T; et al. Involvement of membrane type 1-matrix metalloproteinase (MT1-MMP) in RAGE activation signaling pathways. J. Cell. Physiol. 2011, 226, 1554–1563. [Google Scholar] [CrossRef] [PubMed]
- Valentin, F.; Bueb, J.L.; Kieffer, P.; Tschirhart, E.; Atkinson, J. Oxidative stress activates MMP-2 in cultured human coronary smooth muscle cells. Fundam. Clin. Pharmacol. 2005, 19, 661–667. [Google Scholar] [CrossRef] [PubMed]
- Browatzki, M.; Larsen, D.; Pfeiffer, C.A. M.; Gehrke, S.G.; Schmidt, J.; Kranzhöfer, A.; Katus, H.A.; Kranzhöfer, R. Angiotensin II stimulates matrix metalloproteinase secretion in human vascular smooth muscle cells via nuclear factor-κB and activator protein 1 in a redox-sensitive manner. J. Vasc. Res. 2005, 42, 415–423. [Google Scholar] [CrossRef] [PubMed]
- Luchtefeld, M.; Grote, K.; Grothusen, C.; Bley, S.; Bandlow, N.; Selle, T.; Struber, M.; Haverich, A.; Bavendiek, U.; Drexler, H.; et al. Angiotensin II induces MMP-2 in a p47phox-dependent manner. Biochem. Biophys. Res. Commun. 2005, 328, 183–188. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.S.; Wang, S.Q. Salvianolic acid B from Salvia miltiorrhiza inhibits tumor necrosis factor-α (TNF-α)-induced MMP-2 upregulation in human aortic smooth muscle cells via suppression of NAD(P)H oxidase-derived reactive oxygen species. J. Mol. Cell. Cardiol. 2006, 41, 138–148. [Google Scholar] [CrossRef] [PubMed]
- Castro, M.M.; Rizzi, E.; Ceron, C.S.; Guimaraes, D.A.; Rodrigues, G.J.; Bendhack, L.M.; Gerlach, R.F.; Tanus-Santos, J.E. Doxycycline ameliorates 2K-1C hypertension-induced vascular dysfunction in rats by attenuating oxidative stress and improving nitric oxide bioavailability. Nitric Oxide 2012, 26, 162–168. [Google Scholar] [CrossRef] [PubMed]
- Cau, S.B. A.; Guimaraes, D.A.; Rizzi, E.; Ceron, C.S.; Souza, L.L.; Tirapelli, C.R.; Gerlach, R.F.; Tanus-Santos, J.E. Pyrrolidine dithiocarbamate down-regulates vascular matrix metalloproteinases and ameliorates vascular dysfunction and remodelling in renovascular hypertension. Br. J. Pharmacol. 2011, 164, 372–381. [Google Scholar] [CrossRef] [PubMed]
- Castro, M.M.; Rizzi, E.; Rodrigues, G.J.; Ceron, C.S.; Bendhack, L.M.; Gerlach, R.F; Tanus-Santos, J.E. Antioxidant treatment reduces matrix metalloproteinase-2-induced vascular changes in renovascular hypertension. Free Radic. Biol. Med. 2009, 46, 1298–1307. [Google Scholar] [CrossRef] [PubMed]
- Feihl, F.; Liaudet, L.; Waeber, B.; Levy, B.I. Hypertension: A disease of the microcirculation? Hypertension 2006, 48, 1012–1017. [Google Scholar] [CrossRef] [PubMed]
- Vogt, C.J.; Schmid-Schönbein, G.W. Microvascular endothelial cell death and rarefaction in the glucocorticoid-induced hypertensive rat. Microcirculation 2001, 8, 129–139. [Google Scholar] [CrossRef] [PubMed]
- Gobé, G.; Browning, J.; Howard, T.; Hogg, N.; Winterford, C.; Cross, R. Apoptosis occurs in endothelial cells during hypertension-induced microvascular rarefaction. J. Struct. Biol. 1997, 118, 63–72. [Google Scholar] [CrossRef] [PubMed]
- Doughan, A.K.; Dikalov, S.I. Mitochondrial redox cycling of mitoquinone leads to superoxide production and cellular apoptosis. Antioxid. Redox Signal. 2007, 9, 1825–1836. [Google Scholar] [CrossRef] [PubMed]
- Hao, C.N.; Geng, Y.J.; Li, F.; Yang, T.; Su, D.F.; Duan, J.L.; Li, Y. Insulin-like growth factor-1 receptor activation prevents hydrogen peroxide-induced oxidative stress, mitochondrial dysfunction and apoptosis. Apoptosis 2011, 16, 1118–1127. [Google Scholar] [CrossRef] [PubMed]
- Nako, H.; Kataoka, K.; Koibuchi, N.; Dong, Y.F.; Toyama, K.; Yamamoto, E.; Yasuda, O.; Ichijo, H.; Ogawa, H.; Kim-Mitsuyama, S. Novel mechanism of angiotensin II-induced cardiac injury in hypertensive rats: the critical role of ASK1 and VEGF. Hypertens. Res. 2012, 35, 194–200. [Google Scholar] [CrossRef] [PubMed]
- Kobayashi, N.; DeLano, F.A.; Schmid-Schönbein, G.W. Oxidative stress promotes endothelial cell apoptosis and loss of microvessels in the spontaneously hypertensive rats. Arterioscler. Thromb. Vasc. Biol. 2005, 25, 2114–2121. [Google Scholar] [CrossRef] [PubMed]
- Miller, S.J.; Norton, L.E.; Murphy, M.P.; Dalsing, M.C.; Unthank, J.L. The role of the renin-angiotensin system and oxidative stress in spontaneously hypertensive rat mesenteric collateral growth impairment. Am. J. Physiol. Heart Circ. Physiol. 2007, 292, H2523–H2531. [Google Scholar] [CrossRef] [PubMed]
- Khodo, S.N.; Dizin, E.; Sossauer, G.; Szanto, I.; Martin, P.Y.; Feraille, E.; Krause, K.H.; de Seigneux, S. NADPH-oxidase 4 protects against kidney fibrosis during chronic renal injury. J. Am. Soc. Nephrol. 2012, 23, 1967–1976. [Google Scholar] [CrossRef] [PubMed]
- Antonios, T.F.T.; Rattray, F.M.; Singer, D.R.J.; Markandu, N.D.; Mortimer, P.S.; MacGregor, G.A. Rarefaction of skin capillaries in normotensive offspring of individuals with essential hypertension. Heart 2003, 89, 175–178. [Google Scholar] [CrossRef] [PubMed]
- Antonios, T.F.T.; Raghuraman, R.P.; D’Souza, R.; Nathan, P.; Wang, D.; Manyonda, I.T. Capillary remodeling in infants born to hypertensive pregnancy: Pilot study. Am. J. Hypertens. 2012, 25, 848–853. [Google Scholar] [CrossRef] [PubMed]
- Valcarcel-Ares, M.N.; Gautam, T.; Warrington, J.P.; Bailey-Downs, L.; Sosnowska, D.; de Cabo, R.; Losonczy, G.; Sonntag, W.E.; Ungvari, Z.; Csiszar, A. Disruption of Nrf2 signaling impairs angiogenic capacity of endothelial cells: Implications for microvascular aging. J. Gerontol. 2012, 67, 821–829. [Google Scholar] [CrossRef]
- Wagatsuma, A. Effect of aging on expression of angiogenesis-related factors in mouse skeletal muscle. Exp. Gerontol. 2006, 41, 49–54. [Google Scholar] [CrossRef] [PubMed]
- Kubo, M.; Li, T.S.; Kurazumi, H.; Takemoto, Y.; Ohshima, M.; Murata, T.; Katsura, S.; Morikage, N.; Furutani, A.; Hamano, K. Hypoxic preconditioning enhances angiogenic potential of bone marrow cells with aging-related functional impairment. Circ. J. 2012, 76, 986–994. [Google Scholar] [CrossRef] [PubMed]
- Swift, M.E.; Kleinman, H.K.; DiPietro, L.A. Impaired wound repair and delayed angiogenesis in aged mice. Lab. Investig. 1999, 79, 1479–1487. [Google Scholar] [PubMed]
- Choudhery, M.S.; Khan, M.; Mahmood, R.; Mehmood, A.; Khan, S.N.; Riazuddin, S. Bone marrow derived mesenchymal stem cells from aged mice have reduced wound healing, angiogenesis, proliferation and anti-apoptosis capabilities. Cell. Biol. Int. 2012, 36, 747–753. [Google Scholar] [CrossRef] [PubMed]
- Jesmin, S.; Hattori, Y.; Togashi, H.; Ueno, K.I.; Yoshioka, M.; Sakuma, I. Age-related changes in cardiac expression of VEGF and its angiogenic receptor KDR in stroke-prone spontaneously hypertensive rats. Mol. Cell. Biochem. 2005, 272, 63–73. [Google Scholar] [CrossRef] [PubMed]
- Guzik, T.J.; Mussa, S.; Gastaldi, D.; Sadowski, J.; Ratnatunga, C.; Pillai, R.; Channon, K.M. Mechanisms of increased vascular superoxide production in human diabetes mellitus: Role of NAD(P)H oxidase and endothelial nitric oxide synthase. Circulation 2002, 105, 1656–1662. [Google Scholar] [CrossRef] [PubMed]
- Bravard, A.; Bonnard, C.; Durand, A.; Chauvin, M.A.; Favier, R.; Vidal, H.; Rieusset, J. Inhibition of xanthine oxidase reduces hyperglycemia-induced oxidative stress and improves mitochondrial alterations in skeletal muscle of diabetic mice. Am. J. Physiol. Endocrinol. Metab. 2011, 300, E581–E591. [Google Scholar] [CrossRef] [PubMed]
- Rask-Madsen, C.; King, G.L. Mechanisms of disease: Endothelial dysfunction in insulin resistance and diabetes. Nat. Clin. Pract. Endocrinol. Metab. 2007, 3, 46–56. [Google Scholar] [CrossRef] [PubMed]
- Tepper, O.M.; Galiano, R.D.; Capla, J.M.; Kalka, C.; Gagne, P.J.; Jacobowitz, G.R.; Levine, J.P.; Gurtner, G.C. Human endothelial progenitor cells from type II diabetics exhibit impaired proliferation, adhesion, and incorporation into vascular structures. Circulation 2002, 106, 2781–2786. [Google Scholar] [CrossRef] [PubMed]
- Aicher, A.; Heeschen, C.; Mildner-Rihm, C.; Urbich, C.; Ihling, C.; Technau-Ihling, K.; Zeiher, A.M.; Dimmeler, S. Essential role of endothelial nitric oxide synthase for mobilization of stem and progenitor cells. Nat. Med. 2003, 9, 1370–1376. [Google Scholar] [CrossRef] [PubMed]
- Kowluru, R.A.; Chan, P.-S. Oxidative stress and diabetic retinopathy. Exp. Diabetes Res. 2007, 2007. [Google Scholar] [CrossRef]
- Lu, M.; Kuroki, M.; Amano, S.; Tolentino, M.; Keough, K.; Kim, I.; Bucala, R.; Adamis, A.P. Advanced glycation end products increase retinal vascular endothelial growth factor expression. J. Clin. Investig. 1998, 101, 1219–1224. [Google Scholar] [CrossRef] [PubMed]
- Ushio-Fukai, M. Redox signaling in angiogenesis: Role of NADPH oxidase. Cardiovasc. Res. 2006, 71, 226–235. [Google Scholar] [CrossRef] [PubMed]
- Heerkens, E.H.; Shaw, L.; Ryding, A.; Brooker, G.; Mullins, J.J.; Austin, C.; Ohanian, V.; Heagerty, A.M. alphaV integrins are necessary for eutrophic inward remodeling of small arteries in hypertension. Hypertension 2006, 47, 281–287. [Google Scholar] [CrossRef] [PubMed]
- Hishikawa, K.; Oemar, B.S.; Yang, Z.; Luscher, T.F. Pulsatile stretch stimulates superoxide production and activates nuclear factor-kappa B in human coronary smooth muscle. Circ. Res. 1997, 81, 797–803. [Google Scholar] [CrossRef] [PubMed]
- Grote, K.; Flach, I.; Luchtefeld, M.; Akin, E.; Holland, S.M.; Drexler, H.; Schieffer, B. Mechanical stretch enhances mRNA expression and proenzyme release of matrix metalloproteinase-2 (MMP-2) via NAD(P)H oxidase-derived reactive oxygen species. Circ. Res. 2003, 92, e80–e86. [Google Scholar] [CrossRef] [PubMed]
- Leopold, J.A.; Loscalzo, J. Cyclic strain modulates resistance to oxidant stress by increasing G6PDH expression in smooth muscle cells. Am. J. Physiol. Heart Circ. Physiol. 2000, 279, H2477–H2485. [Google Scholar] [PubMed]
- Lehoux, S. Redox signalling in vascular responses to shear and stretch. Cardiovasc. Res. 2006, 71, 269–279. [Google Scholar] [CrossRef] [PubMed]
- Lehoux, S.; Esposito, B.; Merval, R.; Loufrani, L.; Tedgui, A. Pulsatile stretch-induced extracellular signal-regulated kinase 1/2 activation in organ culture of rabbit aorta involves reactive oxygen species. Arterioscler. Thromb. Vasc. Biol. 2000, 20, 2366–2372. [Google Scholar] [CrossRef] [PubMed]
- Ungvari, Z.; Csiszar, A.; Edwards, J.G.; Kaminski, P.M.; Wolin, M.S.; Kaley, G.; Koller, A. Increased superoxide production in coronary arteries in hyperhomocysteinemia: Role of tumor necrosis factor-alpha, NAD(P)H oxidase, and inducible nitric oxide synthase. Arterioscler. Thromb. Vasc. Biol. 2003, 23, 418–424. [Google Scholar] [CrossRef] [PubMed]
- Ungvari, Z.; Csiszar, A.; Huang, A.; Kaminski, P.M.; Wolin, M.S.; Koller, A. High pressure induces superoxide production in isolated arteries via protein kinase C-dependent activation of NAD(P)H oxidase. Circulation 2003, 108, 1253–1258. [Google Scholar] [CrossRef] [PubMed]
- Oeckler, R.A.; Kaminski, P.M.; Wolin, M.S. Stretch enhances contraction of bovine coronary arteries via an NAD(P)H oxidase-mediated activation of the extracellular signal-regulated kinase mitogen-activated protein kinase cascade. Circ. Res. 2003, 92, 23–31. [Google Scholar] [CrossRef] [PubMed]
- Ungvari, Z.; Csiszar, A.; Kaminski, P.M.; Wolin, M.S.; Koller, A. Chronic high pressure-induced arterial oxidative stress: Involvement of protein kinase C-dependent NAD(P)H oxidase and local renin-angiotensin system. Am. J. Pathol. 2004, 165, 219–226. [Google Scholar] [CrossRef] [PubMed]
- Lemarie, C.A.; Tharaux, P.L.; Esposito, B.; Tedgui, A.; Lehoux, S. Transforming growth factor-α mediates nuclear factor kappaB activation in strained arteries. Circ. Res. 2006, 99, 434–441. [Google Scholar] [CrossRef] [PubMed]
- Gebremedhin, D.; Terashvili, M.; Wickramasekera, N.; Zhang, D.X.; Rau, N.; Miura, H.; Harder, D.R. Redox signaling via oxidative inactivation of PTEN modulates pressure-dependent myogenic tone in rat middle cerebral arteries. PLoS One 2013, 8, e68498. [Google Scholar] [CrossRef] [PubMed]
- Schleifenbaum, J.; Kassmann, M.; Szijarto, I.A.; Hercule, H.C.; Tano, J.Y.; Weinert, S.; Heidenreich, M.; Pathan, A.R.; Anistan, Y.M.; Alenina, N.; et al. Stretch-activation of angiotensin II type 1a receptors contributes to the myogenic response of mouse mesenteric and renal arteries. Circ. Res. 2014, 115, 263–272. [Google Scholar] [CrossRef] [PubMed]
- Staiculescu, M.C.; Ramirez-Perez, F.I.; Castorena-Gonzalez, J.A.; Hong, Z.; Sun, Z.; Meininger, G.A.; Martinez-Lemus, L.A. Lysophosphatidic acid induces integrin activation in vascular smooth muscle and alters arteriolar myogenic vasoconstriction. Front. Physiol. 2014, 5, 413. [Google Scholar] [CrossRef]
- Martinez-Lemus, L.A.; Crow, T.; Davis, M.J.; Meininger, G.A. αvβ3- and α5β1-integrin blockade inhibits myogenic constriction of skeletal muscle resistance arterioles. Am. J. Physiol. Heart Circ. Physiol. 2005, 289, H322–H329. [Google Scholar] [CrossRef] [PubMed]
© 2014 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).